Abstract
This study evaluated the hypothesis that a paclitaxel treatment regimen sufficient to produce mechanical allodynia would alter sensitivities of male and female mice to the conditioned rewarding and reinforcing effects of morphine. Saline or paclitaxel were administered on days 1, 3, 5, and 7 in male and female C57Bl/6 mice to induce morphine-reversible mechanical allodynia as measured by the Von Frey filament test. Paclitaxel treatment did not change sensitivity to morphine conditioned place preference (CPP) relative to saline treatment in either male or female mice. Morphine produced peak self-administration under a fixed ratio-1 schedule of reinforcement for 0.03 mg/kg morphine per infusion in female mice and 0.1 mg/kg morphine per infusion in male mice. During the progressive ratio experiments, saline treatment in male mice decreased the number of morphine infusions for 12 days whereas the paclitaxel-treated male mice maintained responding for morphine similar to baseline levels during the same time period. However, paclitaxel did not have an overall effect on the reinforcing efficacy of morphine assessed over a limited dose range during the course of the repeated self-administration. These results suggest that the reward-related behavioral effects of morphine are overall not robustly altered by the presence of paclitaxel treatment under the current dosing regimen, with the exception of maintaining a small yet significant higher baseline than saline treatment during the development of allodynia in male mice.
Keywords: chemotherapy, chronic pain, conditioned place preference, progressive ratio, self-administration
Chronic pain is a major public health problem that significantly diminishes the quality of life for patients (Brennan, Carr, & Cousins, 2007; Chapman, 2013). The prevalence of prescription opioid abuse among patients for the management of chronic pain has been estimated to be between 18– 45% (Ballantyne & LaForge, 2007; Compton & Volkow, 2006; Fishbain, Cole, Lewis, Rosomoff, & Rosomoff, 2008; Morasco & Dobscha, 2008), although the true incidence is still largely unknown (Bell & Salmon, 2009; Fishbain, Rosomoff, & Rosomoff, 1992; Hojsted & Sjogren, 2007). High risk factors for prescription opioid misuse or abuse among pain patients include personal and/or family history of substance use disorders, any exposure to prescription opioids, duration of therapy as well as psychological, socio-economic, genetic, and environmental factors (Edlund et al., 2013; Manchikanti et al., 2007; Turk, Swanson, & Gatchel, 2008). Although the incidence of prescription opioid abuse and dependence is similar between men and women, gender-specific risk factors do exist. For example, women are more likely to use prescription opioids to cope with negative affect and psychiatric distress (Back, Lawson, Singleton, & Brady, 2011; Jamison, Butler, Budman, Edwards, & Wasan, 2010; McHugh et al., 2013). Other factors, including history of substance abuse, tobacco use, mental health disorders, and childhood psychological trauma appear to serve as risk factors for opioid abuse among cancer pain patients (for review see (Pergolizzi, et al., 2016). More than 33% of head and neck cancer patients treated with radiation and cancer chemotherapeutic agents were unable to stop opioid therapy after 6 months of treatment (Kwon, 2013) and emergency department studies indicate that the occurrence of opioid misuse among cancer patients was 34% with a number of variables such as depression, poor coping, and illicit substance use significantly associated with this high risk of opioid misuse (Reyes-Gibby, Anderson, & Todd, 2016). However, little research has been performed for other potential risk factors such as particular chemotherapy regimens, types of cancer, or different cancer pain states.
Human laboratory studies (Comer, Sullivan, Vosburg, Kowalczyk, & Houser, 2010; Zacny et al., 1996) and clinical studies reporting patient-controlled analgesia procedures (Gil, Ginsberg, Muir, Sykes, & Williams, 1990; Graves, Arrigo, Foster, Baumann, & Batenhorst, 1985; Parker, Holtmann, & White, 1991; Sidebotham, Dijkhuizen, & Schug, 1997) indicate that non drug-abusers self-administer prescription opioids only in the presence of experimentally-induced or current pain while recreational opioid users self-administer opioids (e.g., oxycodone) regardless of the presence or absence of pain (Comer, et al., 2010; Lofwall, Nuzzo, & Walsh, 2012). These findings suggest that the reinforcing effects of opioids in pain patients is most likely due to the ability of these drugs to relieve pain, yet the abuse liability of opioids appears not to vary as a function of pain among drug users and chronic pain patients with a long history of opioid use.
Preclinical studies in animals have advanced the understanding of neuroanatomical and molecular effects within brain regions associated with the development of persistent chronic pain and reward (Narita, Ozaki, Ise, Yajima, & Suzuki, 2003) such as the ventral tegmental area (VTA) (Narita et al., 2004; Niikura, Narita, Butelman, Kreek, & Suzuki, 2010; Ozaki, Narita, Iino, Miyoshi, & Suzuki, 2003; Ozaki et al., 2002), nucleus accumbens (Altier & Stewart, 1999; Baliki et al., 2013), and amygdala (Goncalves et al., 2008; Narita et al., 2006; Neugebauer, Li, Bird, & Han, 2004). Studies on the modulatory effects of arthritic pain (Colpaert FC, 2001), nerve injury-induced neuropathic pain (Cahill CM, 2013; Woller SA, 2012) and persistent inflammatory pain (Sufka, 1994) on measures of opioid reward in rats reveal an increase in opioid self-administration and potentiation in opioid conditioned place preference behaviors. In contrast, other studies found that arthritic rats self-administered fewer infusions of morphine compared to normal rats (Lyness, Smith, Heavner, Iacono, & Garvin, 1989), and nerve injury in rats decreased the rewarding and reinforcing effects of opioids (Martin TJ, 2007; Woller SA, 2012) and the ability of opioids to facilitate mid-brain stimulation in rats (Ewan & Martin, 2011). A recent study demonstrated that nerve injury, chronic inflammation, and administration of cancer chemotherapeutics diminished acquisition of fentanyl self-administration in mice (Wade CL, 2013). However, other studies in rats reported no effect of nerve injury on fentanyl self-administration (Kupers R, 1995), no effect of prolonged inflammation on morphine conditioned place preference (Shippenberg TS, 1988), and no changes of paclitaxel- or oxaliplatin-induced neuropathy on the conditioned rewarding effects of opioids (Mori, et al., 2014). Discrepancies among these studies may be attributed to several factors, including but not limited to, the opioid studied, the dose and route of administration used, the model of chronic pain or abuse liability examined, and/or a combination of these factors.
In the present study, we tested the hypothesis that a paclitaxel treatment regimen sufficient to produce mechanical allodynia will alter sensitivity of male and female C57Bl/6 mice to the: 1) conditioned rewarding effects of morphine using the conditioned place preference (CPP) procedure; and, 2) morphine reinforcing efficacy using intravenous self-administration assays. The administration of taxane-family chemotherapeutics, such as paclitaxel, produces damage to peripheral sensory neurons including neuronal mitochondrial and axonal transport dysfunction, altered signal conduction, and distal axonal degeneration (Flatters & Bennett, 2006; Melli, Keswani, Fischer, Chen, & Hoke, 2006; Nakata & Yorifuji, 1999; Persohn et al., 2005). The persistent behavioral manifestations of these peripheral sensory alterations include reduced threshold and increased responsiveness to non-noxious stimuli (allodynia) in C57Bl/6 mice (Ward, McAllister, Neelakantan, & Walker, 2013; Ward, Ramirez, Neelakantan, & Walker, 2011).
METHODS
Subjects
Male and female C57Bl/6 mice, 5-weeks of age at the beginning of the study, and weighing 20–25 g were purchased from SAGE Labs, (Boyertown, PA, USA) for the self-administration studies (N=20, males; N=20, females), and from Taconic Farms, Inc. (Cranbury, NJ, USA) for the CPP experiments (N=66, males; N=64, females). The original vendor discontinued the sale of C57Bl/6 mice temporarily requiring switching vendors to continue the study. Mice were group housed in plastic cages and allowed to acclimate to the temperature-and-humidity controlled animal facility for 3 to 7 days before the experiments began. Mice were housed under a 12 h light/dark cycle with lights off at 10.00 AM so that all experiments occurred during the dark phase of the mice’s diurnal cycle. For the CPP studies, mice were housed by sex in groups of four with nestlets provided as enrichment. Food and water were available ad libitum. For the self-administration studies, mice were initially group-housed by sex but then were individually housed with nestlets a day prior to the start of the experiment and maintained on 90% of their free feeding body weights by feeding each mouse approximately 2.75 g pellet daily of Purina Rodent Chow Diet 5001 (Ralston-Purina, St. Louis, MO, USA) during the initial food pre-training phase. After food-training, mice received food and water ad libitum for the remaining experiments. All mice were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of Temple University and the Guide for the Care and Use of Laboratory Animals (Institution of Laboratory Animal Research, National Academy Press; Eighth edition, revised 2011).
Drugs
Morphine sulfate was generously donated by the National Institute on Drug Abuse (NIDA) Drug Supply Program (Bethesda, MD, USA). Paclitaxel was obtained as a 6 mg/mL concentration stock solution (Hospira, Inc., Lake Forest, IL, USA) and then further diluted in saline. Morphine was dissolved in 0.9% saline. Systemic injections were administered intraperitoneally (IP) in a volume of 0.01 ml/g of body weight.
Conditioned Place Preference (CPP)
Apparatus
The CPP apparatus consisted of 8 identical experimental chambers (Model ENV-3013, MED Associates, St. Albans, VT, USA), each with a white (mesh floor), middle grey (grey plexiglass floor), and black (bar floor) compartments and distinct light settings. Each chamber was located within a sound-attenuating enclosure and connected to a computer-driven interface (Model SG-6080/D, MED Associates, Georgia, VT USA) that controlled the data collection and experimental conditions.
Procedure
A pseudo-biased place conditioning procedure was used (i.e, a pre-conditioning preference test is not performed) (Ward SJ, 2014). Male (n = 66) and female (n = 64) C57Bl/6 mice were randomly assigned to control and treatment groups. The saline groups of mice received saline injections and the treatment groups of mice received 8 mg/kg paclitaxel injections on days 1, 3, 5, and 7 for a total dose of 32 mg/kg (Ward, et al., 2011). Starting on day 11 and during the period of peak allodynia (increased sensitivity to non-noxious mechanical stimulus) (Ward, et al., 2011), mice were conditioned on alternating days between saline (saline, days 11, 13, and 15) and morphine (0.3, 2.5, or 10 mg/kg, days 12, 14, and 16) with a 15 min drug pretreatment time and 30 min conditioning session. Mice were tested for their preference for the previously drug-paired environment in a drug-free state on day 17 (relative to initiation of paclitaxel treatment). As this was a pseudo-biased procedure, separate groups of saline- and paclitaxel-treated mice were also conditioned with alternating vehicle alone (saline) on both sides of the CPP compartments as controls. The effect of paclitaxel treatment on morphine-reward was determined by comparing the preference for the drug-paired compartments between the control saline-treated and paclitaxel-treated groups of mice.
Data and Statistical Analysis
Time spent in each compartment was recorded on the test day and used to calculate the preference score for each mouse using the following equation:
Preference score = [Time in morphine-paired compartment] – [Time spent in saline-paired compartment]
A three-way ANOVA was performed with sex (male, female), treatment (saline, paclitaxel), and morphine dose (0, 0.3, 2.5, and 10 mg/kg) as factors and a Dunnett’s post hoc test (JMP Pro 12, SAS Institute, Inc., Cary, NC USA). Next, individual one-way ANOVAs within each sex and treatment group were performed to test for differences among doses and Tukey’s multiple comparisons post hoc tests were used (GraphPad Prism 5.0 Software, Inc, La Jolla, CA). Statistical significance was set at p < 0.05 for all analyses.
Morphine Self-Administration
Apparatus
Food pre-training and morphine self-administration experiments were conducted in standard mouse operant experimental chambers (21.6 cm x 17.8 cm x 12.7 cm, Model ENV-307W, Med Associates, Georgia, VT USA). The experimental chambers were located within ventilated sound-attenuating enclosures and each chamber was equipped with the following: two nose-poke holes (1.2 cm diameter), one on the left and one on the right, with internal amber stimulus lights (ENV-313W), a center dipper hole between the two nose-pokes that opened to a motor-driven dipper (ENV-302W) for liquid food presentation, a house light (ENV-315M), a tone generator (ENV-323AW), and a ventilator fan. The receptacle for dipper access contained an amber stimulus light located above (ENV-221M). In addition, an electronic circuit operated a computer-controlled syringe pump designed for intravenous drug-delivery. The syringe was connected to a single-channel fluid swivel mounted on a counter-balanced arm above the operant chamber (MED-307A-CT-B2).
Procedure
Food pre-training
Food-restricted male and female C57Bl/6 mice were trained to respond in the right nose-poke for a 50% Ensure/50% water solution under a Fixed Ratio (FR1) schedule of reinforcement during daily 1 h sessions. During each session, every response in the right illuminated nose-poke hole resulted in illumination of the stimulus light and delivery of liquid food through the center dipper receptacle for 20 s. The number of reinforcers earned during each session, the number of inactive nose-poke responses, and the total number of head entries into the dipper receptacle were recorded. Responses on inactive nose-poke had no scheduled consequences during the experimental sessions. The criteria for acquisition of liquid food self-administration were defined as three consecutive days of stable FR1 responding (<10% changes among the mean number of reinforcers earned for the three days) and at least 75% of total responses corresponding to the active right nose-pokes.
Morphine self-administration: acquisition and dose-response studies (FR1)
Mice that met criteria for food self-administration acquisition were surgically implanted with a chronic indwelling jugular cannula (Caine SB, 1999). Following surgery and 2 days of recovery, all mice were trained to self-administer 0.1 mg/kg per infusion morphine under an FR1 schedule of reinforcement during daily 2 h sessions. This initial training dose for morphine was chosen based on a previous study reporting that morphine maintained maximal response rates at this dose using the FR1 schedule in C57Bl/6 mice (Elmer, et al., 2002). During the session, every response on the active right nose-poke resulted in a single infusion of morphine paired with illumination of the light above the food receptacle and a tone delivery for 1 s. After every morphine infusion, the chamber houselights went off for 60 s and the mouse’s responses had no programmed consequences. After stable responding under FR1 schedule (<10% changes among the mean number of reinforcers earned for three days and with at least 75% of total responses corresponding to the active right nose-pokes), a dose-response curve for morphine (0.01–0.3 mg/kg per infusion) was generated. The doses of morphine were tested in a pseudo-randomized order until stable responding for each dose under the FR1 schedule as described above was achieved for three consecutive days. Saline was substituted for morphine after establishing the dose-response curve for morphine in mice.
Morphine self-administration: maintenance and progressive ratio responding
The doses of morphine that maintained the highest rates of responding under the FR1 schedule of reinforcement in male (0.1 mg/kg per infusion) and female (0.03 mg/kg per infusion) mice were chosen for the subsequent experiments. After stable responding under the FR1 schedule of responding, mice were given access to morphine under a PR schedule of reinforcement. Under this schedule, the response requirements to earn a single reinforcer were increased exponentially in the following progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, etc. (Richardson & Roberts, 1996). The dependent variable measured was the number of infusions earned by the end of the 4 h session and defined as breakpoint. Total cumulative responses made by the mouse on the correct nose-poke during each 4 h session were also recorded. The criterion for stable response under the PR schedule was defined as three consecutive days of stable breakpoints (<20% changes from the mean number of reinforcers earned for three consecutive days). Three female mice and one male mouse failed to acquire reliable PR responding (lower than 4 infusions) and the cannula of two male mice were blocked before they could acquire PR responding fully and move to saline or paclitaxel treatment. These mice were not included in the PR study.
Morphine self-administration: Effect of paclitaxel on progressive ratio responding
Following stable responding on PR, mice were randomly assigned to receive saline or 8 mg/kg paclitaxel injections using the same dosing regimen used above while continuing daily PR self-administration sessions. Mice received the paclitaxel injections after completion of the 4 h PR session on days 1, 3, 5, and 7, and the training dose of morphine (males-0.1 mg/kg per infusion; females 0.03 mg/kg per infusion) continued to be available through day 13, a period during which mechanical allodynia develops (Ward et al., 2011). On days 14–16 and 17–19, mice were tested with two other morphine doses (0.01 to 0.1 mg/kg per infusion) counter-balanced within a treatment group and balanced between the saline- and paclitaxel-treatment groups so that the order of testing was the same in the saline- and paclitaxel-treatment groups.
Data and Statistical Analysis
Response rates measured from the FR experiments were subjected to a two-way ANOVA with sex (male, female) and morphine dose (0, 0.01, 0.03, 0.1, 0.3 mg/kg per infusion) as factors with Bonferroni post hoc tests (GraphPad Prism 5.0 Software, Inc, La Jolla, CA). The data from the PR experiments included total number of responses and breakpoint (equivalent to the number of infusions per session). The breakpoints and total responses measured on the three test days for each of the three doses of morphine were averaged for each mouse, averaged into group means, and compared between saline- and paclitaxel-treated groups. The number of infusions of morphine (breakpoint) was subjected to one-way ANOVA at baseline and two, three-way ANOVAs: 1) development over days; and, 2) morphine dose-response curve. The first three-way ANOVA was performed with sex (male, female), treatment (saline, paclitaxel), and days of treatment (baseline, 2, 7, 12) as factors and a Dunnett’s post hoc test. The second three-way ANOVA was performed with sex (male, female), treatment (saline, paclitaxel), and morphine dose (0.01, 0.03, 0.1) as factors and a Dunnett’s test post hoc test. Statistical significance was set at p < 0.05 for all analyses.
Paclitaxel-Induced Mechanical Allodynia
To compare the results from CPP and self-administration studies (described above) with the anti-allodynia effects of systemic administration of morphine in male and female mice, mechanical allodynia produced by paclitaxel administration was examined under the similar dosing and testing protocols. Separate groups of male and female C57Bl/6 mice were treated with 8 mg/kg paclitaxel or saline on days 1, 3, 5 and 7. Paclitaxel-induced mechanical allodynia was measured using the Von Frey filament assay (Ward, et al., 2011), on days 2, 5, and 8. Starting on day 11, corresponding to the peak allodynia period, separate groups of saline- and paclitaxel-treated mice (n=8 per group) received one injection per day of morphine for three consecutive days (days 11–13). Within each group, half of the mice (n=4) received the lower dose (2.5 mg/kg) while the remaining half (n=4) received the higher dose (10 mg/kg) of morphine. Following the third injection of morphine on day 13, mice were tested for mechanical allodynia 30 min after the injection of morphine. This three-day regimen of dosing and testing was continued for the following two weeks so that morphine injections were administered Monday, Tuesday, and Wednesday (days 18–20 and days 25–27) with either ascending doses (5.0 and 10 mg/kg) or descending doses (5.0 and 2.5 mg/kg) of morphine (counterbalanced within groups). The purpose of the three-day morphine treatment was to directly compare the anti-allodynia effects of morphine after repeated morphine injections as in the CPP and self-administration studies. Mechanical allodynia prior to and following treatment with morphine (2.5–10 mg/kg) in saline-or paclitaxel-treated male and female mice were determined and compared.
Data and Statistical Analysis
Two, three-way ANOVAs were performed with: 1) the factors of sex (male and female), treatment (saline and paclitaxel), and days (0, 5, 8); and, 2) the factors of sex (male and female), treatment (saline and paclitaxel), and morphine dose (0, 2.5, 5, 10) (JMP Pro 12, SAS Institute, Inc., Cary, NC USA). Separate repeated-measures, two-way ANOVAs with Dunnett’s Multiple Comparisons post hoc tests were used to compare treatment and morphine dose factors within each sex (GraphPad Prism 5.0 Software, Inc, La Jolla, CA).
RESULTS
Morphine Conditioned Reward in the Absence and Presence of Paclitaxel-Induced Mechanical Allodynia
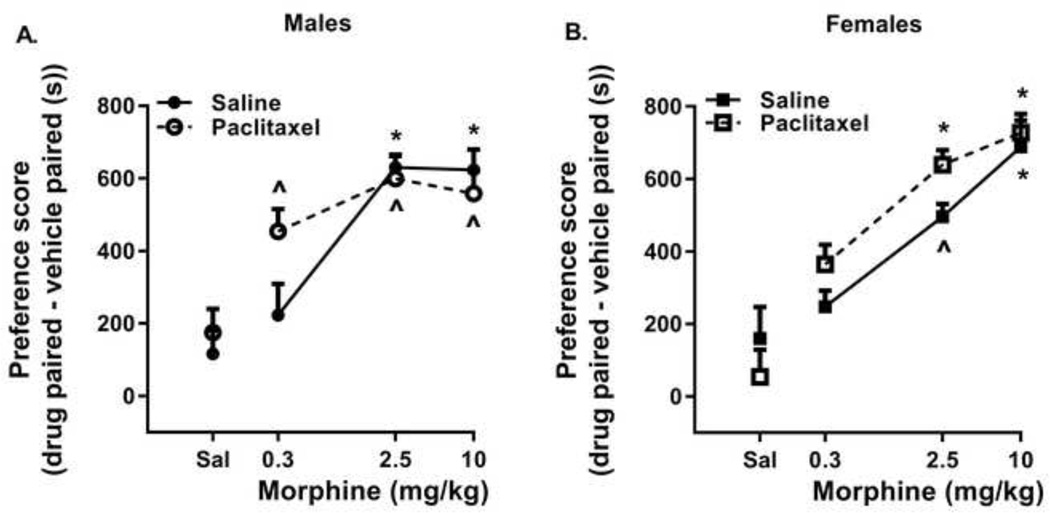
A three-way ANOVA of dose, sex, and treatment in the CPP assay was significant [F(7, 147) = 11.05, p < 0.0001] with Dunnett’s post hoc test indicating dose as the significant contributing factor (p<0.00001). No treatment or sex differences, and no interactions among any of the three factors were found (Figure 1, panels A and B). Individual one-way ANOVAs were then performed for morphine dose in the different groups of mice. In the saline-treated mice, morphine produced significant dose-dependent rewarding effects in male [F(3,36)=14.24; p<0.0001] and female mice [F(3,32)=11.39; p < 0.0001]. Tukey’s multiple comparison post hoc tests revealed significant differences (p<0.05) between saline and 2.5 and 10 mg/kg morphine in all mice and between 0.3 mg/kg morphine and the doses of 2.5 and 10 mg/kg morphine in male, saline-treated mice and between 0.3 and the dose of 10 mg/kg morphine in female, saline-treated mice. In paclitaxel-treated mice, morphine produced significant CPP in male (F(3,35)=9.743; p<0.0001) and female mice (F(3,36)=23.28; p < 0.0001). Post hoc tests revealed significant differences (p<0.05) between saline and all doses of morphine in both male and female mice. Besides being different from saline, morphine failed to reveal any dose-dependent effects in male paclitaxel-treated mice, but a dose of 0.3 mg/kg morphine produced significantly less CPP (p<0.05) than either 2.5 or 10 mg/kg morphine in female paclitaxel-treated mice.
Figure 1.
Paclitaxel treatment does not alter the rewarding effects of morphine in the conditioned place preference assay in male (panel A) or female (panel B) mice. Abscissa: Saline (Sal) or doses of morphine expressed as mg/kg. Ordinate: Preference score, time spent in the drug – vehicle paired sides on the test day in seconds (s). Each point represents the mean preference score ± SEM (n=8/group for morphine, n=16/group for saline). ^, significantly different than saline-conditioned place preference p<0.05; *, significantly different than saline and 0.03 mg/kg morphine-conditioned place preference: p<0.05.
Morphine Self-Administration in the Absence and Presence of Paclitaxel-Induced Mechanical Allodynia
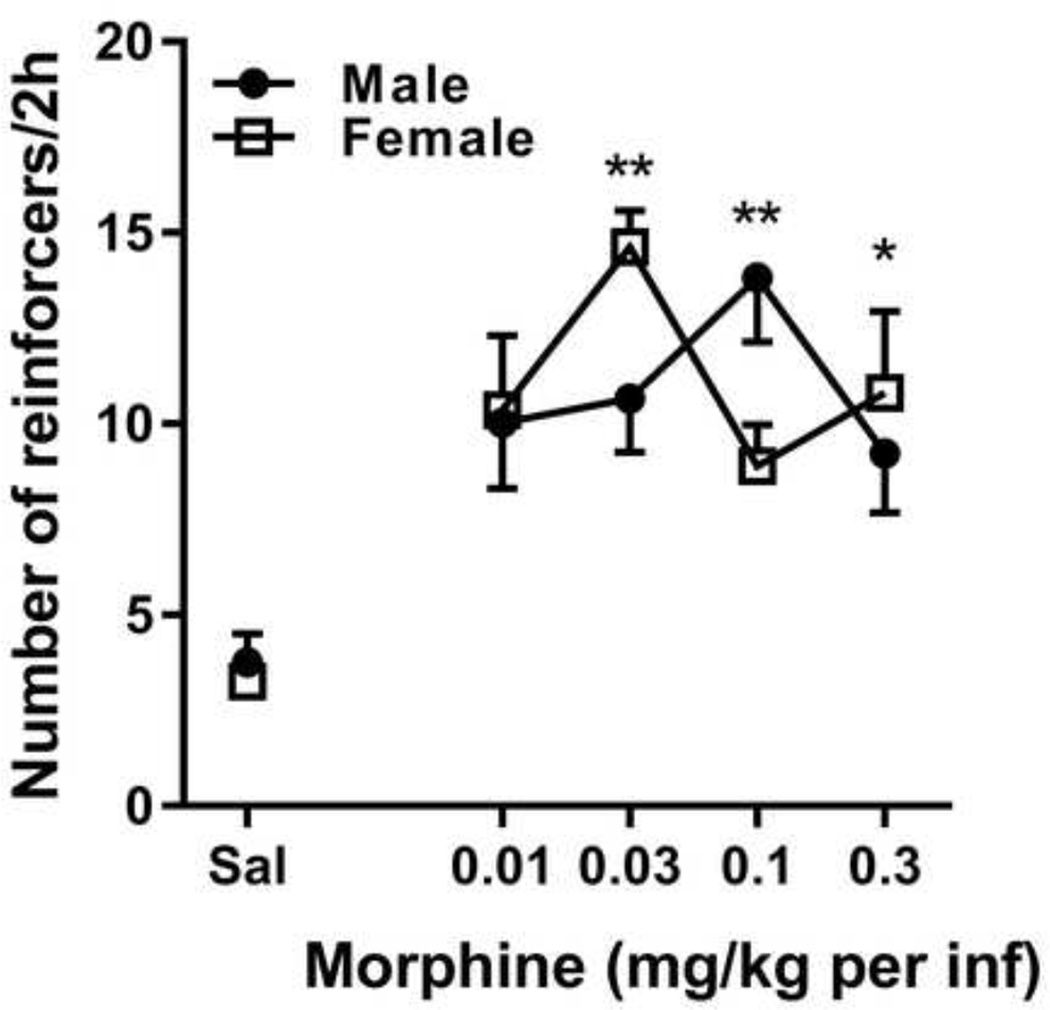
Prior to paclitaxel treatment, morphine produced an inverted-U shaped dose-response curve under an FR1 schedule of reinforcement in male and female C57Bl/6 mice (Figure 2). A two-way ANOVA revealed a significant effect of dose [F(4, 67) = 6.56, p<0.0002], but no effect of sex or an interaction between sex and dose. Bonferroni post tests revealed that the response rates maintained by 0.03 mg/kg per infusion (p < 0.01) and 0.3 mg/kg per infusion (p < 0.05) morphine in female mice and 0.1 mg/kg per infusion of morphine in male mice (p < 0.01) were significantly different from response rates maintained by saline.
Figure 2.
An inverted-U shaped dose-response for morphine under an FR1 schedule of reinforcement in male (filled circles) and female (open squares) mice. Abscissa: Saline (Sal) or unit dose of morphine expressed as mg/kg per infusion. Ordinate: Response rate expressed as the mean number of infusions per 2 h ± SEM (n=5–18/group). Significantly different than saline responding: *, p<0.05; **, p<0.01.
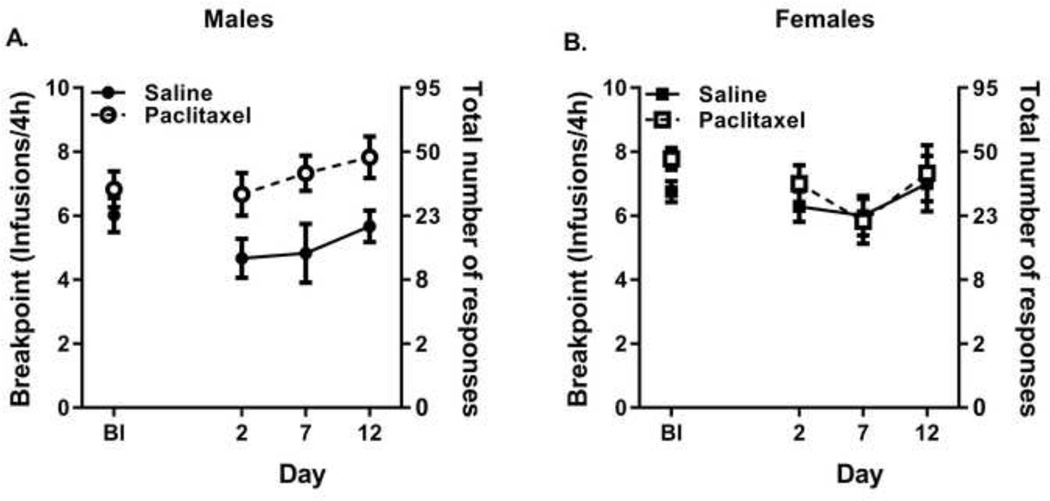
Prior to paclitaxel treatment, morphine supported breakpoint values of 6.0 in the saline group and 6.8 in the paclitaxel group at the training dose of 0.1 mg/kg per infusion in male mice (baseline measures Figure 3A) and breakpoint values of 6.8 in the saline group and 7.8 in the paclitaxel group at the training dose of 0.03 mg/kg per infusion in female mice (baseline measures Figure 3B). There were no significant differences among the four groups in baseline responding for morphine [F(3,21)=2.56, p<0.08); N.S.] as tested by one-way ANOVA. During the development of allodynia in the PR assay, a three-way ANOVA of treatment, sex, and number of days was significant [F(7, 92)= 3.35, p < 0.0031] with Dunnett’s post hoc test indicating treatment as the significant contributing factor (p<0.00031) (Figure 3). No significant differences for sex or number of days of treatment were observed although a significant interaction between sex and treatment was observed (p<0.028). Specifically, the PR responding for saline-treated males was decreased relative to the PR responding for 0.1 mg/kg per infusion morphine in the paclitaxel-treated male (p<0.0003) while PR responding for 0.03 mg/kg per infusion morphine was not different between the saline- and paclitaxel-treated female mice.
Figure 3.
Paclitaxel treatment maintained responding for morphine under a PR schedule in male mice responding for 0.1 mg/kg per infusing compared to saline treatment (panel A) but not in female mice responding for 0.03 mg/kg per infusion (panel B). Abscissa: Baseline (Bl) prior to stating paclitaxel injections, day after first saline (filled symbols) or paclitaxel (closed symbols) injection. Paclitaxel injections occurred on days 1, 3, 5, and 7. Left ordinate: Breakpoint (defined as the number of infusions per session); Right ordinate: Corresponding total cumulative number of responses required in the PR schedule. Each data point represents mean ± SEM (n=6–15/group).
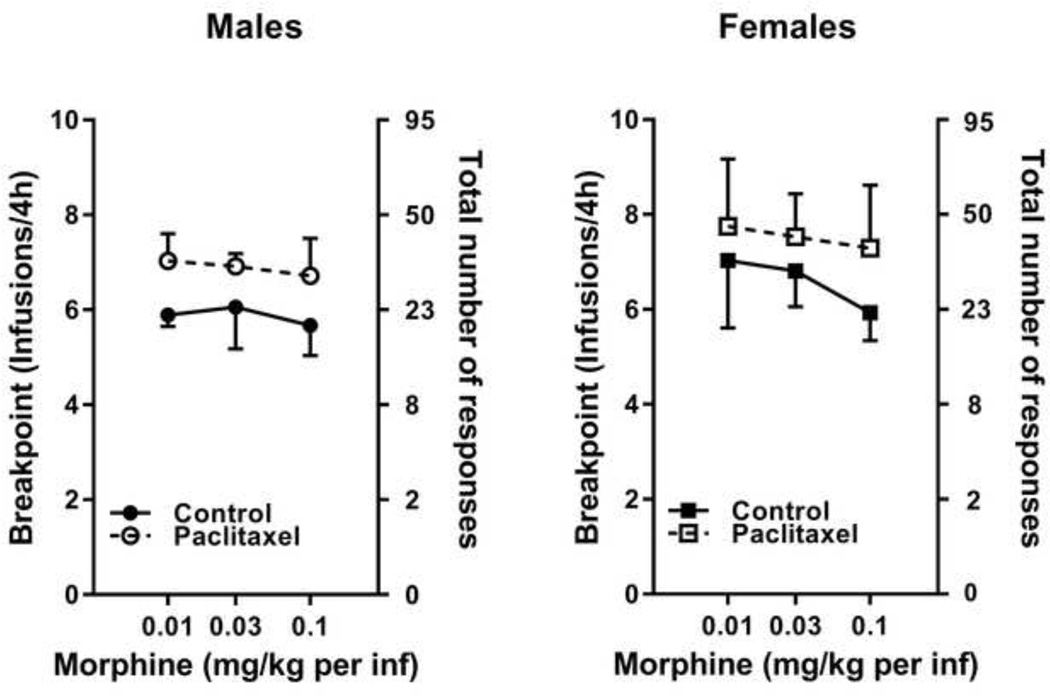
Although paclitaxel treatment produced slightly higher number of total cumulative responses and breakpoints relative to saline treatment for all doses of morphine tested in both male (Figure 4A) and female (Figure 4B) mice, these small differences between treatments were not statistically significant [F(7,70)=1.01, p=0.43 N.S.] as determined by a three-way ANOVA of treatment, sex, or morphine dose. In addition, no differences between sex or morphine dose were observed in the analysis.
Figure 4.
Paclitaxel treatment did not alter morphine dose-response curves under a PR schedule of responding in either male (panel A) or female (panel B) mice. Abscissa: Unit doses of morphine expressed as mg/kg per infusion. Left ordinate: Breakpoint (defined as the number of infusions per session); Right ordinate: Corresponding total cumulative number of responses required in the PR schedule. Each data point represents mean number of infusions achieved in the 4 h session ± SEM (n=6–15/group).
Anti-Allodynia Effects of Morphine
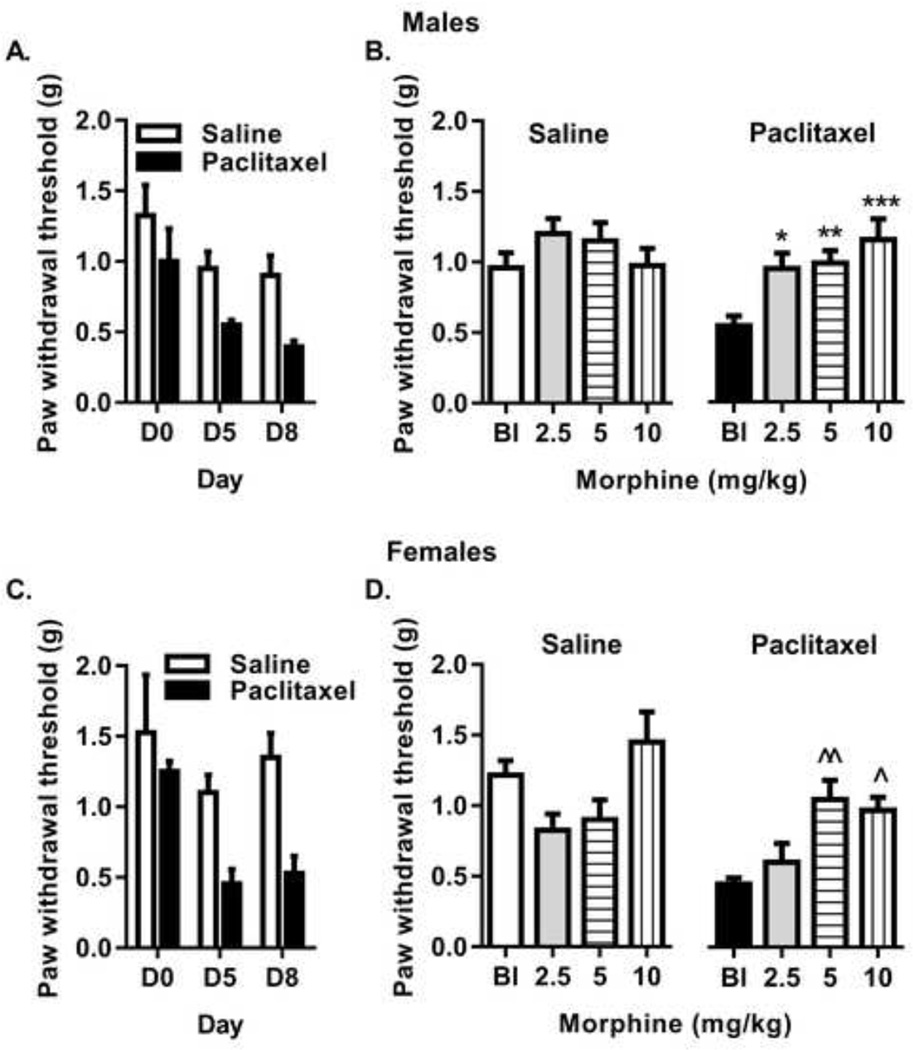
The effects of morphine to reverse paclitaxel-induced mechanical allodynia were assessed in male and female mice after the paclitaxel treatment and similar morphine dosing regimens as were used in the self-administration and CPP experiments. For development of allodynia after treatment with 8 mg/kg paclitaxel (Figure 6, panels A and C), three-way ANOVA of treatment, sex, and number of days was significant [F(7,95) = 6.60, p<0.0001] with Dunnett’s post hoc test indicating number of days (p<0.0009) and treatment (p<0.00001) as significant factors. Specifically, treatment with 8 mg/kg paclitaxel produced measurable allodynia by days 5 and 8 in both male and female mice. No sex differences or interactions were observed. For the potency of morphine to reverse allodynia after paclitaxel treatment (Figure 6, panels B and D), three-way ANOVA of treatment, dose, and sex was significant [F(7,121) = 6.10, p<0.0001] with Dunnett’s post hoc test indicating morphine dose (p<0.0002) and treatment (p<0.00004) as significant factors and treatment by dose as a significant interaction (p<0.02). No sex differences or other interactions were observed. Separate repeated-measures, two-way ANOVAs of treatment and morphine dose were significant in both male [F(3,42) = 3.88, p<0.02] and female [F(3,42) = 4.43, p<0.01] mice. Morphine did not alter the sensitivity to mechanical pressure in the saline-treated male mice but Dunnett’s post hoc tests revealed morphine doses of 2.5 mg/kg (p<0.03), 5 mg/kg (p<0.004), and 10 mg/kg (p<0.0001) significantly decreased mechanical allodynia relative to baseline allodynia measures in the paclitaxel-treated male mice (Figure 6B). In female mice, morphine did not alter sensitivity measures in the saline-treated mice but morphine significantly decreased mechanical allodynia at doses of 5 mg/kg (p<0.006) and 10 mg/kg (p<0.02) doses relative to baseline in the paclitaxel-treated group (Figure 6D).
DISCUSSION
The assessment of reward-related behaviors associated with morphine using two different models with high predictive validity and the evaluation of these behavioral effects in both male and female mice described in the current study are unique to the best of our knowledge. The current findings reveal that a regimen of paclitaxel sufficient to produce morphine reversible mechanical allodynia with no observable sex differences in mice, did not significantly alter the conditioned rewarding effects or the relative reinforcing efficacy of morphine tested within a limited dose range in either male or female mice.
The conditioned rewarding effects of morphine were assessed in the presence and absence of paclitaxel-induced mechanical allodynia in male and female mice using the CPP procedure. Sensitivities to morphine-induced reward in the control saline-treated male and female groups are consistent with previous studies using similar doses in mice (Belzung & Barreau, 2000; Mirbaha, Tabaeizadeh, Shaterian-Mohammadi, Tahsili-Fahadan, & Dehpour, 2009; Ozaki, Narita, Ozaki, Khotib, & Suzuki, 2004; Sahraei et al., 2004). In the CPP experiments, paclitaxel-induced allodynia failed to alter significantly the effects of morphine relative to saline-treated control male and female mice. Our observation that the susceptibility to morphine’s conditioned rewarding effects remains unaltered as a function of paclitaxel treatment is consistent with a recent report in male rats where the conditioned rewarding effects of opioids such as 4 mg/kg morphine, 0.056 mg/kg oxycodone, and 0.017 mg/kg fentanyl, administered subcutaneously, were not altered in the presence of paclitaxel- and oxaliplatin-induced treatment (Mori, et al., 2014). However, our results are in contrast to the reports indicating either an increase (Cahill CM, 2013; Sufka, 1994) or a decrease (Suzuki, Kishimoto, & Misawa, 1996) (Niikura, Kobayashi, et al., 2008; Niikura, Narita, et al., 2008; Ozaki, et al., 2003; Ozaki, et al., 2002; Ozaki, et al., 2004) in opioid reward using alternative chronic pain models such as persistent inflammatory pain and neuropathic pain from nerve injury in male rats and mice.
The discrepancies between these studies may be due to the different underlying mechanisms of the chronic pain models used. Chemotherapeutic agents such as paclitaxel produce modest degeneration and toxicity of peripheral afferent neurons and spinal microglial hypertrophies compared to those evoked by surgical nerve injuries in animals (Xiao & Bennett, 2008; Zheng, Xiao, & Bennett, 2011). Dissociable molecular changes across pain models appear to be consistent with behavioral manifestations, including a more severe reduction in withdrawal thresholds to mechanical stimulus (80% increase in sensitivity to mechanical stimulus – mechanical allodynia) on the ipsilateral paw in mice with nerve injury (Wade CL, 2013) as opposed to our previous observations in mice treated with paclitaxel at the current dosing regimen (50–65% bilateral decrease in the mechanical threshold sensitivity) (Ward SJ, 2014). The supraspinal pathophysiological alterations produced as a result of paclitaxel exposure to higher brain structures are largely unknown. Therefore, a plausible explanation for the lack of effects of paclitaxel on morphine CPP in the current study is that the presence of paclitaxel-induced mechanical allodynia is less likely to modulate key neural substrates such as the VTA and nucleus accumbens that directly regulate opioid reward (Wise, 1989; Leone P, 1991). In comparison, the presence of persistent inflammatory pain can up-regulate kappa-opioid receptors in the nucleus accumbens of the central reward circuitry (Narita et al., 2005; Suzuki, Kishimoto, Misawa, Nagase, & Takeda, 1999) and the presence of peripheral nerve injury via surgical manipulation can down regulate and reduce the functioning of the µ opioid receptors in the VTA thereby decreasing opioid-induced reward (Narita, et al., 2004; Niikura, Narita, et al., 2008; Ozaki, et al., 2003).
Paclitaxel treatment failed to significantly alter morphine CPP data in the current study, yet dose-response analyses for morphine CPP in male paclitaxel-treated mice revealed a relatively flat dose-response curve compared to the saline-treated male mice or any of the female mice. The doses of morphine that induced CPP in the paclitaxel-treated mice were likely lower (~males – 8-fold; females – 17-fold) than those doses that were shown to effectively reverse paclitaxel-induced mechanical allodynia in male and female mice. Our observation suggests that the conditioned rewarding effects of morphine may occur at doses below those required to reverse mechanical allodynia and that morphine may alter the two distinct behavioral endpoints (allodynia vs. CPP) via different mechanisms similar to previous observations using other animal models of pain (Airavaara et al., 2012; Johansen, Fields, & Manning, 2001; Speed et al., 2011) (Qu, et al., 2011; Zhang, et al., 2013). For example, 10-fold increases in the rewarding potency relative to the anti-nociceptive and anti-allodynic effects of morphine were demonstrated in rats in the presence of chronic inflammatory (van der Kam EL, 2008; Hummel M, 2008) and neuropathic pain (Rutten K, 2011). However, as there were no overall paclitaxel treatment effects on the conditioned rewarding effects of morphine in either sex in the current study, full potency estimates or conclusions cannot yet be made until additional lower doses of morphine are tested in both the CPP and allodynia behavioral tests.
The use of drug self-administration models to assess opioid reinforcing behaviors in the context of a developing chronic pain state in rats has been examined previously (Colpaert, Meert, De Witte, & Schmitt, 1982) and supports the notion that the presence of untreated or undertreated chronic pain primarily maintains opioid self-administration behavior via the pain-alleviating effects of different opioids, i.e., negative reinforcement (Colpaert et al., 2001; Lyness, et al., 1989; Martin, Kim, Buechler, Porreca, & Eisenach, 2007). Whether or not the positive reinforcing effects are altered as a function of pain are difficult to examine in these studies by comparing rates of responding using an FR schedule of reinforcement. The PR schedule of reinforcement provides a reliable model to predict the relative reinforcing efficacy of drugs of abuse (Markou et al., 1993; Richardson & Roberts, 1996) and has not been employed previously to assess the reinforcing effects of opioids as a function of pain.
In the present study, male and female C57Bl/6 mice exhibited comparable morphine self-administration behaviors with no sex differences noted in the rate of acquisition under the FR1 schedule. The FR schedule dose-response curve for morphine revealed maximal response rates at the 0.1 mg/kg per infusion dose in male mice and 0.03 mg/kg per infusion dose in female mice. These findings in our male mice are similar to dose-response functions reported in previous studies in mice (Elmer, Pieper, Hamilton, & Wise, 2010; Elmer, et al., 2002). The observed peak response rates for our female mice are in agreement with the limited number of studies in female rats under similar dose conditions (Maisonneuve IM, 1999). Surprisingly, morphine intravenous self-administration in female mice has not been established prior to this study. While previous studies found female rats to acquire intravenous opioid self-administration faster than males (morphine and heroin) (Carroll, Campbell, & Heideman, 2001; Lynch & Carroll, 1999; Cicero TJ, 2003) and infuse greater amounts of opioids intravenously (Carroll et al., 2002; Cicero, Aylward, & Meyer, 2003), the female mice in the current study did not acquire self-administration faster or infuse more morphine than the male mice under the FR1 schedule. These observations suggest that the magnitude or existence of the sex differences in morphine self-administration may be dissimilar across species (Forgie, Beyerstein, & Alexander, 1988; Fattore L1, 2009). Additional studies are needed to resolve these apparent discrepancies.
After the FR portion of the study, male and female mice were switched to a PR schedule of reinforcement and maintained on morphine to stabilize their responding and provide a baseline prior to starting paclitaxel administration. Significant main effects of treatment and treatment by sex interaction suggest that progressive ratio responding for 0.1 mg/kg per infusion morphine was lower in the saline-treated male mice while progressive ratio responding remained at the higher baseline levels after initiation of paclitaxel treatment in the male mice. Paclitaxel treatment therefore prevented the reduction in morphine PR responding observed in the saline-treated male mice for 12 days. No differences in PR responding for the dose of 0.03 mg/kg per infusion morphine were observed between the female mice treated with saline or paclitaxel. Tests of additional doses of morphine after day 12 revealed that paclitaxel treatment did not have an overall effect on the reinforcing efficacy of morphine under the PR schedule of reinforcement. The interpretation of our results under the PR schedule is challenging due to the shallow dose-response curves we obtained for morphine similar to those often observed for opioids under similar PR schedules – [see discussion (Richardson & Roberts, 1996) (Arnold & Roberts, 1997). Future studies employing modified PR schedules where the response increments are more gradual and sensitive to support monotonically increasing dose-dependent response curves for opioids (Grasing K, 2003; Roberts & Bennett, 1993) are needed to dissociate dose-specific positive reinforcing effects and motivational salience of opioids in the context of chronic pain induced by chemotherapy.
Previous studies demonstrated that the presence of chronic pain maintained intravenous self-administration of prescription opioids using an FR schedule of reinforcement in rats in direct relationship with the effectiveness of these compounds to reverse mechanical hypersensitivity (Martin, et al., 2007). Other studies demonstrated a decrease in the positive reinforcing effects of opioids under the FR schedule of reinforcement in rats with chronic pain (Lyness, et al., 1989; Woller et al., 2012), including chronic pain after chemotherapy treatment (Wade CL, 2013). The main discordance among the studies is the use of FR versus PR schedules of reinforcement which can be differentially sensitive to experimental manipulations (Richardson & Roberts, 1996; (Coen KM, 2009), and perhaps mediated by discrete underlying processes and neural systems (McGregor & Roberts, 1993; McGregor & Roberts, 1995) as well as differential pain models with varying underlying mechanisms. Taken together with previous findings in the literature, the most likely explanation for the current pattern of overall similar morphine-seeking behavior in the presence or absence of paclitaxel-induced mechanical allodynia in our mice is that the paclitaxel dosing regimen may produce only a modest negative subjective state and/or sensory component (e.g. allodynia), and perhaps the male mice may be slightly more sensitive to some aspects of the development of allodynia relative to female mice. Only one statistically significant treatment effect and one sex by treatment effect were observed suggesting that if they exist, sex differences were too small to be detected with the sample size used in the current study.
Figure 5.
Mechanical allodynia develops after 5 days of treatment with 8 mg/kg paclitaxel treatment (panels A, C) and is reversed by morphine (2.5–10 mg/kg) (panels B, D) in only the paclitaxel-treated male (upper panels) and female (lower panels) mice. Abscissa: Time points prior to (D0) and after initiation of paclitaxel treatment on days 5 and 8) (panels A and C); baseline (Bl) and morphine dose expressed as mg/kg (panels B and D) on days 11–27. Ordinate: Threshold of sensitivity to the mechanical stimulus (g). Bars represent the mean ± SEM for paw withdrawal thresholds in g; n=8 per group. Significantly different than baseline: *, p<0.03; **, p<0.004; ***, p<0.0001; ^, p<0.02; ^^, p<0.006.
Acknowledgments
This research was supported by R01 CA129092 and the Peter F. McManus Charitable Trust to Ellen Walker. These funding sources had no other role other than financial support.
All authors contributed in a significant way to the manuscript. Harshini Neelakantan was responsible for study design, conduct of experiments, data analysis and interpretation, and manuscript preparation. Sara Jane Ward participated in study design, data interpretation and manuscript preparation. Ellen Walker was responsible for study design, participated in data analysis and interpretation, and manuscript preparation, and provided the funding for this project. All authors have read and approved the final manuscript.
We thank Dr. Sandra Comer for important comments related to the discussion of human laboratory studies cited in the current manuscript.
Footnotes
Portions of these data were presented at the Mid-Atlantic Pharmacology Society 2013 and the College on Problems of Drug Dependence 2014 conferences and appeared in published abstracts in The Pharmacologist (Volume 55(4), 2013) and online (http://www.cpdd.org/Pages/Meetings/CPDD14AbstractBook.pdf), respectively.
Disclosures
The authors declare no conflict of interest.
Contributor Information
Harshini Neelakantan, Department of Pharmaceutical Sciences, Temple University.
Sara Jane Ward, Center for Substance Abuse Research, Temple University.
Ellen Ann Walker, Department of Pharmaceutical Sciences & Center for Substance Abuse Research, Temple University.
REFERENCES
- Airavaara M, Harvey BK, Voutilainen MH, Shen H, Chou J, Lindholm P, Wang Y. CDNF protects the nigrostriatal dopamine system and promotes recovery after MPTP treatment in mice. Cell Transplantation. 2012;21(6):1213–1223. doi: 10.3727/096368911X600948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier N, Stewart J. The role of dopamine in the nucleus accumbens in analgesia. Life Sciences. 1999;65(22):2269–2287. doi: 10.1016/s0024-3205(99)00298-2. [DOI] [PubMed] [Google Scholar]
- Arnold J, Roberts D. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacology, Biochemistry & Behavior. 1997;57(3):441–7. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Back SE, Lawson KM, Singleton LM, Brady KT. Characteristics and correlates of men and women with prescription opioid dependence. Addictive Behaviors. 2011;36(8):829–834. doi: 10.1016/j.addbeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, Apkarian AV. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. The Journal of Neuroscience. 2013;33(41):16383–16393. doi: 10.1523/JNEUROSCI.1731-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129(3):235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Bell K, Salmon A. Pain, physical dependence and pseudoaddiction: redefining addiction for ‘nice’ people? The International Journal on Drug Policy. 2009;20(2):170–178. doi: 10.1016/j.drugpo.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Belzung C, Barreau S. Differences in drug-induced place conditioning between BALB/c and C57Bl/6 mice. Pharmacology, Biochemistry, and Behavior. 2000;65(3):419–423. doi: 10.1016/s0091-3057(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesthesia and Analgesia. 2007;105(1):205–221. doi: 10.1213/01.ane.0000268145.52345.55. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Xue L, Grenier P, Magnussen C, Lecour S, Olmstead MC. Changes in morphine reward in a model of neuropathic pain. Behavioral Pharmacology. 2013;24(3):207–213. doi: 10.1097/FBP.0b013e3283618ac8. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology (Berl) 1999;147(1):22–24. doi: 10.1007/s002130051134. [DOI] [PubMed] [Google Scholar]
- Carroll M, Campbell U, Heideman P. Ketoconazole suppresses food restriction-induced increases in heroin self-administration in rats: sex differences. Experimental and Clinical Psychopharmacology. 2001;9(3):307–316. doi: 10.1037//1064-1297.9.3.307. [DOI] [PubMed] [Google Scholar]
- Chapman CR. Opioid pharmacotherapy for chronic noncancer pain: the american experience. The Korean Journal of Pain. 2013;26(1):3–13. doi: 10.3344/kjp.2013.26.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacology, Biochemistry, and Behavior. 2003;74(3):541–549. doi: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- Coen KM, Adamson KL, Corrigall WA. Medication-related pharmacological manipulations of nicotine self-administration in the rat maintained on fixed- and progressive-ratio schedules of reinforcement. Psychopharmacology (Berl) 2009;201(4):557–568. doi: 10.1007/s00213-008-1321-6. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Meert T, De Witte P, Schmitt P. Further evidence validating adjuvant arthritis as an experimental model of chronic pain in the rat. Life Sciences. 1982;31(1):67–75. doi: 10.1016/0024-3205(82)90402-7. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W. Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain. 2001;91(1–2):33–45. doi: 10.1016/s0304-3959(00)00413-9. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Kowalczyk WJ, Houser J. Abuse liability of oxycodone as a function of pain and drug use history. Drug and Alcohol Dependence. 2010;109(1–3):130–138. doi: 10.1016/j.drugalcdep.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Abuse of prescription drugs and the risk of addiction. Drug and Alcohol Dependence. 2006;(83 Suppl 1):S4–S7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Edlund MJ, Martin BC, Russo JE, Devries A, Braden JB, Sullivan MD. The Role of Opioid Prescription in Incident Opioid Abuse and Dependence Among Individuals with Chronic Non-cancer Pain: The Role of Opioid Prescription. The Clinical Journal of Pain. 2013;29(8):689–95. doi: 10.1097/AJP.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer G, Pieper J, Hamilton L, Wise R. Qualitative differences between C57BL/6J and DBA/2J mice in morphine potentiation of brain stimulation reward and intravenous self-administration. Psychopharmacology. 2010;208(2):309–321. doi: 10.1007/s00213-009-1732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer G, Pieper J, Rubinstein M, Low M, Grandy D, Wise R. Failure of intravenous morphine to serve as an effective instrumental reinforcer in dopamine D2 receptor knock-out mice. Journal of Neuroscience. 2002;22(10):RC224. doi: 10.1523/JNEUROSCI.22-10-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ. Opioid facilitation of rewarding electrical brain stimulation is suppressed in rats with neuropathic pain. Anesthesiology. 2011;114(3):624–632. doi: 10.1097/ALN.0b013e31820a4edb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology. 2009;34(1):S227–S236. doi: 10.1016/j.psyneuen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Medicine. 2008;9(4):444–459. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Rosomoff HL, Rosomoff RS. Drug abuse, dependence, and addiction in chronic pain patients. The Clinical Journal of Pain. 1992;8(2):77–85. doi: 10.1097/00002508-199206000-00003. [DOI] [PubMed] [Google Scholar]
- Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122(3):245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgie M, Beyerstein B, Alexander B. Contributions of taste factors and gender to opioid preference in C57BL and DBA mice. Psychopharmacology. 1988;95(2):237–44. doi: 10.1007/BF00174516. [DOI] [PubMed] [Google Scholar]
- Gil KM, Ginsberg B, Muir M, Sykes D, Williams DA. Patient-controlled analgesia in postoperative pain: the relation of psychological factors to pain and analgesic use. The Clinical Journal of Pain. 1990;6(2):137–142. doi: 10.1097/00002508-199006000-00012. [DOI] [PubMed] [Google Scholar]
- Goncalves L, Silva R, Pinto-Ribeiro F, Pego JM, Bessa JM, Pertovaara A, Almeida A. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Experimental Neurology. 2008;213(1):48–56. doi: 10.1016/j.expneurol.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Grasing K, Li N, He S, Parrish C, Delich J, Glowa J. A new progressive ratio schedule for support of morphine self-administration in opiate dependent rats. Psychopharmacology (Berl) 2003;168(4):387–396. doi: 10.1007/s00213-003-1442-x. [DOI] [PubMed] [Google Scholar]
- Graves DA, Arrigo JM, Foster TS, Baumann TJ, Batenhorst RL. Relationship between plasma morphine concentrations and pharmacologic effects in postoperative patients using patient-controlled analgesia. Clinical Pharmacy. 1985;4(1):41–47. [PubMed] [Google Scholar]
- Hojsted J, Sjogren P. Addiction to opioids in chronic pain patients: a literature review. European Journal of Pain. 2007;11(5):490–518. doi: 10.1016/j.ejpain.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Hummel M, Lu P, Cummons TA, Whiteside GT. The persistence of a long-term negative affective state following the induction of either acute or chronic pain. Pain. 2008;140(3):436–445. doi: 10.1016/j.pain.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Kupers R, Gybels J. The consumption of fentanyl is increased in rats with nociceptive but not with neuropathic pain. Pain. 1995;60(2):137–141. doi: 10.1016/0304-3959(94)00106-O. [DOI] [PubMed] [Google Scholar]
- Kwon JH. Predictors of long term opioid treatment among patients that receive chemoradiation for head and neck cancer. The Oncologist. 2013;18:768–774. doi: 10.1634/theoncologist.2013-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison RN, Butler SF, Budman SH, Edwards RR, Wasan AD. Gender differences in risk factors for aberrant prescription opioid use. The Journal of Pain. 2010;11(4):312–320. doi: 10.1016/j.jpain.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proceedings of the National Academy of Sciences. 2001;98(14):8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone P, Pocock D, Wise RA. Morphine-dopamine interaction: ventral tegmental morphine increases nucleus accumbens dopamine release. Pharmacology Biochemisrty and Behavior. 1991;39(2):469–472. doi: 10.1016/0091-3057(91)90210-s. [DOI] [PubMed] [Google Scholar]
- Lofwall MR, Nuzzo PA, Walsh SL. Effects of cold pressor pain on the abuse liability of intranasal oxycodone in male and female prescription opioid abusers. Drug and Alcohol Dependence. 2012;123(1–3):229–238. doi: 10.1016/j.drugalcdep.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch W, Carroll M. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lyness WH, Smith FL, Heavner JE, Iacono CU, Garvin RD. Morphine self-administration in the rat during adjuvant-induced arthritis. Life Sciences. 1989;45(23):2217–2224. doi: 10.1016/0024-3205(89)90062-3. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Giordano J, Boswell MV, Fellows B, Manchukonda R, Pampati V. Psychological factors as predictors of opioid abuse and illicit drug use in chronic pain patients. Journal of Opioid Management. 2007;3(2):89–100. doi: 10.5055/jom.2007.0045. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick S. Attenuation of the reinforcing efficacy of morphine by 18-methoxycoronaridine. European Journal of Pharmacology. 1999;383(1):15–21. doi: 10.1016/s0014-2999(99)00560-9. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112(2–3):163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Opioid self-administration in the nerve-injured rat: relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology. 2007;106(2):312–322. doi: 10.1097/00000542-200702000-00020. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Devito EE, Dodd D, Carroll KM, Potter JS, Greenfield SF, Weiss RD. Gender differences in a clinical trial for prescription opioid dependence. Journal of Substance Abuse Treatment. 2013;45(1):38–43. doi: 10.1016/j.jsat.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Roberts D. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Research. 1993;624(1–2):245–252. doi: 10.1016/0006-8993(93)90084-z. [DOI] [PubMed] [Google Scholar]
- McGregor A, Roberts D. Effect of medial prefrontal cortex injections of SCH 23390 on intravenous cocaine self-administration under both a fixed and progressive ratio schedule of reinforcement. Behavioral Brain Research. 1995;67(1):75–80. doi: 10.1016/0166-4328(94)00106-p. [DOI] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129(Pt 5):1330–1338. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- Mori T, Kanbara T, Harumiya M, Iwase Y, Masumoto A, Komiya S, Suzuki T. Establishment of opioid-induced rewarding effects under oxaliplatin- and Paclitaxel-induced neuropathy in rats. Journal of Pharmacological Science. 2014;126(1):47–55. doi: 10.1254/jphs.14134fp. [DOI] [PubMed] [Google Scholar]
- Mirbaha H, Tabaeizadeh M, Shaterian-Mohammadi H, Tahsili-Fahadan P, Dehpour AR. Estrogen pretreatment modulates morphine-induced conditioned place preference in ovariectomized mice. Pharmacology, Biochemistry, and Behavior. 2009;92(3):399–403. doi: 10.1016/j.pbb.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Nakata T, Yorifuji H. Morphological evidence of the inhibitory effect of taxol on the fast axonal transport. Neuroscience Research. 1999;35(2):113–122. doi: 10.1016/s0168-0102(99)00074-7. [DOI] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31(4):739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Narita M, Kishimoto Y, Ise Y, Yajima Y, Misawa K, Suzuki T. Direct evidence for the involvement of the mesolimbic kappa-opioid system in the morphine-induced rewarding effect under an inflammatory pain-like state. Neuropsychopharmacology. 2005;30(1):111–118. doi: 10.1038/sj.npp.1300527. [DOI] [PubMed] [Google Scholar]
- Narita M, Ozaki S, Ise Y, Yajima Y, Suzuki T. Change in the expression of c-fos in the rat brain following sciatic nerve ligation. Neuroscience Letters. 2003;352(3):231–233. doi: 10.1016/j.neulet.2003.08.052. [DOI] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Imai S, Ozaki S, Kishimoto Y, Oe K, Suzuki T. Molecular mechanism of changes in the morphine-induced pharmacological actions under chronic pain-like state: suppression of dopaminergic transmission in the brain. Life Sciences. 2004;74(21):2655–2673. doi: 10.1016/j.lfs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. The Neuroscientist. 2004;10(3):221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Niikura K, Kobayashi Y, Okutsu D, Furuya M, Kawano K, Maitani Y, Narita M. Implication of spinal protein kinase Cgamma isoform in activation of the mouse brain by intrathecal injection of the protein kinase C activator phorbol 12,13-dibutyrate using functional magnetic resonance imaging analysis. Neuroscience Letters. 2008;433(1):6–10. doi: 10.1016/j.neulet.2007.12.049. [DOI] [PubMed] [Google Scholar]
- Niikura K, Narita M, Butelman ER, Kreek MJ, Suzuki T. Neuropathic and chronic pain stimuli downregulate central mu-opioid and dopaminergic transmission. Trends in Pharmacological Sciences. 2010;31(7):299–305. doi: 10.1016/j.tips.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Niikura K, Narita M, Okutsu D, Tsurukawa Y, Nanjo K, Kurahashi K, Suzuki T. Implication of endogenous beta-endorphin in the inhibition of the morphine- induced rewarding effect by the direct activation of spinal protein kinase C in mice. Neuroscience Letters. 2008;433(1):54–58. doi: 10.1016/j.neulet.2007.12.042. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Iino M, Miyoshi K, Suzuki T. Suppression of the morphine-induced rewarding effect and G-protein activation in the lower midbrain following nerve injury in the mouse: involvement of G-protein-coupled receptor kinase 2. Neuroscience. 2003;116(1):89–97. doi: 10.1016/s0306-4522(02)00699-1. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Iino M, Sugita J, Matsumura Y, Suzuki T. Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: implication of the reduction in mu-opioid receptor functions in the ventral tegmental area. Journal of Neurochemistry. 2002;82(5):1192–1198. doi: 10.1046/j.1471-4159.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Ozaki M, Khotib J, Suzuki T. Role of extracellular signal-regulated kinase in the ventral tegmental area in the suppression of the morphine-induced rewarding effect in mice with sciatic nerve ligation. Journal of Neurochemistry. 2004;88(6):1389–1397. doi: 10.1046/j.1471-4159.2003.02272.x. [DOI] [PubMed] [Google Scholar]
- Parker RK, Holtmann B, White PF. Patient-controlled analgesia. Does a concurrent opioid infusion improve pain management after surgery? Journal of the American Medical Association. 1991;266(14):1947–1952. doi: 10.1001/jama.266.14.1947. [DOI] [PubMed] [Google Scholar]
- Pergolizzi J, Zampogna G, Taylor R, Gonima E, Posada J, Raffa R. A guide for pain management in low and middle income communities. Managing the risk of opioid abuse in patients with cancer pain. Frontiers in Pharmacology. 2016;7:42. doi: 10.3389/fphar.2016.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persohn E, Canta A, Schoepfer S, Traebert M, Mueller L, Gilardini A, Cavaletti G. Morphological and morphometric analysis of paclitaxel and docetaxel-induced peripheral neuropathy in rats. European Journal of Cancer. 2005;41(10):1460–1466. doi: 10.1016/j.ejca.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Qu C, King T, Okun A, Lai J, Fields H, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152(7):1641–8. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Gibby C, Anderson K, Todd K. Risk for opioid misuse among emergency department cancer patients. Academic Emergency Medicine. 2016;23(2):151–8. doi: 10.1111/acem.12861. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. [Review] Journal of Neuroscience Methods. 1996;66(1):1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts D, Bennett S. Heroin self-administration in rats under a progressive ratio schedule of reinforcement. Psychopharmacology. 1993;111(2):215–218. doi: 10.1007/BF02245526. [DOI] [PubMed] [Google Scholar]
- Rutten K, van der Kam EL, De Vry J, Tzschentke TM. Dissociation of rewarding, anti-aversive and anti-nociceptive effects of different classes of anti-nociceptives in the rat. European Journal of Pain. 2011;15(3):299–305. doi: 10.1016/j.ejpain.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Sahraei H, Ghazzaghi H, Zarrindast MR, Ghoshooni H, Sepehri H, Haeri-Rohan A. The role of alpha-adrenoceptor mechanism(s) in morphine-induced conditioned place preference in female mice. Pharmacology, Biochemistry, and Behavior. 2004;78(1):135–141. doi: 10.1016/j.pbb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Stein C, Huber A, Millan MJ, Herz A. Motivational effects of opioids in an animal model of prolonged inflammatory pain: alteration in the effects of kappa- but not of mu-receptor agonists. Pain. 1988;35(2):179–86. doi: 10.1016/0304-3959(88)90225-4. [DOI] [PubMed] [Google Scholar]
- Sidebotham D, Dijkhuizen MR, Schug SA. The safety and utilization of patient-controlled analgesia. Journal of Pain and Symptom Management. 1997;14(4):202–209. doi: 10.1016/s0885-3924(97)00182-6. [DOI] [PubMed] [Google Scholar]
- Speed N, Saunders C, Davis AR, Owens WA, Matthies HJ, Saadat S, Galli A. Impaired striatal Akt signaling disrupts dopamine homeostasis and increases feeding. PLoS ONE. 2011;6(9):e25169. doi: 10.1371/journal.pone.0025169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufka K. Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain. 1994:355–366. doi: 10.1016/0304-3959(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kishimoto Y, Misawa M. Formalin- and carrageenan-induced inflammation attenuates place preferences produced by morphine, methamphetamine and cocaine. Life Sciences. 1996;59(19):1667–1674. doi: 10.1016/0024-3205(96)00498-5. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kishimoto Y, Misawa M, Nagase H, Takeda F. Role of the kappa-opioid system in the attenuation of the morphine-induced place preference under chronic pain. Life Sciences. 1999;64(1):PL1–PL7. doi: 10.1016/s0024-3205(98)00537-2. [DOI] [PubMed] [Google Scholar]
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. The Clinical Journal of Pain. 2008;24(6):497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- van der Kam EL, Vry JD, Schiene K, Tzschentke TM. Differential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the rat. Pain. 2008;136(3):373–379. doi: 10.1016/j.pain.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Wade CL, Krumenacher P, Kitto KF, Peterson CD, Wilcox GL, Fairbanks CA. Effect of chronic pain on fentanyl self-administration in mice. PLoS ONE. 2013;8(11):e79239. doi: 10.1371/journal.pone.0079239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, McAllister SD, Neelakantan H, Walker EA. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT1A receptors without diminishing nervous system function or chemotherapy efficacy. British Journal of Pharmacology. 2013;171(3):636–45. doi: 10.1111/bph.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Ramirez MD, Neelakantan H, Walker EA. Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesthesia and Analgesia. 2011;113(4):947–950. doi: 10.1213/ANE.0b013e3182283486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. Opiate reward: sites and substrates. Neuroscience Biobehavioral Reviews. 1989;13(2–3):129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Woller SA, Moreno GL, Hart N, Wellman PJ, Grau JW, Hook MA. Analgesia or addiction?: implications for morphine use after spinal cord injury. Journal of Neurotrauma. 2012;29(8):1650–1662. doi: 10.1089/neu.2011.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: Abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-L-carnitine. Pain. 2008;135(3):262–270. doi: 10.1016/j.pain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, McKay MA, Toledano AY, Marks S, Young CJ, Klock PA, Apfelbaum JL. The effects of a cold-water immersion stressor on the reinforcing and subjective effects of fentanyl in healthy volunteers. Drug and Alcohol Dependence. 1996;42(2):133–142. doi: 10.1016/0376-8716(96)01274-4. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang M, Li A, Pan L, Berman B, Ren K, Lao L. DAMGO in the central amygdala alleviates the affective dimension of pain in a rat model of inflammatory hyperalgesia. Neuroscience. 2013;252:359–66. doi: 10.1016/j.neuroscience.2013.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng FY, Xiao WH, Bennett GJ. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience. 2011;176:447–454. doi: 10.1016/j.neuroscience.2010.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]