Abstract
Background
Pulmonary hypertension (PH) is a common and morbid complication of left heart disease with 2 subtypes: isolated post-capillary PH (Ipc-PH) and combined post-capillary and pre-capillary PH (Cpc-PH). Little is known about the clinical or physiological characteristics that distinguish these 2 subphenotypes, and if Cpc-PH shares molecular similarities to pulmonary arterial hypertension (PAH).
Objectives
We sought to test the hypothesis that the hemodynamic and genetic profile of Cpc-PH would more closely resemble PAH than Ipc-PH.
Methods
We used Vanderbilt’s electronic medical record linked to a DNA biorepository to extract demographics, clinical data, invasive hemodynamics, echocardiography, and vital status for all patients referred for right heart catheterization between 1998 and 2014. We identified shared genetic variants between PAH and Cpc-PH compared with Ipc-PH using pre-existing single-nucleotide polymorphism data.
Results
We identified 2,817 patients with PH (13% Cpc-PH, 52% Ipc-PH, and 20% PAH). Cpc-PH patients were on average 6 years younger, with more severe pulmonary vascular disease than Ipc-PH patients, despite similar comorbidities and prevalence, severity, and chronicity of left heart disease. After adjusting for relevant covariates, the risk of death was similar between Cpc-PH and Ipc-PH (HR: 1.14, 95% CI: 0.96 to 1.35, p = 0.15) when defined by diastolic pressure gradient. We identified 75 shared exonic single-nucleotide polymorphisms between Cpc-PH and PAH enriched in pathways involving cell structure, extracellular matrix, and immune function. These genes are expressed, on average, 32% higher in lungs relative to other tissues.
Conclusions
Cpc-PH patients develop pulmonary vascular disease similar to PAH patients, despite younger age and similar prevalence of obesity, diabetes mellitus, and left heart disease compared with Ipc-PH patients. An exploratory genetic analysis in Cpc-PH identified genes and biological pathways in the lung known to contribute to PAH pathophysiology, suggesting that Cpc-PH may be a distinct and highly morbid PH subphenotype.
Keywords: Diastolic Pressure Gradient, Heart Failure With Preserved Ejection Fraction, Heart Failure With Reduced Ejection Fraction, Isolated Post-Capillary Pulmonary Hypertension, Pulmonary Arterial Hypertension
Introduction
Pulmonary hypertension (PH) often complicates left ventricular (LV) dysfunction (1) and is associated with worse prognosis (2–6). No therapy improves outcomes in PH associated with left heart disease (PH-LHD) (7–9), perhaps due to the heterogeneity of patients with PH-LHD. Although patients may develop isolated post-capillary PH (Ipc-PH) from passive transmission of left-sided pressure, up to 15% develop pulmonary vascular disease disproportionate to their LV disease (5), which was recently termed combined post- and pre-capillary PH (Cpc-PH). Cpc-PH may represent a subphenotype of PH-LHD that shares physiological and molecular similarities with pulmonary arterial hypertension (PAH).
Cpc-PH is diagnosed at right heart catheterization (RHC) on the basis of a diastolic pressure gradient (DPG) ≥7 mm Hg (10). We recently showed that pulmonary vascular physiology in Cpc-PH, as measured by the resistance-compliance relationship, resembles PAH more than Ipc-PH, suggesting a potential shared etiology (11). Although prior papers have provided valuable information (2,3,5,12–16), our understanding of Cpc-PH etiology and pathophysiology is limited, as no biological data have been reported in this population (17). A better understanding of the clinical and molecular features associated with severe pulmonary vascular disease in patients with LV disease may facilitate tailored therapeutic strategies and targeted inclusion in clinical trials for Cpc-PH patients.
We hypothesized that Cpc-PH would resemble PAH more than Ipc-PH in terms of hemodynamic and genetic characteristics. Using custom data extraction methods, we analyzed patients from Vanderbilt’s electronic medical record and linked DNA biorepository in all patients undergoing RHC over a 17-year period. We built on prior studies by integrating clinical, hemodynamic, echocardiographic, and genetic data in a large cohort with extensive follow-up.
Methods
Study population
The Vanderbilt University Institutional Review Board (IRB #140544) approved this study. Data for this study were extracted from Vanderbilt’s Synthetic Derivative database, a deidentified version of Vanderbilt’s electronic medical record originating in 1995 that was linked to a prospective DNA biorepository (BioVU) in 2007. The design and implementation of the Synthetic Derivative and BioVU were previously described (18,19). The Synthetic Derivative contained over 2.1 million unique patients at the time data were extracted for this study.
Hemodynamic Data and Group Definitions
We queried the database for all patients who underwent RHC between 1998 (when RHC reports were digitalized) and 2014. A unique algorithm using regular expressions and pattern matching was created to extract structured, quantitative data from all RHC reports. For this study, if a patient had multiple catheterizations or provocative testing, only resting data from the first procedure were analyzed. Inpatients and outpatients were included in this study, but patients with profound hemodynamic instability (bradycardia, tachycardia, hypertension, hypotension, or shock) were excluded. Additional exclusion criteria and the number of patients excluded are detailed in Online Table 1. Nonphysiological data suggestive of entry error (e.g., arterial saturation >100%, negative cardiac output) were deleted, and missing or deleted data were imputed (see Statistical Analysis for details).
For our primary analysis, patients were categorized according to contemporary guidelines by the integrated mean hemodynamic values on the RHC report (Table 1) (10,20). Zero-level in the Vanderbilt catheterization lab is midthoracic cavity, and has been consistent over the study period. Briefly, PH was defined as a mean pulmonary arterial pressure ≥25 mm Hg. PAH was defined as PH with a pulmonary arterial wedge pressure (PAWP) ≤15 mm Hg and pulmonary vascular resistance (PVR) >3 Wood units, while excluding patients with World Health Organization (WHO) III and IV PH by International Classification of Diseases, 9th edition (ICD-9) coding for chronic obstructive pulmonary disease (COPD), interstitial lung disease, and chronic pulmonary embolism (Online Table 2). Finally, for patients with PH-LHD (PH and a PAWP >15 mm Hg), Cpc-PH and Ipc-PH were identified as a DPG ≥7 mm Hg and <7 mm Hg, respectively. If multiple possible etiologies of PH were present, patients were categorized according to their initial RHC hemodynamics. In addition, the following sensitivity analyses were performed: 1) only patients with complete data for classification were analyzed; 2) patients with COPD were excluded from the entire cohort; 3) Cpc-PH was defined by a PVR >3 Wood units; 4) Cpc-PH was defined by a DPG ≥7 mm Hg and a PVR >3 Wood units; 5) Cpc-PH was defined by DPG ≥7 mm Hg and PAWP >18 mm Hg.
Table 1.
Hemodynamic Classification of Pulmonary Hypertension*
| Category | mPAP | PAWP | PVR | DPG |
|---|---|---|---|---|
| No PH | <25 mm Hg | ----- | ----- | ----- |
| PCPH | ≥25 mm Hg | ≤15 mm Hg | ----- | ----- |
| PAH* | ≥25 mm Hg | ≤15 mm Hg | > 3 Wood units | ----- |
| Ipc-PH | ≥25 mm Hg | >15 mm Hg | ----- | <7 mm Hg |
| Cpc-PH | ≥25 mm Hg | >15 mm Hg | ----- | ≥7 mm Hg |
In addition, PAH categorization required the exclusion of patients with International Classification of Diseases, 9th edition codes for chronic obstructive pulmonary disease, interstitial lung disease, and chronic pulmonary embolism.
Cpc-PH = combined post-capillary and pre-capillary pulmonary hypertension; DPG = diastolic pressure gradient; Ipc-PH = isolated post-capillary pulmonary hypertension; mPAP = mean pulmonary artery pressure; PAH = pulmonary arterial hypertension; PCPH = pre-capillary pulmonary hypertension; PH = pulmonary hypertension; PAWP = pulmonary arterial wedge pressure.
Clinical and Outcome Data
Demographic data were extracted from the date of RHC. Comorbidity, echocardiographic, and laboratory data were restricted to within 6 months before or after RHC. Comorbidities were defined on the basis of ICD-9 coding or previously validated algorithms (21) (Online Table 2). We extracted laboratory values that report on disease severity (B-type natriuretic peptide [BNP]) or reflect quantitative measures of comorbidities (hemoglobin, glomerular filtration rate, glycosylated hemoglobin [HbA1c], lipid profiles). Quantitative and semiquantitative echocardiographic data were extracted as described previously (22). LV hypertrophy and left atrial enlargement were defined as LV posterior wall thickness ≥12 mm and anterior-posterior left atrial diameter >40 mm, respectively. In order to determine medications prescribed without knowledge of invasive hemodynamics, medications were restricted to those included on the subject’s medication list 6 months prior to RHC. The Synthetic Derivative is linked to the Social Security Death Index, which was used to determine vital status. Follow-up time was calculated from the date of RHC. Patients were censored at the time of death from any cause or the date of last Social Security Death Index search (June 1, 2016). For the primary survival analysis, mortality was compared between PH-LHD patients (defined as mean pulmonary arterial pressure ≥25 mm Hg and PAWP >15 mm Hg) with Ipc-PH (defined as DPG <7 mm Hg) and Cpc-PH (defined as DPG ≥7 mm Hg) in unadjusted and adjusted models. As a secondary survival analysis, we also compared the survival of both groups by the 5 sensitivity analyses listed earlier.
Genetics Data
Pre-existing genotyping data was available for 297 patients with PAH, Cpc-PH, and Ipc-PH, as defined in our primary analysis. To allow genetic comparisons across groups, we only analyzed data for subjects who were genotyped on the Illumina Infinium HumanExome BeadChip, the most commonly used platform in BioVU to date. Due to the small sample sizes of other ancestries, analyses were limited to European Americans. Principal components of all polymorphic single-nucleotide polymorphisms (SNPs) were estimated, with visual inspection of plots to confirm ancestral grouping, and 1 subject was excluded as an outlier to the European-American group. SNPs with minor allele frequencies <5%, multiple locations in the genome, deviation from Hardy-Weinberg proportions (p < 0.001), and genotyping success rate <95% were excluded. In total, 27,997 polymorphic SNPs were included in the analysis. Logistic regression was used to calculate the odds ratio for the association between alleles in Cpc-PH or PAH versus Ipc-PH as the common reference group in unadjusted models. An allelic association test was run in PLINK v.1.9, and significance was determined by permutation testing with up to 1 million replicates, under an adaptive design. Significant SNPs were identified by genome-wide association testing comparing Cpc-PH and PAH to Ipc-PH controls. Significance was defined in 1 of 2 ways: either a p value <0.05 or a large effect size (absolute value of log [odds ratio] >1) for both comparisons (Cpc-PH vs. Ipc-PH and PAH vs. Ipc-PH), regardless of the p value. SNPs meeting these criteria were further restricted to SNPs located in coding regions only.
We used the Genotype-Tissue Expression (GTEx) project (23), a database of tissue-specific gene expression profiles from 449 genotyped deceased donors, to determine additional genes that were associated with these significant SNPs by expression quantitative trait loci (eQTL) mapping. We compiled a final list of genes associated with the significant SNPs, either by the exonic location of the SNP or by eQTL mapping. We further used the GTEx database to determine the relative tissue expression for the genes of interest. Pathway analysis was performed on the genes represented by these SNPs using the WEB-based Gene SeT AnaLysis Toolkit (WebGestalt)(24,25). We performed gene ontology analysis of the associated genes to determine the presence of highly significant gene ontology groups (defined as a false discovery rate <15% for this exploratory analysis).
Statistical Analyses
Data are expressed as mean ± standard deviation for continuous variables, and absolute value and percent for categorical variables, unless stated otherwise. Differences between 2 groups were assessed using the Mann-Whitney U or chi-square test, as appropriate. For regression analyses, imputation of missing and deleted data was performed using multiple imputations with additive regression, bootstrapping, and predictive mean matching. Univariate and multivariate logistic regression was used to identify variables associated with Cpc-PH in patients with PH-LHD. Survival was analyzed using the Kaplan-Meier and Cox proportional hazard methods, and compared by the log-rank test or Cox regression, respectively. Results are reported as odds ratio with 95% confidence intervals and p values. Statistical analysis was performed using R (Version 3.3.1)(26) and Stata for Macintosh (Version 14.0, StataCorp).
Results
Demographics and Clinical Characteristics
We identified 5,797 unique patients referred for RHC (Figure 1); 1,011 patients met pre-specified exclusion criteria. Of the remaining 4,786 patients, 2,817 had PH: 564 with PAH (20%), 364 with Cpc-PH (13%), and 1,456 with Ipc-PH (52%) were included in our subsequent analyses.
Figure 1. Flow Diagram for Patient Categorization.
This schematic represents the initial dataset and subsequent exclusions. The disease classification is according to the 5th World Symposium on Pulmonary Hypertension. All units are mm Hg, unless stated otherwise. Cpc-PH = combined post-capillary and pre-capillary pulmonary hypertension; COPD = chronic obstructive pulmonary disease; DPG = diastolic pressure gradient; ICD-9 = International Classification of Diseases, 9th edition; ILD = interstitial lung disease; Ipc-PH = isolated postcapillary pulmonary hypertension; mPAP = mean pulmonary artery pressure; PAH = pulmonary arterial hypertension; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; PAWP = pulmonary arterial wedge pressure; RHC = right heart catheterization; WHO = World Health Organization.
In unadjusted analyses, Cpc-PH patients were similar in age to those with PAH (p>0.05), but significantly younger than Ipc-PH, patients (p < 0.001, Table 2). Cpc-PH patients were more likely to be female than Ipc-PH patients, although less likely than PAH patients (p < 0.05 for both comparisons). Cpc-PH and Ipc-PH patients had similar clinical characteristics, with a higher point prevalence of diabetes mellitus, coronary artery disease (CAD), hypertension, heart failure, obesity, and anemia than PAH patients (p < 0.001 for all comparisons). A similar proportion of patients with heart failure in the Cpc-PH and Ipc-PH groups had reduced ejection fraction (50% vs. 55%, p > 0.05) defined as <50%. Guideline-based heart failure medications, including beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretic agents, and lipid-lowering medications, were used more often in Cpc-PH and Ipc-PH patients compared with PAH patients (p < 0.001 for all comparisons). ICD-9 coding for valvular heart disease was rare in our cohort, with a similar prevalence across all groups (p > 0.05).
Table 2.
Demographic and Clinical Characteristics of the Cohort
| Characteristics | PAH (n = 564) |
Cpc-PH (n = 364) |
Ipc-PH (n =1,456) |
|---|---|---|---|
| Age, years | 55 ± 15 | 56 ± 14 | 62 ± 14* |
| Female (%) | 69y | 49 | 43* |
| BMI, kg/m2(n =1,909) | 29 ± 7y | 32 ± 9 | 31 ± 8 |
| Race (%) | |||
| White | 78 | 72 | 83* |
| Black | 17 | 21 | 13* |
| Other | 5 | 7 | 4* |
| Comorbidities (%) | |||
| Hypertension | 64y | 80 | 85 |
| Diabetes mellitus | 24y | 49 | 49 |
| Obesity | 35y | 54 | 48 |
| CAD | 45y | 66 | 81* |
| COPD | 0y | 16 | 14 |
| ILD | 0y | 9 | 3* |
| OSA | 7y | 15 | 14 |
| Anemia | 34y | 51 | 61* |
| ASD | 8 | 6 | 5 |
| Atrial fibrillation | 18y | 32 | 45* |
| Valvular disease | 7 | 9 | 8 |
| Heart failure | 50y | 65 | 64 |
| Lupus | 4y | 1 | 1 |
| Scleroderma | 11y | 3 | <1y |
| Medications (%) | |||
| Anticoagulant agents | 38 | 38 | 38 |
| Lipid-lowering medications | 27y | 42 | 55* |
| CCBs | 15 | 15 | 16 |
| Βeta-blockers | 22y | 43 | 51* |
| ACE inhibitors | 20 | 25 | 31* |
| ARBs | 7y | 15 | 13 |
| Diuretic agents | 58y | 72 | 73 |
| ERAs | 11y | 3 | <1* |
| PDE5 inhibitors | 10y | 6 | 2* |
| Prostacyclins | 13y | 4 | <1* |
| Labs | |||
| BNP, pg/ml (n = 1,691) | 637 ± 894y | 970 ± 1,216 | 945 ± 1,125 |
| GFR, ml/min/1.73 m2(n = 2,347) | 68 ± 25y | 65 ± 34 | 61 ± 29 |
| Hemoglobin, g/dl (n = 2,333) | 13.6 ± 2.2y | 12.7 ± 2.3 | 12.1 ± 2.1* |
| Hemoglobin A1c, % (n = 1,364) | 6.3 ± 1.4y | 6.7 ± 1.6 | 6.5 ± 1.4* |
p < 0.05 for Ipc-PH vs. Cpc-PH;
p < 0.05 for PAH vs. Cpc-PH.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; ASD = atrial septal defect; BMI = body mass index; BNP = B-type natriuretic protein; CAD = coronary artery disease; CCB = calcium-channel blocker; COPD = chronic obstructive pulmonary disease; ERA = endothelin receptor antagonist; GFR = glomerular filtration rate; ILD = interstitial lung disease; OSA = obstructive sleep apnea; PDE5 = phosphodiesterase 5. Other abbreviations as in Table 1.
BNP values were elevated in all groups versus reference values in our laboratory (10 to 100 pg/ml; p < 0.001); however, BNP was higher in Cpc-PH and Ipc-PH patients compared with PAH patients (p < 0.001). Cpc-PH patients had increased HbA1c compared with Ipc-PH patients, and HbA1c in both groups was higher than in PAH patients (p < 0.05). Renal function was worse in the Cpc-PH and Ipc-PH groups than in the PAH group (p < 0.01), and all groups had similar lipid profiles (p > 0.05, data not shown).
In patients with heart failure, median times to RHC after heart failure diagnosis in Cpc-PH (median time 116 days, interquartile range [IQR]: 5 to 1,314 days) and Ipc-PH (median time 101 days, IQR: 6 to 1,078 days) were nearly identical (p = 0.98), suggesting the same chronicity of disease in both groups. As expected, PAH-specific medication use was more frequent in patients with PAH than with Cpc-PH, and more frequent in patients with Cpc-PH than with Ipc-PH (p < 0.001 for all comparisons).
Echocardiographic and Hemodynamic Data
A total of 2,074 (82%) patients had at least 1 transthoracic echocardiogram performed within 6 months of the RHC. The median time between echocardiogram and RHC was 2 days prior (IQR: 17 days prior to 1 day after). The degree of LV remodeling was similar or less severe in Cpc-PH compared to Ipc-PH patients (Table 3): both had similar LV ejection fraction and prevalence of LV hypertrophy (p > 0.05), with larger LV and left atrial diameters and LV mass index seen in patients with Ipc-PH (p < 0.05 for all comparisons). All echocardiographic measures of LV remodeling and stress were less severe in PAH compared with either Cpc-PH or Ipc-PH (p < 0.01 for all comparisons).
Table 3.
Echocardiographic Characteristics of the Cohort
| Variable | PAH (n = 484) |
Cpc-PH (n = 312) |
Ipc-PH (n = 1,222) |
|---|---|---|---|
| LVEDD, cm (n = 1,996) | 4.3 ± 1.1y | 5.2 ± 1.4 | 5.4 ± 1.2* |
| LVESD, cm (n = 1,947) | 2.9 ± 1.2y | 4.0 ± 1.7 | 4.1 ± 1.5* |
| LV IVS thickness, mm (n = 1,730) |
11 ± 3y | 12 ± 3 | 12 ± 3 |
| LA diameter, cm (n = 1,935) | 3.9 ± 0.8y | 4.5 ± 0.9 | 4.7 ± 0.8* |
| LV ejection fraction, % (n = 1,991) |
51 ± 12y | 42 ± 19 | 40 ± 19 |
| LV mass index, g/m2 (n = 1,644) |
86 ± 41y | 113 ± 51 | 124 ± 47* |
| LVH, % (n = 1,975) | 23y | 33 | 35 |
| LAE, % (n = 1,935) | 40y | 69 | 78y |
p < 0.05 for Ipc-PH vs. Cpc-PH;
p < 0.05 for PAH vs. Cpc-PH.
IVS = interventricular septal; LA = left atrial; LAE = left atrial enlargement; LV = left ventricular; LVEDD = left ventricular end-diastolic diameter; LVESD = left ventricular end-systolic diameter; LVH = left ventricular hypertrophy. Other abbreviations as in Table 1.
Cpc-PH patients exhibited hemodynamics distinct from Ipc-PH and closely resembling PAH (Table 4). Despite lower PAWP in Cpc-PH than Ipc-PH (p < 0.001), all indexes of pulmonary vascular disease were more severe in Cpc-PH than Ipc-PH patients, including right atrial and pulmonary arterial pressures, PVR, and pulmonary arterial compliance (p < 0.001 for all comparisons). In addition, cardiac function was worse in Cpc-PH than Ipc-PH patients, as evidenced by lower stroke volume, cardiac index, and pulmonary arterial saturation (p < 0.001 for all comparisons). We performed 5 sensitivity analyses: 1) excluding all patients classified with imputed values; 2) excluding all patients with COPD; 3) using a PVR >3 Wood units to define Cpc-PH; 4) using a PVR >3 Wood units and DPG ≥7 mm Hg to define Cpc-PH; and 5) using a PAWP >18 mm Hg to diagnose PH-LHD. All sensitivity analyses yielded similar results with respect to hemodynamic differences between Cpc-PH, Ipc-PH, and PAH (Online Tables 3–7).
Table 4.
Hemodynamic Characteristics of the Cohort
| Characteristic | PAH (n = 564) |
Cpc-PH (n = 364) |
Ipc-PH (n =1,456) |
|---|---|---|---|
| Heart rate, beats/min (n = 1,318) | 78 ± 14 y | 82 ± 16 | 76 ± 16* |
| Systolic BP, mm Hg(n = 2,167) | 126 ± 24 | 128 ± 26 | 126 ± 26 |
| Diastolic BP, mm Hg(n = 2,125) | 76 ± 16 | 76 ± 13 | 70 ± 14* |
| Mean RA pressure, mm Hg (n = 2,200) | 8 ± 5 y | 14 ± 7 | 12 ± 6* |
| Systolic PA pressure, mm Hg(n = 2,293) | 72 ± 23 | 69 ± 20 | 53 ± 13* |
| Diastolic PA pressure, mm Hg(n = 2,294) | 29 ± 11y | 34 ± 8 | 23 ± 6* |
| Mean PA pressure, mm Hg(n = 2,293) | 45 ± 14y | 47 ± 11 | 36 ± 8* |
| PAWP, mm Hg (n = 2,187) | 9 ± 4y | 22 ± 5 | 24 ± 6* |
| DPG, mm Hg(n = 2,147) | 19 ± 12y | 12 ± 6 | −1 ± 5* |
| Transpulmonary gradient, mm Hg(n = 2,146) | 35 ± 15y | 25 ± 10 | 12 ± 5* |
| PVR, Wood units (n = 2,175) | 8.6 ± 5.0y | 5.8 ± 3.2 | 2.6 ± 1.6* |
| Cardiac index, l/min/m2 (n = 2,018) | 2.5 ± 0.8 | 2.5 ± 0.8 | 2.7 ± 0.9* |
| Stroke volume, ml(n = 1,105) | 60 ± 24 | 64 ± 30 | 71 ± 30* |
| PA oxygen saturation, %(n = 2,051) | 64 ± 9y | 61 ± 10 | 63 ± 10* |
| Pulmonary arterial compliance, ml/mm Hg(n = 1,036) |
1.6 ± 1.0y | 2.4 ± 1.8 | 2.7 ± 1.7* |
| RC time, s (n = 1,013) | 0.67 ± 0.22y | 0.61 ± 0.28 | 0.37 ± 0.19* |
p < 0.05 for Ipc-PH vs. Cpc-PH;
p < 0.05 for PAH vs. Cpc-PH.
BP = blood pressure; PA = pulmonary arterial; PVR = pulmonary vascular resistance; RA = right atrial; RC = resistance-compliance. Other abbreviations as in Table 1.
Clinical and Hemodynamic Correlates of Cpc-PH in PH-LHD
In univariate analysis of patients with PH-LHD, younger age and female sex were predictors of Cpc-PH. After adjusting for age and sex, clinical predictors of Cpc-PH included increased hemoglobin, decreased prevalence of CAD and anemia, and increased prevalence of COPD (Table 5). Decreased beta-blocker and angiotensin-converting enzyme inhibitor use, as well as increased PAH-specific therapy use before RHC were predictive of Cpc-PH. Echocardiographic indexes of LV remodeling, specifically larger LV mass index, and left atrial and LV internal dimensions, remained predictive of Ipc-PH.
Table 5.
Univariate and Multivariate Associations of Cpc-PH in PH-LHD.
| Variable | Unadjusted OR (95% CI) |
Age- and Sex-Adjusted OR (95% CI) |
P Value |
|---|---|---|---|
| Age (yrs) | 0.97 (0.96–0.98) | 0.97 (0.96–0.98) | <0.001 |
| Female sex | 1.27 (1.01–1.60) | 1.27 (1.01–1.61) | 0.043 |
| BMI (kg/m2) | 1.02 (1.00–1.03) | 1.01 (0.997–1.03) | 0.39 |
| Comorbidities | |||
| Hypertension | 0.75 (0.56–1.00) | 0.84 (0.62–1.14) | 0.27 |
| Heart failure | 1.06 (0.83–1.35) | 1.08 (0.84–1.38) | 0.55 |
| Diabetes mellitus | 0.97 (0.77–1.22) | 1.00 (0.79–1.26) | 0.98 |
| Obesity | 1.28 (1.02–1.62) | 1.13 (090–1.44) | 0.30 |
| CAD | 0.46 (0.36–0.60) | 0.59 (0.45–0.77) | <0.001 |
| COPD | 1.24 (0.90–1.70) | 1.48 (1.07–2.05) | 0.017 |
| Anemia | 0.69 (0.55–0.87) | 0.76 (0.60–0.96) | 0.021 |
| Medications | |||
| CCB | 0.89 (0.64–1.22) | 0.91 (0.66–1.27) | 0.60 |
| Diuretic agents | 0.93 (0.72–1.20) | 0.99 (0.76–1.29) | 0.96 |
| Βeta-blockers | 0.71 (0.56–0.89) | 0.72 (0.57–0.91) | 0.007 |
| ACE inhibitors | 0.74 (0.57–0.96) | 0.71 (0.54–0.93) | 0.013 |
| ARB | 1.13 (0.81–1.56) | 1.27 (0.91–1.77) | 0.17 |
| Endothelin blockers | 7.06 (2.76–18.05) | 5.10 (1.96–13.25) | 0.001 |
| PDE5 inhibitors | 3.37 (1.87–6.06) | 3.07 (1.69–5.58) | < 0.001 |
| Prostacyclins | 9.62 (3.89–23.31) | 6.99 (2.79–17.52) | < 0.001 |
| Labs | |||
| BNP (pg/ml) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 0.185 |
| HbA1c (%) | 1.07 (0.99–1.15) | 1.06 (0.98–1.15) | 0.15 |
| GFR (per 10 ml/min/1.73 m2) | 1.04 (1.01–1.08) | 1.00 (1.0–1.04) | 0.89 |
| Hemoglobin (g/dl) | 1.15 (1.09–1.21) | 1.15 (1.09–1.22) | <0.001 |
| Hemodynamics | |||
| Heart rate (beats/min) | 1.01(1.01–1.02) | 1.01 1.00–1.02) | 0.007 |
| Systolic BP mm Hg) | 1.00 (1.00–1.01) | 1.01(1.00–1.01) | 0.008 |
| Diastolic BP (mm Hg) | 1.03 (1.02–1.04) | 1.02 (1.02–1.03) | < 0.001 |
| Mean RA pressure (mm Hg) | 1.05 (1.03–1.07) | 1.05 (1.03–1.06 | < 0.001 |
| Systolic PA pressure (mm Hg) | 1.06 (1.05–1.07) | 1.06 (1.05–1.07) | < 0.001 |
| Diastolic PA pressure (mm Hg) | 1.24 (1.21–1.26) | 1.23 (1.20–1.26) | <0.001 |
| Mean PA pressure (mm Hg) | 1.14 (1.12–1.15) | 1.13 (1.11–1.15) | < 0.001 |
| PAWP (mm Hg) | 0.93 (0.91–0.95) | 0.92 (0.90–0.94) | < 0.001 |
| PVR (Wood units) | 1.96 (1.81–2.12) | 1.99 (1.83–2.16) | < 0.001 |
| Cardiac index (l/min/m2) | 0.80 (0.70–0.91) | 0.81 (0.71–.93) | 0.002 |
| Stroke volume (ml) | 0.99 (0.99–0.99) | 0.99 (0.99–1.0) | < 0.001 |
| PA oxygen saturation (%) | 0.98 (0.97–0.99) | 0.98 (0.97–0.99) | < 0.001 |
| Capacitance (ml/mm Hg) | 0.83 (0.77–0.90) | 0.83 (0.77–0.90) | < 0.001 |
| RC time (0.1 s) | 3.82 (2.24–6.54) | 3.78 (2.19–6.52) | <0.00 |
| Echocardiography | |||
| LVEDD (cm) | 0.99 (0.98–1.00) | 0.98 (0.97–0.99) | 0.001 |
| LVESD (cm) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 0.03 |
| LV posterior wall (cm) | 0.94 (0.89–0.99) | 0.96 (0.92–1.02) | 0.18 |
| LV mass index (g/m2) | 0.99 (0.99–1.00) | 1.0 (0.99–1.00) | 0.03 |
| LV ejection fraction (%) | 1.00 (1.00–1.01) | 1.01 (1.00–1.01) | 0.059 |
| Left atrial enlargement | 0.65 (0.50–0.85) | 0.75 (0.56–0.99) | 0.043 |
| LVH | 0.89 (0.69–1.16) | 1.04 (0.80–1.36) | 0.75 |
Outcomes
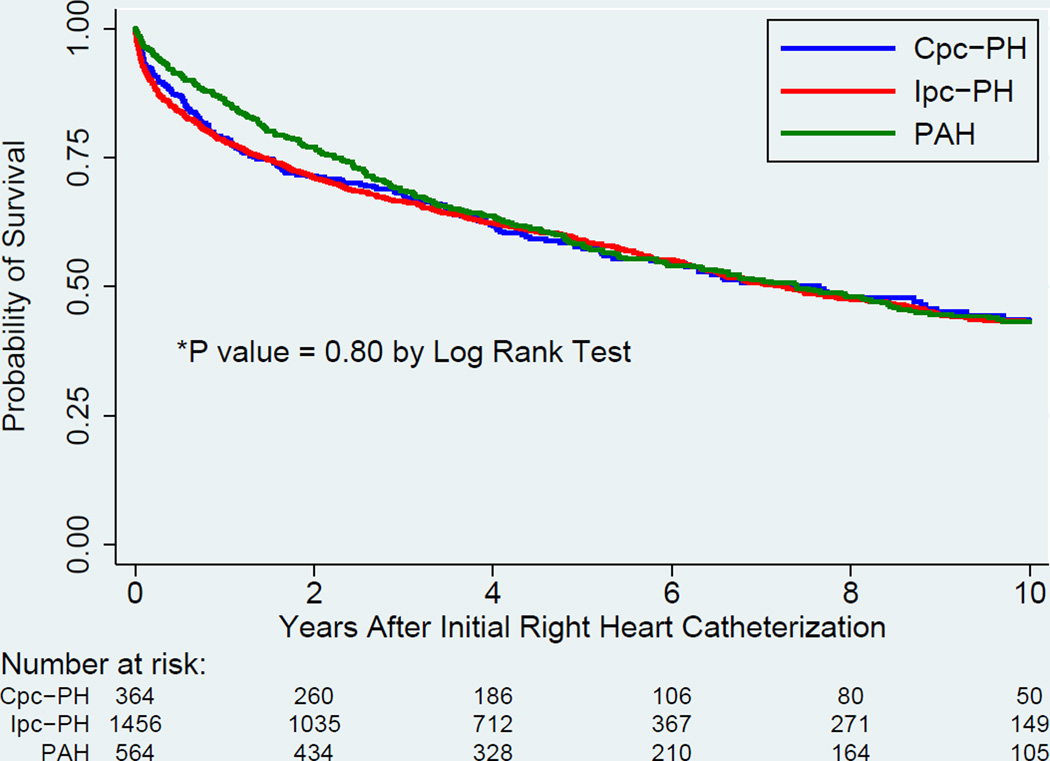
Unadjusted survival was no different among the 3 groups (p > 0.05 by log-rank test, Figure 2). After adjusting for age, sex, body mass index, and relevant comorbidities (COPD, interstitial lung disease, obstructive sleep apnea, CAD, atrial fibrillation, valvular heart disease, lupus, and scleroderma) in a Cox proportional hazards regression model, the risk of death was no different in Cpc-PH patients compared to Ipc-PH patients (hazard ratio [HR]: 1.14, 95% confidence interval [CI]: 0.96 to 1.35, p = 0.15, Table 6). Defining Cpc-PH by our pre-specified sensitivity analyses yielded similar results, with 1 exception: when defined by PVR >3 Wood units, Cpc-PH had increased mortality compared with Ipc-PH (HR: 1.35; 95% CI: 1.18 to 1.55, p < 0.001).
Figure 2. Unadjusted Survival Analysis for Patients With Cpc-PH, Ipc-PH, and PAH.
Unadjusted survival between Cpc-PH, Ipc-PH, and PAH is no different (p>0.05 by log rank test). Abbreviations as in Figure 1.
Table 6.
Unadjusted and Adjusted Hazard Ratio for Mortality in Cpc-PH Compared to Ipc-PH.
| PH-LHD Category |
Cpc-PH Definition | Unadjusted Mortality |
P Value | Adjusted Mortality* |
P Value |
|---|---|---|---|---|---|
| Ipc-PH | ------- | 1.0 (reference) | ------- | 1.0 (reference) | ------- |
| Cpc-PH | DPG ≥7 mm Hg, PAWP >15 mm Hg (primary analysis): |
0.99 (0.84–1.17) | 0.91 | 1.14 (0.96–1.35) | 0.15 |
| + Classified with complete data | 0.99 (0.83–1.19) | 0.95 | 1.12 (0.93–1.34) | 0.23 | |
| + COPD excluded | 1.01 (0.84–1.22) | 0.92 | 1.16 (0.95–1.40) | 0.14 | |
| + PVR >3 Wood units | 1.03 (0.87–1.23) | 0.73 | 1.17 (0.98–1.41) | 0.084 | |
| DPG ≥7 mm Hg, PAWP >18 mm Hg | 1.04 (0.85–1.26) | 0.71 | 1.17 (0.96–1.44) | 0.12 | |
| PVR >3 Wood units, PAWP >15 mm Hg | 1.29 (1.13–1.47) | <0.001 | 1.35 (1.18–1.55) | <0.001 | |
Mortality adjusted for the following variables determined within 6 months of right heart catheterization: age, sex, body mass index, and International Classification of Diseases, 9th edition coding for CAD, atrial fibrillation, valvular disease, COPD, interstitial lung disease, lupus, and scleroderma.
Genetics Analysis
We analyzed 254 Caucasian subjects with pre-existing genotyping in BioVU: 79 with PAH, 139 with Ipc-PH, and 36 with Cpc-PH (Online Table 8). We identified 141 SNPs that were differentially expressed between PAH and Cpc-PH versus Ipc-PH controls. After exclusion of SNPs not associated with any known genes, 75 exonic SNPs remained (Online Tables 9 and 10), including 2 genes previously associated with PAH progression (COL18A1)(27) and modulation (SMCR7)(28). These 75 exonic SNPs in 73 genes were associated with an additional 68 genes by eQTL mapping using the GTEx database, yielding a total of 141 genes associated with shared risk for both Cpc-PH and PAH versus Ipc-PH (Online Table 11).
Gene ontology analysis revealed enrichment in genes related to cytoskeletal structure and immune function. Highly significant pathways included actin binding, extracellular matrix, basement membrane, transferase activity, pre-ribosome structure, and the major histocompatibility complex (MHC) Class II protein complex (p < 0.005, with a false discovery rate <15% for all comparisons, Table 7). The 141 genes were expressed an average of 32% higher in lungs relative to other tissues (6th highest expression levels of 53 tissues) in the GTEx database. When GTEx analysis was restricted to genes in the statistically significant pathways for actin binding, basement membrane, extracellular matrix, and MHC Class II proteins, expression was increased 105%, 140%, 83%, and 619% in the lung, respectively. These findings suggest that these genes and pathways may be of functional importance in the lung, and support the possibility of pathophysiological overlap between PAH and Cpc-PH in the development of pulmonary vascular disease.
Table 7.
Significant Gene Ontology Groups Identified for the 141 Genes Associated With Cpc-PH and PAH Compared With Ipc-PH Controls
| GO Group | Total Number of Genes in GO Group |
Observed Number of Variant Genes in GO Group |
Expected Number of Variant Genes in GO Group |
P Value | FDR | Genes |
|---|---|---|---|---|---|---|
| Actin binding | 353 | 8 | 2.1 | 0.001 | 0.06 |
PARVB, FLII, SSH3, SYNE2, LIMCH1, MYO9B, MACF1, MYH11 |
| Structural molecule activity |
608 | 10 | 3.6 | 0.003 | 0.11 |
ROPN1B, MOSPD3, FLG, RPSA, CCDC108, COL4A3, COL11A2, COL18A1, LAMA5, MYH11 |
| Extracellular matrix |
184 | 5 | 1.1 | 0.004 | 0.14 |
COL18A1, COL4A3, COL11A2, FREM1, LAMA5 |
| Basement membrane |
88 | 4 | 0.5 | 0.002 | 0.14 |
COL18A1, COL4A3, FREM1, LAMA5 |
| Transferase activity |
8 | 2 | 0.05 | 0.0009 | 0.06 | SHMT1, ALDH1L1 |
| Pre-ribosome | 16 | 2 | 0.1 | 0.004 | 0.14 | UTP14C, RPSA |
| MHC Class II protein complex |
16 | 2 | 0.1 | 0.004 | 0.14 |
HLA-DPA1, HLA-DPB1 |
FDR = false discovery rate; GO = gene ontology. Other abbreviations as in Table 1.
Discussion
We sought to compare clinical and genetic features of Cpc-PH, Ipc-PH, and PAH in a large database, testing the hypothesis that Cpc-PH would more closely resemble PAH than Ipc-PH hemodynamically and genetically. We found that Cpc-PH patients had more severe pulmonary vascular disease and were substantially younger than Ipc-PH patients, despite similar comorbidities and LV remodeling, with similar survival after adjusting for clinically relevant covariates. Furthermore, we found shared genetic variants between PAH and Cpc-PH, involving biologically plausible pathways in the development of vascular remodeling, with high expression in the lungs (Central Illustration). Our study demonstrates the potential for integrated electronic medical record-based cohorts to provide biological insight in pulmonary vascular diseases.
Central Illustration. Cpc-PH and PAH Patients: Clinical and Genetic Similarities.
Despite a similar prevalence of systemic, vascular, and cardiac comorbidities, Cpc-PH patients develop more severe pulmonary vascular disease at a younger age than Ipc-PH patients. When compared with Ipc-PH patient controls, we identified 141 SNPs shared between Cpc-PH and PAH patients. These SNPs were associated with genes that are highly expressed in lung tissue and involve gene ontology groups previously implicated in PAH pathophysiology, such as cytoskeletal structure and function. Cpc-PH = combined post-capillary and pre-capillary pulmonary hypertension; Ipc-PH = isolated post-capillary pulmonary hypertension; LV = left ventricular; PAH = pulmonary arterial hypertension; SNP = single-nucleotide polymorphism.
PH is a frequently observed complication of left heart disease and associated with worse clinical outcomes (2,3,5,6,14,29); however, little is known about the etiology and prognosis of its various subphenotypes. PH-LHD is dichotomized by the presence or absence of a pre-capillary component, although the ideal metric for identifying the pre-capillary component is debated (30,31). The most current recommendations by the 5th World Symposium on PH are a DPG ≥7mm Hg to define Cpc-PH (10). An elevated DPG has been linked histopathologically to vascular remodeling (5); thus, we used this hemodynamic metric to identify Cpc-PH in our cohort. In their joint 2015 statement, the European Respiratory Society and European Society of Cardiology recommend use of the DPG ≥7 mm Hg and/or PVR >3 Wood units to diagnose Cpc-PH (32). To further challenge the validity of our findings, we performed sensitivity analyses defining Cpc-PH by an elevated PVR alone, and by an elevated DPG and PVR, and found similar results (Online Tables 5 and 6).
In our cohort, the clinical characteristics of Cpc-PH are nearly identical to those of patients with Ipc-PH, with a high burden of the metabolic syndrome and vascular disease at rates exceeding those seen in PAH. These findings are consistent with previously published reports by our group and others (2,33). In fact, HbA1c was higher in Cpc-PH than in Ipc-PH patients, and both human and animal studies have implicated insulin resistance in the development of pulmonary vascular disease (34–36). One important demographic distinction is a younger age in Cpc-PH compared with Ipc-PH patients, despite a similar time between heart failure diagnosis and RHC, an observation corroborated by several previously published reports (5,12). Echocardiography demonstrated smaller left atrial and LV dimensions, smaller LV mass, and similar degrees of LV hypertrophy in Cpc-PH compared with Ipc-PH patients. However, despite similar, or less severe, left heart dysfunction, the hemodynamic derangements seen in Cpc-PH are more pronounced. These findings raise doubts that Cpc-PH is simply a consequence of prolonged exposure to elevated left-sided pressures. Younger age, despite similar chronicity and severity of LV dysfunction, suggests an alternative explanation (perhaps a predisposition) for the development of exaggerated pulmonary vascular disease in Cpc-PH patients.
Compared with Ipc-PH patients in our adjusted model, we observed a similar mortality in Cpc-PH when defined by DPG, but increased mortality in Cpc-PH defined by PVR. This corroborates the findings of several (13,16,37), but not all (5,14) previous publications. The only study to demonstrate increased mortality in PH-LHD patients with a DPG ≥7 mm Hg evaluated Cpc-PH in 80 and 49 patients with systolic and diastolic heart failure, respectively (14). Their survival analysis adjusted for 4 covariates: age; sex; stable ischemic heart disease; and creatinine clearance <60 ml/min. The same group also showed increased mortality in a subset of subjects with both elevated transpulmonary gradient and DPG, after adjusting for the same covariates (5). In contrast, our survival analysis involved a large and diverse Cpc-PH cohort (351 patients with all types of left heart disease) after adjustment for 10 clinically relevant and highly morbid covariates (including body mass index, COPD, interstitial lung disease, atrial fibrillation, and connective tissue disease). These differences in study design and statistical analysis may account for the discrepant all-cause mortality seen in our studies. Of note, we observed increased mortality in PH-LHD patients with an elevated PVR in one of our sensitivity analyses. PVR is a function of cardiac output, whereas DPG is merely a small difference in pressures. This indicates that right heart function is the major determinant of survival in PH-LHD, regardless of etiology. Our data demonstrate that DPG is more useful than PVR as a diagnostic tool to differentiate PH-LHD phenotypes, but is less helpful than PVR in predicting mortality (38,39).
We used pre-existing genetic data in Vanderbilt’s BioVU database to test the hypothesis that Cpc-PH develops, in part, due to shared genetic risk with PAH. We identified 75 exonic SNPs differentially expressed between Cpc-PH and PAH compared with Ipc-PH control patients. Risk factors for PH-LHD are similar in Ipc-PH and Cpc-PH, making Ipc-PH patients an ideal reference group for isolation of genes associated with the disproportionate pulmonary vascular disease seen in Cpc-PH. One of the identified SNPs involved COL18A1, the gene encoding the antiangiogenic protein endostatin, which was recently associated with PAH (27). Another SNP involved SMCR7, a modulator of dynamin-related protein 1, a mitochondrial fissure stimulant shown to regulate PAH (28). On gene ontology analysis, we found that the identified SNPs were associated with pathways previously identified in vascular remodeling, including cytoskeletal, cell structure, and immune function. Moreover, these genes, especially those in highly significant gene ontology groups, have increased expression in lung tissue. Our group and others have shown that many of these pathways are deranged in PAH (40–45). Although these findings are exploratory, they suggest that Cpc-PH and PAH may share an underlying genetic susceptibility to pulmonary vascular disease.
Our study has several limitations. Our data are derived from a single center, although our large and heterogeneous population strengthens the generalizability of our findings. Treatment options and guidelines have evolved throughout the course of our 17-year study, and we were unable to account for this in our analysis. Hemodynamic tracings and echocardiographic images are not available for review in the Synthetic Derivative. Therefore, it is possible that individual subjects are misclassified due to errors in the computer-generated hemodynamic values on the RHC and echocardiographic reports. We previously showed strong agreement between the manual and computer-generated integrated mean PAWP in this database (11) and modest error in echocardiographic data extraction on a population level (22). We addressed the problem of potential misclassification several ways: we used histograms to evaluate outliers, deleting values believed to be nonphysiological; we excluded patients with pre-capillary PH and diagnoses known to cause WHO III and IV PH; and we defined Cpc-PH several ways, according to pre-specified sensitivity analyses. Finally, the genotyping platform used has limited scope to identify common or noncoding variants. Accordingly, we focused our analysis on exonic SNPs in European Americans to maximize our power. Although our genetic findings are biologically plausible, they require replication in additional cohorts, with functional validation of the genes of interest. Therefore, these findings should be considered hypothesis generating. Given the paucity of biological data in this population, these observations may represent an important step forward in understanding the pathobiology of Cpc-PH.
Conclusions
Cpc-PH is a highly morbid and incompletely understood subset of PH, with a high prevalence of obesity, diabetes mellitus, and cardiovascular disease. Our study suggests that the severity and chronicity of LV disease may not fully explain the development of severe pulmonary vascular disease in these patients. In this initial genetic analysis in the Cpc-PH population, we found evidence of genetic abnormalities in pathways that are highly active in the lungs and known to contribute to the pathophysiology of PAH. Our clinical and exploratory genetic findings suggest that Cpc-PH may have a distinct pathophysiology from Ipc-PH.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
Patients with combined pre-capillary and post-capillary pulmonary hypertension (Cpc-PH) develop more severe pulmonary vascular disease than those with isolated post-capillary pulmonary hypertension (Ipc-PH), despite similar comorbidities, chronicity and severity of left heart disease.
Translational Outlook
Exonic single nucleotide polymorphisms shared between patients with Cpc-PH and pulmonary arterial hypertension patients provide novel targets for future functional studies. A molecular signature of Cpc-PH may permit targeted therapeutic management and specific inclusion of patients with this PH subtype in clinical trials.
Acknowledgments
Dr. Hemnes has served as a consultant for Pfizer, United Therapeutics, Bayer and Actelion. She has received research support from United Therapeutics. This research was supported by NIH #1 U01 HL125212-01 (Dr. Hemnes), American Heart Association Fellow to Faculty Grant #13FTF16070002, Pulmonary Hypertension Association Proof-of-Concept Award, and Actelion Entelligence Young Investigator Award (Dr. Brittain). Dr. Assad is supported by NIH # 5T32HL087738-08. The dataset used in the analyses described were obtained from Vanderbilt University Medical Centers BioVU, which is supported by institutional funding and by the Vanderbilt CTSA grant UL1 TR000445 from NCATS/NIH. Genome-wide genotyping was funded by NIH grants RC2GM092618 from NIGMS/OD and U01HG004603 from NHGRI/NIGMS.
Abbreviations and Acronyms
- Cpc-PH
combined post-capillary and pre-capillary pulmonary hypertension
- DPG
diastolic pressure gradient
- Ipc-PH
isolated post-capillary pulmonary hypertension
- LV
left ventricular
- PAH
pulmonary arterial hypertension
- PH
pulmonary hypertension
- PH-LHD
pulmonary hypertension associated with left heart disease
- PAWP
pulmonary arterial wedge pressure
- PVR
pulmonary vascular resistance
- RHC
right heart catheterization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: There are no other industry disclosures.
References
- 1.Fang JC, DeMarco T, Givertz MM, et al. World Health Organization Pulmonary Hypertension Group 2: pulmonary hypertension due to left heart disease in the adult—a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2012;31:913–933. doi: 10.1016/j.healun.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. J Am Coll Cardiol HF. 2013;1:290–299. doi: 10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Bursi F, McNallan SM, Redfield MM, et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59:222–231. doi: 10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam CSP, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in "out-of-proportion" pulmonary hypertension. Chest. 2013;143:758–766. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 6.Guglin M, Khan H. Pulmonary hypertension in heart failure. J Card Fail. 16:461–474. doi: 10.1016/j.cardfail.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Califf RM, Adams KF, McKenna WJ, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1997;134:44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 8.Anand I, McMurray J, Cohn JN, et al. EARTH Investigators. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet. 364:347–354. doi: 10.1016/S0140-6736(04)16723-8. [DOI] [PubMed] [Google Scholar]
- 9.Kaluski E, Cotter G, Leitman M, et al. Clinical and hemodynamic effects of bosentan dose optimization in symptomatic heart failure patients with severe systolic dysfunction, associated with secondary pulmonary hypertension--a multi-center randomized study. Cardiology. 2008;109:273–280. doi: 10.1159/000107791. [DOI] [PubMed] [Google Scholar]
- 10.Vachiéry JL, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–D108. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Assad TR, Brittain EL, Wells QS, et al. Hemodynamic evidence of vascular remodeling in combined post- and precapillary pulmonary hypertension. Pulm Circ. 2016;6:313–321. doi: 10.1086/688516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thenappan T, Shah SJ, Gomberg-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Failure. 2011;4:257–265. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 13.Tampakakis E, Leary PJ, Selby VN, et al. The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. J Am Coll Cardiol HF. 2015;3:9–16. doi: 10.1016/j.jchf.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerges M, Gerges C, Pistritto AM, et al. Pulmonary hypertension in heart failure. Epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med. 2015;192:1234–1246. doi: 10.1164/rccm.201503-0529OC. [DOI] [PubMed] [Google Scholar]
- 15.Zotter-Tufaro C, Duca F, Kammerlander AA, et al. Diastolic pressure gradient predicts outcome in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2015;66:1308–1310. doi: 10.1016/j.jacc.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Tedford RJ, Beaty CA, Mathai SC, et al. Prognostic value of the pre-transplant diastolic pulmonary artery pressure-to-pulmonary capillary wedge pressure gradient in cardiac transplant recipients with pulmonary hypertension. J Heart Lung Transplant. 2014;33:289–297. doi: 10.1016/j.healun.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupuis J, Guazzi M. Pathophysiology and clinical relevance of pulmonary remodelling in pulmonary hypertension due to left heart diseases. Can J Cardiol. 2015;31:416–429. doi: 10.1016/j.cjca.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Pulley J, Clayton E, Bernard GR, et al. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci. 2010;3:42–48. doi: 10.1111/j.1752-8062.2010.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roden DM, Pulley JM, Basford MA, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Gottesman O, Kuivaniemi H, Tromp G, et al. eMERGE Network. The Electronic Medical Records and Genomics (eMERGE) Network: past, present, and future. Genet Med. 2013;15:761–771. doi: 10.1038/gim.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells QS, Farber-Eger E, Crawford DC. Extraction of echocardiographic data from the electronic medical record is a rapid and efficient method for study of cardiac structure and function. J Clin Bioinforma. 2014;4:12. doi: 10.1186/2043-9113-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–W748. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Duncan D, Shi Z, et al. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013;41:W77–W83. doi: 10.1093/nar/gkt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Accessed September 26, 2016]. Available at: http://www.R-project.org/ [Google Scholar]
- 27.Damico R, Kolb TM, Valera L, et al. Serum endostatin is a genetically determined predictor of survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;191:208–218. doi: 10.1164/rccm.201409-1742OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsboom G, Toth PT, Ryan JJ, et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999;34:1802–1806. doi: 10.1016/s0735-1097(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 30.Gerges M, Gerges C, Lang IM. How to define pulmonary hypertension due to left heart disease. Eur Respir J. 2016;48:553–555. doi: 10.1183/13993003.00432-2016. [DOI] [PubMed] [Google Scholar]
- 31.Naeije R, Hemnes AR. The difficult diagnosis of pulmonary vascular disease in heart failure. Eur Respir J. 2016;48:308–310. doi: 10.1183/13993003.00789-2016. [DOI] [PubMed] [Google Scholar]
- 32.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 33.Robbins IM, Newman JH, Johnson RF, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest. 2009;136:31–36. doi: 10.1378/chest.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugh ME, Robbins IM, Rice TW, et al. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant. 2011;30:904–911. doi: 10.1016/j.healun.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West J, Niswender KD, Johnson JA, et al. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J. 2013;41:861–871. doi: 10.1183/09031936.00030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansmann G, Wagner RA, Schellong S, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation. 2007;115:1275–1284. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 37.Opitz CF, Hoeper MM, Gibbs JS, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol. 2016;68:368–378. doi: 10.1016/j.jacc.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 38.Brittain EL, Assad TR, Hemnes AR, et al. The diastolic pressure gradient does not—and should not—predict outcomes. J Am Coll Cardiol HF. 2015;3:845. doi: 10.1016/j.jchf.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Naeije R. Measurement to predict survival: the case of diastolic pulmonary gradient. J Am Coll Cardiol HF. 2015;3:425. doi: 10.1016/j.jchf.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 40.West JD, Austin ED, Gaskill C, et al. Identification of a common Wnt-associated genetic signature across multiple cell types in pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2014;307:C415–C430. doi: 10.1152/ajpcell.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M, Austin ED, Hemnes AR, et al. An evidence-based knowledgebase of pulmonary arterial hypertension to identify genes and pathways relevant to pathogenesis. Mol Biosyst. 2014;10:732–740. doi: 10.1039/c3mb70496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jesus Perez VA, Alastalo TP, Wu JC, et al. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways. J Cell Biol. 2009;184:83–99. doi: 10.1083/jcb.200806049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson JA, Hemnes AR, Perrien DS, et al. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302:L474–L484. doi: 10.1152/ajplung.00202.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemnes AR, Trammell AW, Archer SL, et al. Peripheral blood signature of vasodilator-responsive pulmonary arterial hypertension. Circulation. 2015;131:401–409. doi: 10.1161/CIRCULATIONAHA.114.013317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West J. Cross talk between Smad, MAPK, and actin in the etiology of pulmonary arterial hypertension. Adv Exp Med Biol. 2010;661:265–278. doi: 10.1007/978-1-60761-500-2_17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.