Abstract
Goals
To investigate trends in colorectal cancer (CRC) incidence and survival among Hispanics in Texas.
Background
The incidence of CRC is rising among young adults in the US. Given Texas’ large Hispanic population, investigating CRC trends in Texas may provide valuable insight into the future of CRC epidemiology in an ever-diversifying US population.
Study
Data from the Texas Cancer Registry (1995–2010) were used to calculate age-adjusted CRC rates based on the 2000 US standard population. Annual percentage change (APC) and five-year cancer-specific survival (CSS) rates were reported by age, race/ethnicity, stage, and anatomical location.
Results
Of 123,083 CRC cases, 11% occurred in individuals <50 years old, 26% of whom were Hispanic. Incidence was highest among African Americans (AAs; 76.3/100,000), followed by non-Hispanic whites (NHWs; 60.2/100,000) and Hispanics (50.8/100,000). Although overall CRC incidence declined between 1995 and 2010 (APC −1.8%, p<0.01), trends differed by age and race/ethnicity. Among individuals ≥50, the rate of decline was statistically significant among NHWs (APC −2.4%, p<0.01) and AAs (APC −1.3%, p<0.01) but not among Hispanics (APC −0.6%, p=0.13). In persons aged 20–39, CRC incidence rose significantly among Hispanics (APC 2.6%, p<.01) and NHWs (APC 2.4%, p<0.01), but not AAs (APC 0.3%, p =0.75). CSS rates among Hispanics and NHWs were comparable across most age groups and cancer stages whereas CSS rates among AAs were generally inferior to those observed among NHWs and Hispanics.
Conclusion
Although CRC incidence has declined in Texas, it is rising among young Hispanics and NHWs while declining more slowly among older Hispanics than among older NHWs and AAs.
Keywords: Hispanic, Texas, colorectal cancer, incidence, survival
INTRODUCTION
As the third most commonly diagnosed cancer and the second most common cause of cancer-related death among men and women in the United States, colorectal cancer (CRC) is projected to account for 132,700 new cases and 49,700 deaths in 201. CRC incidence and mortality rates have declined since 19981, likely due to improved adherence to CRC screening guidelines2 and more effective cancer-directed therapy. However, recently published data from the Surveillance, Epidemiology, and End Results (SEER) database clearly show a rising incidence of CRC among younger individuals, with annual percentage change (APC) rates highest for distal (sigmoid and rectal) tumors diagnosed in those between the ages of 20 and 393–8.
Hispanics represent the youngest (median age 27 years old) and most rapidly growing ethnic group in the US. In fact, by 2060, Hispanics are expected to account for >30% of the entire US population. Although the designation “Hispanic” encompasses a population diverse in racial and geographic origin, individuals of Mexican ancestry currently account for the majority of Hispanics in the US.9 As compared to non-Hispanic Whites (NHWs), Hispanics have poorer access to health care and tend to present with later stage CRC, which likely account for their inferior survival after CRC diagnosis10–12. Given the rapid growth of the young Hispanic population in the US, the rising incidence of CRC among 20–39 year-old Hispanics warrants further investigation.
With >10 million Hispanic residents, Texas is home to 20% of the US Hispanic population. By 2040, Hispanics will account for about half of Texas’ total population (18.8 million Hispanics), representing a growth rate of 530% between 1980 and 204013. In light of the rising incidence of young-onset CRC, the rapid growth of the US Hispanic population, and the enrichment of Texas’ population for Hispanics of Mexican ancestry, we analyzed data from the Texas Cancer Registry (TCR) (TCR) to assess trends in CRC incidence and survival among Texans diagnosed with CRC between 1995 and 2010. We hypothesized that an in-depth analysis of the TCR, which includes a Hispanic population larger than that included in published SEER analyses of young-onset CRC, would shed light on a rising health care problem among the most rapidly growing segment of the US population.
MATERIALS AND METHODS
Study Population
Cases of CRC diagnosed in the State of Texas between January 1, 1995 and December 31, 2010 were identified using the TCR, a non-SEER active and passive surveillance system that maintains the most comprehensive cancer database in Texas14. The TCR meets the Centers for Disease Control and Prevention’s high quality data standards, as well as gold certification from the North American Association of Central Cancer Registries with numerous internal and external quality assurance procedures14.
We used anatomic site and histologic codes of the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) to define invasive CRCs: adenocarcinoma (814_3), adenocarcinoma in adenomatous polyps (821_3), adenocarcinoma in adenomatous polyposis coli (APC) (82203), papillary adenocarcinoma (826_3), mucinous (colloid) adenocarcinoma (848_3), and signet ring adenocarcinoma (84903). Individuals were classified as NHW, African American (AA), Hispanic, or Asian/Pacific Islander based on the standards of the North American Association of Central Cancer Registries, Version 1315. We restricted our analysis to persons aged 20 years or older at CRC diagnosis.
Clinical Characteristics
Data pertaining to primary tumor anatomical location, stage at diagnosis, and survival were collected and analyzed. The anatomical location of each tumor was classified according to the ICD-O-3 classification system: right colon (C18.0–C18.4), left colon (C18.5–C18.7, C19.9, C20.9), large intestine NOS (C18.8–C18.9, C26.0), rectosigmoid colon (C18.7, C19.9, C20.9), and colon excluding rectosigmoid colon (C18.0–C18.6, C18.8–C18.9). For the purposes of this study, tumors of the cecum, ascending colon, hepatic flexure, or transverse colon were designated as right-sided tumors, while tumors of the splenic flexure, descending colon, sigmoid colon, rectosigmoid junction, and rectum were designated as left-sided tumors. The subsite defined as rectosigmoid colon consisted of the sigmoid colon, rectosigmoid junction and rectum. We classified tumors by these anatomical subsites based on published data suggesting that the distribution of tumors across these subsites has been shifting and that trends vary by ethnicity and race5,6,16. Stage of disease at diagnosis, as defined by the 1997 and 2000 editions of the SEER Summary Staging Manual, was analyzed by age group, race/ethnicity and year of diagnosis.
Statistical Analysis
We used annual population estimates for Texas as denominators to calculate incidence rates for CRC. All incidence rates were age-adjusted to the 2000 US standard population by the direct method. We calculated overall incidence rates and incidence rates according to age group (20–39, 40–49, 50–74, 75+ years old), sex, race/ethnicity (non-Hispanic white, Hispanic, African American), and anatomical subsite (right-sided colon, left-sided colon, rectosigmoid). We selected age categories based on the commonly accepted definition for adolescent and young adults (AYA, 20–39 years old)4, adults who are not routinely offered CRC screening (40–49 years old), and adults who are routinely offered CRC screening (≥50 years old). Annual percentage change (APC) of CRC incidence rates and their corresponding 95% confidence intervals (95%CI) were used to characterize changes in CRC incidence rates over time. We considered trends statistically significant if the 95% CIs surrounding the APC excluded zero. Observed cause-specific survival (CSS) at five years was calculated and stratified by age, stage at diagnosis, and anatomic subsite. Relative survival could not be calculated because expected survival of Texans by race and ethnicity is currently unknown at this time. We compared 5-year survival percentage between race/ethnicity using Chi-square test. We used SEER*Stat version 8.2.1 (http://www.seer.cancer.gov/seerstat) to conduct all analyses.
RESULTS
Incidence
Between January 1, 1995 and December 31, 2010, 123,083 individuals were diagnosed with CRC in Texas (Table 1). Median age at diagnosis was 69 years, with 89% of patients diagnosed at 50 years old or older, 8% diagnosed between 40 and 49 years old, and 3% diagnosed between 20 and 39 years old. Fifty-six percent of colorectal cancers were left-sided; 39% were right-sided; and the remaining 5% were NOS. The majority of patients were NHW (69%), followed by Hispanic (17%), AA (12%), and other (2%). Of note, Hispanics accounted for 26% of CRC patients ≤50 years old, a proportion which increased from 22% between 1995 and 1998 to 29% between 2007 and 2010 (Supplementary Table 1).
Table 1.
Demographic and clinical characteristics of CRC cases, Texas, 1995–2010
| Entire Cohort N=123,083 |
Right Colon N=47,600 |
Left Colon N=69,223 |
Colon NOS N=6,260 |
|
|---|---|---|---|---|
| Age Groups | ||||
|
| ||||
| 20–39 years | 3,218 (3%) | 904 (2%) | 2,114 (3%) | 200 (3%) |
| 40–49 years | 9,810 (8%) | 2,830 (6%) | 6,525 (9%) | 455 (7%) |
| 50–74 years | 69,154 (56%) | 24,297 (51%) | 41,477 (60%) | 3,380 (54%) |
| 75+ years | 40,901 (33%) | 19,569 (41%) | 19,107 (28%) | 2,225 (36%) |
|
| ||||
| Race/Ethnicity | ||||
|
| ||||
| Non-Hispanic White | 84,405 (69%) | 33,605 (71%) | 46,684 (67%) | 4,116 (66%) |
| Hispanic White | 21,064 (17%) | 7,096 (15%) | 12,921 (19%) | 1,047 (17%) |
| African American | 15,050 (12%) | 6,162 (13%) | 7,934 (11%) | 954 (15%) |
| Other | 2,564 (2%) | 737 (1%) | 1694 (3%) | 143 (2%) |
|
| ||||
| Years | ||||
|
| ||||
| 1995–1998 | 28,153 (23%) | 10,390 (22%) | 16,196 (23%) | 1,567 (25%) |
| 1999–2002 | 30,618 (25%) | 11,761 (25%) | 17,196 (25%) | 1,661 (27%) |
| 2003–2006 | 32,185 (26%) | 12,699 (27%) | 17,973 (26%) | 1,513 (24%) |
| 2007–2010 | 32,127 (26%) | 12,750 (27%) | 17,858 (26%) | 1,519 (24%) |
|
| ||||
| Sex | ||||
|
| ||||
| Male | 66,203 (54%) | 22,839 (48%) | 40,095 (58%) | 3,269 (52%) |
| Female | 56,880 (45%) | 24,761 (52%) | 29,128 (42%) | 2,991 (48%) |
|
| ||||
| Stage | ||||
|
| ||||
| Localized | 45,157 (37%) | 17,060 (36%) | 26,505 (38%) | 1,592 (25%) |
| Regional | 47,198 (38%) | 19,965 (42%) | 25,518 (37%) | 1,715 (27%) |
| Distant | 21,951 (18%) | 7,982 (17%) | 12,232 (18%) | 1,737 (28%) |
| Unknown | 8,777 (7%) | 2,593 (5%) | 4,968 (7%) | 1,216 (19%) |
CRC incidences by age, sex, and race/ethnicity are listed in Table 2 while APC rates are listed in Table 3. Overall, the absolute incidence of CRC was highest among AAs (76.3/100,000), followed by NHWs (60.2/100,000) and Hispanics (50.8/100,000). Between 1995 and 2010, the age-standardized CRC incidence rate for the entire cohort declined from 62.9/100,000 to 48.3/100,000 (APC −1.8%,CI −2.3% to −1.3%, p<0.01). When we stratified by race/ethnicity, CRC incidence showed statistically significant linear declines in age-standardized incidence among NHWs (APC −2.0%, CI −2.5% to −1.5%, p<0.01) and AAs (APC −1.2%, CI −2.0% to −0.5%, p<0.01), but not among Hispanics (APC −0.5%, CI −1.2% to 0.3%, p=0.18). Incidence patterns differed by age (Table 3; Figure 1). While CRC incidence declined over the study period for individuals aged 50–74 years (APC −1.6%, CI −2.3% to −1.0%, p<0.01) and 75+ years (APC −2.6%, CI −3.2% to −2.1%, p<0.01), CRC incidence in individuals aged 20–39 years increased by 1.8% (CI 1.2% to 2.5%, p<0.01) annually while remaining relatively stable in individuals aged 40–49 years (APC 0.3%, CI −0.1% to 0.7%, p=0.08).
Table 2.
Absolute Incidence of CRC by race/ethnicity, sex, and age, Texas, 1995–2010
| Race | Gender | Whole Cohort | 20–39 years | 40–49 years | 50–74 years | 75+ years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| 1995–2010 | 1995 | 2010 | 1995–2010 | 1995 | 2010 | 1995–2010 | 1995 | 2010 | 1995–2010 | 1995 | 2010 | 1995–2010 | 1995 | 2010 | ||
| All | Both | 59.6 | 62.9 | 48.3 | 3.3 | 3.0 | 3.7 | 19.8 | 17.7 | 20.0 | 103.3 | 107.4 | 82.2 | 268.0 | 300.0 | 208.5 |
| Male | 72.7 | 78.3 | 59.7 | 3.5 | 3.3 | 3.8 | 21.4 | 20.9 | 22.9 | 127.6 | 133.6 | 102.4 | 331.1 | 378.7 | 263.0 | |
| Female | 49.4 | 51.8 | 39.2 | 3.2 | 2.7 | 3.7 | 18.1 | 14.7 | 17.1 | 81.9 | 84.6 | 63.9 | 229.6 | 258.8 | 173.6 | |
|
| ||||||||||||||||
| NHW | Both | 60.2 | 65.2 | 48.2 | 3.6 | 3.5 | 4.0 | 19.4 | 17.5 | 22.1 | 103.5 | 110.9 | 79.3 | 274.2 | 312.6 | 210.9 |
| Male | 73.2 | 80.8 | 58.7 | 3.9 | 3.8 | 4.1 | 21.3 | 20.0 | 25.6 | 127.1 | 136.8 | 98.5 | 337.2 | 395.8 | 256.4 | |
| Female | 49.9 | 54.1 | 39.4 | 3.4 | 3.2 | 3.8 | 17.6 | 15.0 | 18.6 | 81.9 | 88.1 | 61.4 | 235.9 | 270.9 | 180.8 | |
|
| ||||||||||||||||
| Hispanics | Both | 50.8 | 46.2 | 45.2 | 2.9 | 2.0 | 3.6 | 18.3 | 13.9 | 18.3 | 89.9 | 81.6 | 78.3 | 217.6 | 208.9 | 190.8 |
| Male | 65.1 | 62.4 | 60.4 | 2.9 | 2.0 | 3.4 | 19.5 | 18.7 | 20.1 | 117.7 | 110.2 | 104.3 | 285.1 | 285.8 | 273.8 | |
| Female | 39.7 | 34.0 | 33.7 | 2.9 | 2.0 | 3.8 | 17.1 | 9.3 | 16.5 | 66.6 | 58.3 | 55.8 | 174.1 | 160.5 | 138.6 | |
|
| ||||||||||||||||
| African American | Both | 76.3 | 77.8 | 63.3 | 3.7 | 3.6 | 3.8 | 26.5 | 28.4 | 22.3 | 139.2 | 141.3 | 121.2 | 318.5 | 324.5 | 239.9 |
| Male | 91.0 | 93.3 | 76.3 | 4.0 | 4.4 | 4.1 | 28.6 | 32.4 | 24.0 | 166.1 | 170.4 | 143.5 | 389.3 | 388.8 | 306.9 | |
| Female | 66.2 | 66.8 | 54.5 | 3.5 | 2.9 | 3.5 | 24.6 | 24.9 | 20.7 | 117.9 | 117.8 | 103.6 | 281.1 | 289.9 | 203.9 | |
Rates per 100,000 and age-adjusted to the 2000 US Std Population
Table 3.
Annual percentage change in age-adjusted CRC incidence rates by race/ethnicity, age, and anatomic site, Texas, 1995–2010
| Entire Cohort | Right Colon | Left Colon | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Age Group | Race | APC | P-value | 95% CI | APC | P-value | 95% CI | APC | P-value | 95% CI | |||
| All ages | All | −1.80 | <0.01 | −2.34 | −1.25 | −1.18 | <0.01 | −1.85 | −0.49 | −2.12 | <0.01 | −2.63 | −1.62 |
| NHW | −2.03 | <0.01 | −2.53 | −1.53 | −1.41 | <0.01 | −2.05 | −0.77 | −2.37 | <0.01 | −2.85 | −1.88 | |
| African American | −1.24 | <0.01 | −1.95 | −0.53 | −0.35 | 0.47 | −1.37 | 0.68 | −1.72 | <0.01 | −2.37 | −1.07 | |
| Hispanic | −0.50 | 0.18 | −1.24 | 0.26 | 0.29 | 0.57 | −0.77 | 1.35 | −0.85 | 0.04 | −1.65 | −0.04 | |
|
| |||||||||||||
| 20–39 | All | 1.82 | <0.01 | 1.16 | 2.48 | 1.54 | 0.02 | 0.31 | 2.77 | 2.24 | <0.01 | 1.32 | 3.17 |
| NHW | 2.41 | <0.01 | 1.26 | 3.57 | 0.70 | 0.38 | −0.96 | 2.40 | 3.33 | <0.01 | 2.03 | 4.64 | |
| African American | 0.27 | 0.75 | −1.49 | 2.06 | 2.51 | 0.08 | −0.31 | 5.42 | −1.12 | 0.38 | −3.71 | 1.54 | |
| Hispanic | 2.60 | <0.01 | 1.34 | 3.88 | 2.63 | 0.08 | −0.39 | 5.73 | 2.99 | <0.01 | 1.20 | 4.82 | |
|
| |||||||||||||
| 40–49 | All | 0.33 | 0.08 | −0.05 | 0.71 | 0.60 | 0.17 | −0.29 | 1.50 | 0.23 | 0.44 | −0.39 | 0.86 |
| NHW | 1.11 | 0.01 | 0.46 | 1.76 | 0.83 | 0.24 | −0.62 | 2.30 | 1.21 | 0.01 | 0.33 | 2.10 | |
| African American | −0.94 | 0.15 | −2.24 | 0.38 | 0.35 | 0.70 | −1.56 | 2.29 | −1.52 | 0.06 | −3.12 | 0.10 | |
| Hispanic | −0.23 | 0.64 | −1.23 | 0.78 | 0.11 | 0.92 | −2.11 | 2.38 | −0.33 | 0.60 | −1.63 | 0.99 | |
|
| |||||||||||||
| 50–74 | All | −1.62 | <0.01 | −2.25 | −0.99 | −0.92 | 0.04 | −1.80 | −0.04 | −1.95 | <0.01 | −2.52 | −1.37 |
| NHW | −2.04 | <0.01 | −2.68 | −1.39 | −1.28 | <0.01 | −2.17 | −0.38 | −2.42 | <0.01 | −3.04 | −1.81 | |
| African American | −1.03 | <0.01 | −1.67 | 0.65 | −0.19 | 0.73 | −1.34 | 0.97 | −1.39 | <0.01 | −2.01 | −0.78 | |
| Hispanic | −0.17 | 0.66 | −0.99 | 0.65 | 0.65 | 0.28 | −0.58 | 1.89 | −0.49 | 0.23 | −1.30 | 0.34 | |
|
| |||||||||||||
| 75+ | All | −2.64 | <0.01 | −3.22 | −2.06 | −1.73 | <0.01 | −2.38 | −1.07 | −3.41 | <0.01 | −4.06 | −2.76 |
| NHW | −2.88 | <0.01 | −3.41 | −2.35 | −1.85 | <0.01 | −2.45 | −1.25 | −3.78 | <0.01 | −4.43 | −3.12 | |
| African American | −1.66 | <0.01 | −2.77 | −0.54 | −0.64 | 0.41 | −2.26 | 1.00 | −2.40 | <0.01 | −3.59 | −1.21 | |
| Hispanic | −1.20 | 0.01 | −2.06 | −0.33 | −0.19 | 0.72 | −1.31 | 0.94 | −1.91 | <0.01 | −3.14 | −0.68 | |
|
| |||||||||||||
| 50+ | All | −2.05 | <0.01 | −2.64 | −1.47 | −1.32 | <0.01 | −2.03 | −0.61 | −2.48 | <0.01 | −3.04 | −1.92 |
| NHW | −2.40 | <0.01 | −2.96 | −1.83 | −1.47 | <0.01 | −2.25 | −0.88 | −2.91 | <0.01 | −3.47 | −2.35 | |
| African American | −1.30 | <0.01 | −2.06 | −0.52 | −0.45 | 0.40 | −1.55 | 0.67 | −1.76 | <0.01 | −2.44 | −1.07 | |
| Hispanic | −0.60 | 0.13 | −1.39 | 0.20 | 0.24 | 0.64 | −0.82 | 1.30 | −1.01 | 0.02 | −1.85 | −0.16 | |
Figure 1.
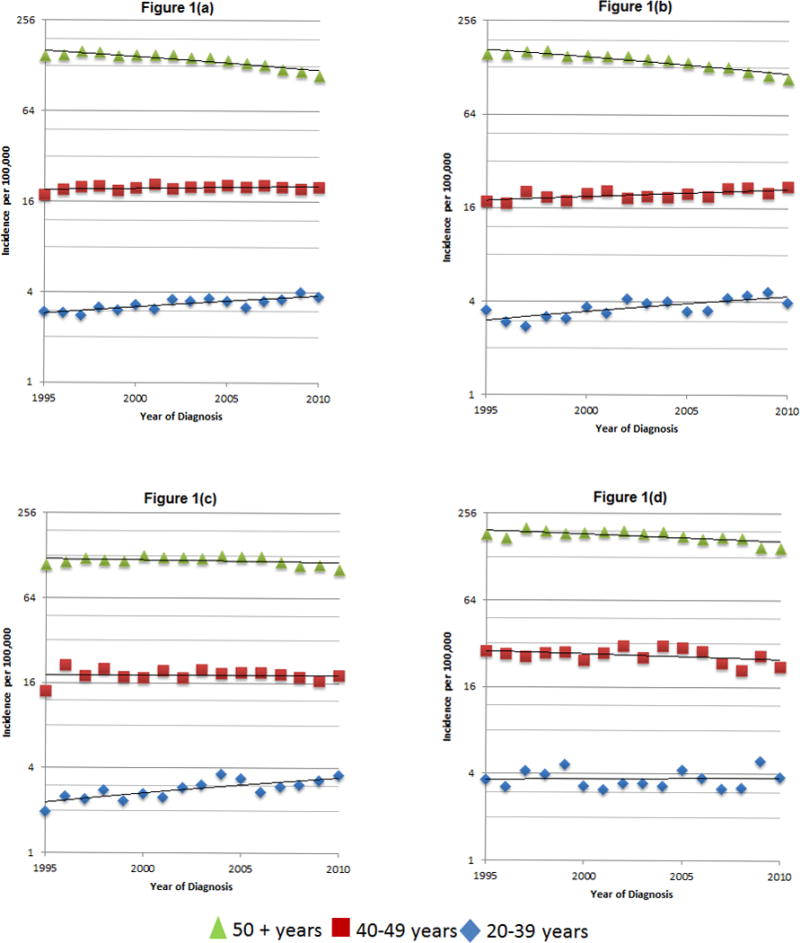
Time trends of age-adjusted CRC incidence rates, overall (a) and by race/ethnicity (b, non-Hispanic white; c, Hispanic; d, African American), Texas, 1995–2010
When we stratified by race/ethnicity within age groups, age-standardized incidence rates for CRC among individuals ≥50 years old declined by 2.4% (CI −3.0 to −1.8, p<0.01) annually in NHWs and 1.3% (CI −2.1% to −0.5%, p<0.01) annually in AAs. There was no statistically significant decline in CRC incidence among Hispanics ≥50 (APC −0.6%, CI −1.4 % to 0.2%, p=0.13); however, closer examination of this age group (Figure 1c) suggests an initial upward trend in age-adjusted CRC incidence (1995–2000), followed by a plateau in 2000–2005, then a slightly downward trend in 2005–2010. In individuals 20–39 years old, age-standardized incidence rates for CRC rose by 2.4% per year in NHWs (CI 1.3% to 3.6%, p<0.01) and 2.6% per year in Hispanics (CI 1.3% to 3.9%, p<0.01) but remained stable among AAs (APC 0.3%, CI −1.5% to 2.1%, p=0.75) (Table 3). Stratification by sex revealed a rising incidence of CRC among both male and female Hispanics and NHWs ages 20–39, but no change among AA men or women ages 20–39 (Supplementary Table 2b).
Incidence patterns within age and race/ethnicity subgroups varied by anatomical subsite. In older individuals (≥50 years old), the rate of decline in incidence of left-sided tumors was greater than that for right-sided tumors (Table 3). Although the incidence of both right-sided tumors (APC 1.5%, CI 0.2 to 2.8, p=0.02) and left-sided tumors (APC 2.2%, CI 1.3 to 3.2, p<0.01) increased in the 20–39 age group, further analysis revealed some important differences among race/ethnicity subgroups. Whereas the incidence of left-sided tumors markedly increased among NHWs (APC 3.3%, CI 2.0 to 4.6, p<0.01) and Hispanics (APC 3.0%, CI 1.2 to 4.8, p = 0.01) aged 20–39 years, it remained essentially unchanged among AAs (APC −1.1%, CI −3.7 to 1.5, p=0.38) in this age group. There was, however, a non-significant increase in right-sided tumors among AAs (APC 2.6%, CI −0.4 to 5.7, p=0.08) and Hispanics (APC 2.5%, CI −0.3 to 5.4, p=0.08) aged 20–39 years without such a trend in NHWs (APC 0.7%, CI −1.0 to 2.4, p=0.38).
Stage at Presentation and Survival
Individuals between the ages 20 and 39 years were more likely to have distant disease at the time of CRC diagnosis (26%) than those diagnosed between ages 50 and 74 years (19%; p<0.01) or those diagnosed when 75 or older (14%; p<0.01) (Table 4). When we examined five-year cancer-specific survival (CSS) by stage, survival rates were inferior among persons 75 years or older for all stages of disease when compared to individuals in the younger age groups (Table 5). Five-year CSS of 20–39 age group was comparable to that of the 40–49 age group for each stage of disease and comparable to that of the 50–74 age group for localized and regional disease, but superior for distant disease (19% vs 12.2%, p<0.05). Survival rates for AAs were generally inferior to those observed in NHWs and Hispanics for each stage of disease and across all age groups while survival rates for Hispanics and NHWs were equivalent across most age groups and stages of disease (Supplementary Table 3). While survival rates for distant disease remained lowest among the AA group throughout the study period, the disparity between five-year CSS for AAs and the other groups diagnosed with localized disease between 1995 and 1998 no longer existed between 2007 and 2010 (p=0.70).
Table 4.
Distribution of CRC stage by race/ethnicity, age, and year of diagnosis, Texas, 1995–2010
| Entire Cohort | 20–39 years | 40–49 years | 50–74 years | 75+ years | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||||
| Race | Years | Local | Region | Dist | Unk | Local | Region | Dist | Unk | Local | Region | Dist | Unk | Local | Region | Dist | Unk | Local | Region | Dist | Unk |
| All Races | 1995–1998 | 33% | 42% | 17% | 9% | 23% | 46% | 24% | 7% | 28% | 41% | 24% | 7% | 33% | 42% | 18% | 7% | 34% | 42% | 13% | 11% |
| 1999–2002 | 35% | 41% | 16% | 8% | 25% | 41% | 26% | 8% | 29% | 41% | 24% | 6% | 35% | 41% | 17% | 7% | 37% | 40% | 13% | 10% | |
| 2003–2006 | 39% | 36% | 18% | 6% | 25% | 41% | 27% | 6% | 29% | 40% | 26% | 5% | 39% | 36% | 19% | 5% | 42% | 35% | 14% | 8% | |
| 2007–2010 | 40% | 35% | 19% | 6% | 28% | 40% | 26% | 6% | 31% | 39% | 25% | 5% | 40% | 35% | 20% | 6% | 43% | 34% | 16% | 7% | |
| All | 37% | 38% | 18% | 7% | 26% | 42% | 26% | 7% | 29% | 40% | 25% | 6% | 37% | 38% | 19% | 6% | 39% | 38% | 14% | 9% | |
|
| |||||||||||||||||||||
| NHW | 1995–1998 | 34% | 42% | 16% | 8% | 25% | 46% | 22% | 7% | 29% | 40% | 24% | 7% | 34% | 42% | 17% | 7% | 35% | 42% | 13% | 10% |
| 1999–2002 | 37% | 40% | 16% | 8% | 28% | 38% | 26% | 8% | 31% | 40% | 24% | 6% | 37% | 40% | 17% | 7% | 38% | 40% | 12% | 9% | |
| 2003–2006 | 41% | 36% | 17% | 6% | 27% | 42% | 24% | 6% | 31% | 40% | 24% | 5% | 40% | 36% | 18% | 5% | 43% | 35% | 14% | 8% | |
| 2007–2010 | 41% | 35% | 18% | 6% | 31% | 38% | 25% | 6% | 32% | 38% | 24% | 6% | 41% | 34% | 19% | 5% | 44% | 34% | 15% | 7% | |
| All | 38% | 38% | 17% | 7% | 28% | 41% | 24% | 7% | 31% | 39% | 24% | 6% | 38% | 38% | 18% | 6% | 40% | 38% | 13% | 9% | |
|
| |||||||||||||||||||||
| Hispanic | 1995–1998 | 29% | 43% | 19% | 8% | 21% | 47% | 26% | 6% | 27% | 45% | 22% | 6% | 29% | 44% | 20% | 7% | 33% | 42% | 14% | 12% |
| 1999–2002 | 32% | 43% | 18% | 7% | 24% | 43% | 25% | 8% | 26% | 44% | 25% | 5% | 32% | 43% | 18% | 7% | 36% | 40% | 14% | 10% | |
| 2003–2006 | 36% | 38% | 20% | 6% | 25% | 41% | 29% | 6% | 30% | 40% | 25% | 5% | 37% | 38% | 20% | 5% | 40% | 38% | 14% | 7% | |
| 2007–2010 | 37% | 37% | 20% | 6% | 25% | 43% | 27% | 5% | 30% | 42% | 25% | 3% | 38% | 37% | 21% | 5% | 42% | 34% | 16% | 8% | |
| All | 34% | 40% | 19% | 7% | 24% | 43% | 27% | 6% | 29% | 42% | 24% | 5% | 35% | 40% | 20% | 6% | 38% | 38% | 15% | 9% | |
|
| |||||||||||||||||||||
| African American | 1995–1998 | 27% | 41% | 22% | 10% | 23% | 46% | 23% | 8% | 26% | 39% | 28% | 6% | 27% | 42% | 24% | 8% | 28% | 39% | 17% | 16% |
| 1999–2002 | 29% | 41% | 21% | 9% | 19% | 44% | 31% | 6% | 26% | 42% | 25% | 7% | 30% | 42% | 21% | 8% | 30% | 40% | 18% | 13% | |
| 2003–2006 | 34% | 35% | 24% | 7% | 21% | 39% | 31% | 9% | 24% | 40% | 32% | 5% | 35% | 34% | 25% | 6% | 36% | 33% | 19% | 11% | |
| 2007–2010 | 35% | 34% | 25% | 6% | 20% | 45% | 28% | 7% | 28% | 39% | 29% | 4% | 36% | 33% | 26% | 5% | 38% | 32% | 20% | 10% | |
| All | 32% | 37% | 23% | 8% | 21% | 44% | 28% | 8% | 26% | 40% | 29% | 5% | 33% | 37% | 24% | 6% | 33% | 36% | 19% | 12% | |
Table 5.
Five-year colorectal cancer-specific survival by age, stage, and anatomic site, Texas, 1995–2010
| Anatomic Site | Stage | Age groups
|
||||
|---|---|---|---|---|---|---|
| 20–39 years | 40–49 years | 50–74 years | 75+ years | All ages | ||
|
| ||||||
| Entire Colon | Localized | 90.7% | 91.6% | 89.6% | 77.6% | 85.9% |
| Regional | 70.2% | 72.3% | 68.6% | 55.6% | 65.6% | |
| Distant | 19.0% | 17.3% | 12.2% | 6.5% | 12.3% | |
| All stages | 62.4% | 63.9% | 64.8% | 55.9% | 62.5% | |
|
| ||||||
| Right Colon | Localized | 92.2% | 92.4% | 90.9% | 80.9% | 87.5% |
| Regional | 72.8% | 72.2% | 68.3% | 57.2% | 65.2% | |
| Distant | 20.7% | 17.3% | 10.5% | 5.9% | 11.1% | |
| All stages | 63.7% | 62.8% | 64.6% | 58.7% | 62.7% | |
|
| ||||||
| Left Colon | Localized | 90.7% | 91.6% | 89.6% | 77.6% | 86.1% |
| Regional | 70.2% | 72.3% | 68.6% | 55.6% | 66.5% | |
| Distant | 19.0% | 17.3% | 12.2% | 6.5% | 13.8% | |
| All stages | 62.4% | 63.9% | 64.8% | 55.9% | 63.7% | |
DISCUSSION
Our analysis of Texas Cancer Registry (TCR) data show that, between 1995 and 2010, the incidence of CRC declined among Texans ≥50 years old and increased among Texans 20–39 years old. Among those ≥50 years old, CRC incidence declined significantly among NHWs and AAs, but not Hispanics. While the incidence of both left- and right-sided tumors decreased in older patients, the rate of decline was more pronounced for left-sided tumors. Among the youngest (20–39 years old) group, CRC incidence increased in NHWs and Hispanics but not AAs. This trend appears to have been driven by a rising incidence of left-sided tumors among NHWs (APC 3.3%) and Hispanics (APC 3.0%). Finally, the demographic findings of our study parallel national data showing a relatively young and growing Hispanic population. Although Hispanics represented only 17% of the total cohort, they represented nearly 30% of those younger than 50 towards the end of the study period.
CRC screening data are not available in the TCR, making it impossible to demonstrate an association between trends in CRC screening and CRC incidence. However, given that CRC screening facilitates both the excision of premalignant polyps and the detection of earlier stage cancer, improved adherence to CRC screening recommendations may account for the declining CRC incidence and downward stage migration among Texans ≥50 years of age. Furthermore, since endoscopy has been shown to detect distal polyps more easily than proximal ones,17 more widespread screening could also account for the more rapid decline in the incidence of left-sided CRC when compared to right-sided CRC in individuals of screening age. The disparate rates of decline in CRC incidence among older individuals of different racial/ethnic groups may reflect either divergent rates of polyp incidence due to shifts in culturally-dependent CRC risk factors or simply the well-documented lower CRC screening rates among minority populations18. The decline in CRC incidence among older AAs may be “catching-up” to that in NHWs due to heightened awareness of the elevated CRC risk in AAs and improved compliance with CRC screening recommendations, whereas the slower rate of decline among older Hispanics may result from slower adoption of CRC screening in this ethnic group19,20. Despite the absence of a statistically significant decline in CRC incidence among Hispanics ≥50 over the entire study period, the suggestion that CRC incidence may have reached its peak in 2000 and started to decline thereafter (see Figure 1c) may reflect a rising frequency of CRC screening among older Hispanics.21.
In contrast, the rising CRC incidence and stable distribution of stage across the study period among the younger, unscreened population suggest a true increase in CRC incidence rather than an apparent increase resulting from detection bias. In many respects, our findings from the TCR parallel those from SEER. Siegel et al. reported that, between 1992 and 2005, the overall incidence of CRC among young adults ages 20–49 increased by 1.5% per year in men and 1.5% per year in women. APC rates were highest in the youngest (20–29) age group (5.2% in men and 5.6% in women), with a rise in left-sided tumors (distal colon and rectum) driving these positive trends16. Similar to our study, Siegel’s analysis by race/ethnicity revealed a rising incidence of CRC in young NHWs and Hispanics but not AAs. In contrast to our study, however, Siegel et al reported no change in the incidence of right-sided tumors in NHWs, and they did not report data for right-sided tumors in Hispanics or AAs. In another SEER analysis, Jafri et al. reported an overall decline in the incidence of CRC among NHWs, Hispanics, and AAs ≥50 years old between 1993 and 2007 and a concomitant rise in individuals < 50 years8. Similar to our findings, they reported a rising incidence of CRC in young Hispanics and NHWs; however, they also reported a small but nevertheless positive trend among AAs (relative increase of 15%). In addition to confirming the declining incidence of CRC in older individuals and the rising incidence in younger individuals, the most recent SEER analysis by Bailey et al. projects that, by 2030, the incidence rates of colon and rectal cancers will increase by 90.0% and 124.2%, respectively, for 20–34 year olds and by 27.7% and 46.0%, respectively, for 35–49 year olds3.
The etiology underlying the rising incidence of young-onset CRC remains unknown. While inherited predisposition should always be considered in young patients with CRC, only a minority of young-onset CRC cases can be attributed to a recognized genetic syndrome, and there are no data to suggest that the frequency of any genetic syndrome associated with young-onset CRC is rising22–24. Investigation into potential etiologies underlying the rising incidence of young-onset CRC has therefore shifted towards environmental factors – either a single factor or a combination of factors, with or without concomitant genetic predisposition.
Certain established factors for CRC traditionally seen in older individuals - obesity, diabetes, and metabolic syndrome, to name a few - have become more prevalent in children and adolescents nationwide. However, to date, obesity, metabolic syndrome, and diabetes, have not been shown to be independently associated with young onset CRC25–27. Over the last few decades, the so-called “Western” diet – characterized by high intake of red meat, fat, and refined sugars and low intake of fiber and whole grains – has become more common in the US, Europe, and Asia, and particularly among children and adolescents28. Red meat consumption in particular appears to increase risk for developing CRC, especially left-sided CRC, in a dose-dependent manner while a high-fiber diet appears to lower risk. Whether red meat increases risk for CRC by exposing the colonic mucosa to potentially carcinogenic preservatives used during processing or by introducing foreign pathogens or hormones that change the colon’s microbial and inflammatory milieu is unknown29–33.
Hispanics living in the US consume a larger proportion of red meat than do NHWs and AAs28. African Americans, when compared to native Africans, consume more animal protein and fat and are colonized with higher levels potentially toxic-producing colonic bacteria34,35. Our data show that, although the absolute incidence of CRC among young Hispanics remained lower than CRC incidence among NHWs and AAs throughout the study period, it rose at a rate similar to that seen in NHWs (APC 2.6% for Hispanics and 2.4% NHWs) and higher than that seen in AAs (APC 0.3%). Worldwide, CRC incidence remains higher among developed countries compared to developing countries, but the incidence of CRC among developing countries (including Latin American countries) is rising36. Furthermore, CRC incidence rises with income level, likely due to a higher prevalence of unhealthy diet and sedentary lifestyle among the wealthy37. Taken together, these data suggest that “acculturation,” in particular the adoption of the Western diet and lifestyle, could explain the rising incidence of CRC among Hispanics in the US.
On the other hand, even within a particular racial/ethnic group, diet and lifestyle vary by socioeconomic status and geographical location and tend to change over time. These confounding factors not only explain some key differences between findings from SEER and regional cancer registries but also make pinpointing a cause of young-onset CRC on epidemiological grounds alone extremely difficult, especially in the context of rapidly shifting demographics. Moving beyond the epidemiology of young-onset CRC into its biological underpinnings will undoubtedly prove critical in future efforts to personalize preventative measures and treatment options.
Although the TCR population is smaller than the SEER population, our study has several strengths. First, as the largest-to-date descriptive analysis of CRC incidence among Hispanics, our study has important implications on the future of CRC epidemiology in an ever-diversifying population8. Specifically, our data suggest that the slower rate of decline of CRC in Hispanics ≥50 years of age may reflect slower adoption of CRC screening among the Hispanic population. As the US Hispanic population both grows and ages, reinforcing the importance of CRC screening in Hispanic communities will become increasingly important. At the same time, the rising incidence of CRC among young Hispanics should drive research exploring potentially modifiable risk factors for CRC in this growing population and should raise suspicion among clinicians in any young individual who presents with obstructive symptoms or bleeding. Second, our population was confined to one geographic region, which becomes particularly important when implicating environmental exposure as a potential risk factor for disease. Jafri et al showed dramatic regional differences in CRC incidence among Hispanics (from 11.2 per 100,000 in Atlanta to 39.2 per 100,000 in New Mexico), supporting the importance of studying large geographic regions separately.
Finally, in contrast to SEER, the TCR includes CRC incidence and cancer-specific survival data for all age groups and all three major racial/ethnic groups. According to the TCR, five-year CSS among 20–39 year olds was, stage for stage, either comparable or superior to that of older age groups. Because younger cancer patients are, in general, more likely than older patients to receive aggressive antineoplastic therapy due to higher performance status, fewer comorbidities, and more willingness to accept risk, the superior survival among younger CRC patients in the TCR and other population-based studies may simply reflect selection bias rather than inherent tumor biology38,39. Indeed, a pooled analysis of 20,023 patients enrolled in randomized controlled trials for first-line stage IV CRC40 revealed that, after adjustment for performance status and sex, the youngest patients (those near 18 years old) had inferior overall and progression-free survival when compared to middle-aged patients (those near 57 years old), especially during the first year of treatment. These data suggest more aggressive tumor biology in young patients, who, in the context of these highly controlled trials, would have received the same treatment as their older counterparts40. Since treatment data are not available in the TCR, we cannot ascertain whether CSS among young patients with stage IV disease in our study fared at least as well as middle-aged and elderly individuals due to more favorable tumor biology or more aggressive antineoplastic therapy. Similarly, we cannot tell whether differences in administration of anti-neoplastic therapy or inherent tumor biology account for the inferior survival of AAs across all stage of disease. However, the resolution of the initially observed disparity between CSS in AAs with localized CRC compared to CSS in NHWs/Hispanics with localized CRC argues against differences in tumor biology, at least for cases of CRC highly curable with surgery alone.
In summary, our findings from the TCR show that the incidence of CRC is increasing among young adults in Texas and that trends differ by race/ethnicity and tumor location. Of particular concern is the rising incidence of CRC in Texas’ rapidly expanding young Hispanic population in the absence of a significant decline among Hispanics of CRC screening age. At the present time, the absolute incidence of young-onset CRC is likely not high enough to warrant universal CRC screening among average-risk individuals younger than 50. However, CRC trends reported from SEER and TCR should alert clinicians to maintain suspicion for CRC in younger patients who present with rectal bleeding, obstructive symptoms, or unexplained anemia and to address any cultural or socioeconomic factors that may compromise CRC screening in populations with documented lower screening rates. Meanwhile, these data should drive clinical and laboratory investigators to better characterize the pathophysiology of young-onset CRC, to account for racial/ethnic disparities in CRC predisposition and survival, and ultimately to propose novel strategies for preventing, detecting, and treating this emerging disease.
Supplementary Material
Acknowledgments
Funding for this research is from the Dan L. Duncan Cancer Center at Baylor College of Medicine (P30CA125123).
Footnotes
The authors disclose no potential conflicts of interest.
Contributor Information
Daniel Y. Wang, Department of Medicine, Vanderbilt University School of Medicine.
Aaron P. Thrift, Department of Medicine, Baylor College of Medicine.
Neda Zarrin-Khameh, Department of Pathology, Baylor College of Medicine.
Alexandra Wichmann, Department of Medicine, Baylor College of Medicine.
Georgina N. Armstrong, Dan L Duncan Cancer Center, Baylor College of Medicine.
Patricia A. Thompson, Department of Pathology, Stony Brook School of Medicine.
Melissa L. Bondy, Department of Pediatrics, Baylor College of Medicine.
Benjamin L. Musher, Department of Medicine, Section of Hematology & Oncology, Baylor College of Medicine, One Baylor Plaza, MS:305, Houston, TX 77030.
References
- 1.Ries L, Melbert D, Krapcho M, Stinchcomb D. SEER Cancer Statistics Review, 1975–2005. Natl Cancer Inst. 2008 [Google Scholar]
- 2.Preventive US, Task S. Clinical Guidelines Annals of Internal Medicine Screening for Colorectal Cancer: A Targeted, Updated Systematic. 2002;(13) doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CE, Hu C-Y, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015;150(1):17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hubbard J, Grothey A. Adolescent and Young Adult Colorectal Cancer. J Natl Compr Cancer Netw. 2013:1219–1225. doi: 10.6004/jnccn.2013.0144. Available at: http://www.jnccn.org/content/11/10/1219.short. Accessed January 1, 2014. [DOI] [PubMed]
- 5.Meyer JE, Narang T, Schnoll-Sussman FH, Pochapin MB, Christos PJ, Sherr DL. Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116(18):4354–9. doi: 10.1002/cncr.25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1695–8. doi: 10.1158/1055-9965.EPI-09-0186. [DOI] [PubMed] [Google Scholar]
- 7.Austin H, Jane Henley S, King J, Richardson LC, Eheman C. Changes in colorectal cancer incidence rates in young and older adults in the United States: what does it tell us about screening. Cancer Causes Control. 2013;(1) doi: 10.1007/s10552-013-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jafri NS, Gould M, El-Serag HB, Duan Z, Davila JA. Incidence and survival of colorectal cancer among Hispanics in the United States: a population-based study. Dig Dis Sci. 2013;58(7):2052–60. doi: 10.1007/s10620-012-2454-3. [DOI] [PubMed] [Google Scholar]
- 9.Bureau UC. Population Projections. 2012 Available at: http://www.census.gov/population/projections/data/national/2012.html.
- 10.Stefanidis D, Pollock BH, Miranda J, et al. Colorectal cancer in Hispanics: a population at risk for earlier onset, advanced disease, and decreased survival. Am J Clin Oncol. 2006;29(2):123–6. doi: 10.1097/01.coc.0000199918.31226.f8. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez MN, Sussman DA, Lee DJ, Mackinnon JA, Fleming LE. Trends in colorectal cancer among hispanics by stage and subsite location: 1989–2006. Clin Transl Gastroenterol. 2012;3:e21. doi: 10.1038/ctg.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cokkinides VE, Bandi P, Siegel RL, Jemal A. Cancer-related risk factors and preventive measures in US Hispanics/Latinos. CA Cancer J Clin. 62(6):353–63. doi: 10.3322/caac.21155. [DOI] [PubMed] [Google Scholar]
- 13.Texas State Data Center and Office of the State Demographer, “Table 1: Population by Race/Ethnicity in 2000 and Projections of the Population by Race/Ethnicity from 2010 to 2040 for Texas Under Alternative Assumptions of Age, Sex and Race/Ethnicity-Specif.
- 14.About the Texas Cancer Registry. Available at: https://www.dshs.state.tx.us/tcr/background.shtm. Accessed January 5, 2014.
- 15.Thornton M. Standards for Cancer Registries Volume II: Data Standards and Data Dictionary, Record Layout Version 13. 17th. Springfield, Ill: North American Association of Central Cancer Registries; 2012. [Google Scholar]
- 16.Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992–2008. Cancer Epidemiol Biomarkers Prev. 2012;21(3):411–6. doi: 10.1158/1055-9965.EPI-11-1020. [DOI] [PubMed] [Google Scholar]
- 17.Boroff ES, Gurudu SR, Hentz JG, Leighton JA, Ramirez FC. Polyp and adenoma detection rates in the proximal and distal colon. Am J Gastroenterol. 2013;108(6):993–9. doi: 10.1038/ajg.2013.68. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Sussman DA, Doubeni CA, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014;106(4):dju032. doi: 10.1093/jnci/dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enard KR, Nevarez L, Hernandez M, et al. Patient navigation to increase colorectal cancer screening among Latino Medicare enrollees: a randomized controlled trial. Cancer Causes Control. 2015 doi: 10.1007/s10552-015-0620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Cancer screening—United States, 2010. MMWR. 2012;61(3):41–45. [PubMed] [Google Scholar]
- 21.National Health Interview Survey Public Use Data File 2010. National Center for Health Statistics, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 22.Hampel H, Frankel WL, Martin E, et al. Feasibility of Screening for Lynch Syndrome Among Patients With Colorectal Cancer. J Clin Oncol. 2008;26(35):5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308(15):1555–65. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stigliano V, Sanchez-Mete L, Martayan A, et al. Early-onset colorectal cancer patients without family history are “at very low risk” for lynch syndrome. J Exp Clin Cancer Res. 2014;33:1. doi: 10.1186/1756-9966-33-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aleksandrova K, Boeing H, Jenab M, et al. Metabolic syndrome and risks of colon and rectal cancer: the European prospective investigation into cancer and nutrition study. Cancer Prev Res (Phila) 2011;4(11):1873–83. doi: 10.1158/1940-6207.CAPR-11-0218. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqui AA. Metabolic syndrome and its association with colorectal cancer: a review. Am J Med Sci. 2011;341(3):227–31. doi: 10.1097/MAJ.0b013e3181df9055. [DOI] [PubMed] [Google Scholar]
- 27.Rosato V, Bosetti C, Levi F, et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control. 2013;24(2):335–41. doi: 10.1007/s10552-012-0119-3. [DOI] [PubMed] [Google Scholar]
- 28.Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr. 2011;14(4):575–83. doi: 10.1017/S1368980010002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bingham SA, Hughes R, Cross AJ. Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response. J Nutr. 2002;132(11 Suppl):3522S–3525S. doi: 10.1093/jn/132.11.3522S. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12421881. Accessed January 21, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Povey AC, Hall CN, Badawi AF, Cooper DP, O’Connor PJ. Elevated levels of the pro-carcinogenic adduct, O(6)-methylguanine, in normal DNA from the cancer prone regions of the large bowel. Gut. 2000;47(3):362–5. doi: 10.1136/gut.47.3.362. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1728032&tool=pmcentrez&rendertype=abstract. Accessed January 21, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119(11):2657–64. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 32.Marx A, Simon P, Simon R, et al. AMACR expression in colorectal cancer is associated with left-sided tumor localization. Virchows Arch. 2008;453(3):243–8. doi: 10.1007/s00428-008-0646-1. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Z, Fanger GR, Banner BF, et al. A dietary enzyme: alpha-methylacyl-CoA racemase/P504S is overexpressed in colon carcinoma. Cancer Detect Prev. 2003;27(6):422–6. doi: 10.1016/j.cdp.2003.07.003. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14642549. Accessed February 9, 2014. [DOI] [PubMed] [Google Scholar]
- 34.O’Keefe SJD, Chung D, Mahmoud N, et al. Why do African Americans get more colon cancer than Native Africans? J Nutr. 2007;137(1 Suppl):175S–182S. doi: 10.1093/jn/137.1.175S. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17182822. [DOI] [PubMed] [Google Scholar]
- 35.Ou J, Carbonero F. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J. 2013;(4) doi: 10.3945/ajcn.112.056689.INTRODUCTION. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman DBF. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; Available from http//globocan.iarc.fr.2013. [Google Scholar]
- 37.Rabeneck L, Horton S, Zauber A, Earle C. Colorectal Cancer. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Disease Control Priorities, Third Edition (Volume 3): Cancer. Third. Washington, DC: The World Bank; 2015. pp. 101–119. [DOI] [PubMed] [Google Scholar]
- 38.Keating NL, Landrum MB, Klabunde CN, et al. Adjuvant chemotherapy for stage III colon cancer: do physicians agree about the importance of patient age and comorbidity? J Clin Oncol. 2008;26(15):2532–7. doi: 10.1200/JCO.2007.15.9434. [DOI] [PubMed] [Google Scholar]
- 39.Jorgensen ML, Young JM, Solomon MJ. Adjuvant chemotherapy for colorectal cancer: age differences in factors influencing patients’ treatment decisions. Patient Prefer Adherence. 2013;7:827–34. doi: 10.2147/PPA.S50970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lieu CH, Renfro La, de Gramont A, et al. Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD Clinical Trials Program. J Clin Oncol. 2014;32(27):2975–84. doi: 10.1200/JCO.2013.54.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


