Abstract
Introduction:
Few studies have evaluated exercise interventions for smokers with depression or other psychiatric comorbidities. This pilot study evaluated the potential role of supervised vigorous exercise as a smoking cessation intervention for depressed females.
Methods:
Thirty adult women with moderate–severe depressive symptoms were enrolled and randomly assigned to 12 weeks of thrice weekly, in person sessions of vigorous intensity supervised exercise at a YMCA setting (EX; n = 15) or health education (HE; n = 15). All participants received behavioral smoking cessation counseling and nicotine patch therapy. Assessments were done in person at baseline, at the end of 12 weeks of treatment, and at 6 months post-target quit date. Primary end points were exercise adherence (proportion of 36 sessions attended) and biochemically confirmed 7-day point prevalence abstinence at Week 12. Biomarkers of inflammation were explored for differences between treatment groups and between women who smoked and those abstinent at Week 12.
Results:
Treatment adherence was high for both groups (72% for EX and 66% for HE; p = .55). The Week 12 smoking abstinence rate was higher for EX than HE (11/15 [73%] vs. 5/15 [33%]; p = .028), but no significant differences emerged at 6-month follow-up. Interleukin-6 levels increased more for those smoking than women abstinent at Week 12 (p = .040).
Conclusions:
Vigorous intensity supervised exercise is feasible and enhances short-term smoking cessation among depressed female smokers. Innovative and cost-effective strategies to bolster long-term exercise adherence and smoking cessation need evaluation in this population. Inflammatory biomarkers could be examined in future research as mediators of treatment efficacy.
Implications:
This preliminary study found that vigorous intensity supervised exercise is feasible and enhances short-term smoking cessation among depressed female smokers. This research addressed an important gap in the field. Despite decades of research examining exercise interventions for smoking cessation, few studies were done among depressed smokers or those with comorbid psychiatric disorders. A novel finding was increases in levels of a pro-inflammatory biomarker observed among women who smoked at the end of the intervention compared to those who did not.
Introduction
In 2014, the prevalence of cigarette smoking among US women was 14.8%.1 Women have lower quit rates than men.2,3 Women are more likely than men to report weight concerns and negative affect as barriers to quitting.4,5 Also, women smokers tend to have higher levels of depression than nonsmokers,6 and smokers with elevated depressive symptoms have poorer smoking treatment outcomes.7–9 Thus, designing effective smoking cessation interventions for women with depression—a tobacco use disparity group—is a public health priority.10,11 Few studies targeted smokers with current depression12–17 and only one of these targeted women specifically.12 This pilot study evaluated the potential role of supervised vigorous exercise as a smoking cessation intervention for depressed females.
A recent Cochrane review of 20 randomized trials concluded there was little evidence that exercise was effective as a smoking cessation intervention.18 These trials were limited by small sample sizes, inadequate control groups, interventions of insufficient exercise intensity, and a lack of support being provided to ensure adherence to the exercise intervention. The only trial to show a long-term effect on smoking abstinence targeted women using 12 weeks of thrice weekly (ie, 36 sessions) supervised, facility-based, vigorous intensity exercise19; an intervention also effective for treating depression.20 When this treatment was streamlined to only four supervised exercise sessions (vs. 36) and held in a community (YMCA) setting, it was not effective for smoking cessation and there was poor adherence to exercise.21 Moreover, two studies of exercise counseling encouraging home-based, unsupervised exercise for depressed smokers found no effect on smoking abstinence compared with a health education contact control group.12,13
A supervised exercise program has the potential to benefit depressed smokers by providing reinforcement, guidance, and support for exercise; thus improving adherence.18 As an initial step, we examined if the same effective intervention tested among women smokers19 could also be efficacious for female smokers with depression, as individuals with psychiatric disorders including depression are understudied in the exercise for smoking cessation treatment literature.16 In addition to the positive results obtained for women smokers,19 vigorous exercise was used in this pilot study because of the potential benefits of greater intensity exercise22–24 and increased cardiorespiratory fitness25–29 on depressive symptoms. One difference from the Marcus et al.19 study is that the exercise was delivered in a community YMCA setting, instead of a research facility, enhancing the potential reach and dissemination of the intervention.
One limitation of the Marcus et al.19 study was that exercise was tested without a pharmacological adjunct for smoking cessation. To be clinically useful and consistent with best practices for smoking cessation,30 the effects of exercise should be additive with cessation medication. Including a pharmacological adjunct also minimizes the chance that participants would seek out pharmacotherapy on their own (eg, to manage withdrawal symptoms), potentially confounding treatment effects. As depressed smokers are usually excluded from trials, there are limited data on the efficacy of pharmacological adjuncts for this population. Thus, we tested the potential effect of exercise when added to nicotine replacement therapy (NRT), a first-line, evidence-based medication treatment.30 We used the nicotine patch due to its safety and low cost.
An exploratory aim was to examine potential biological impact of treatment response, through assessment of the intervention (exercise), and smoking cessation outcome on biomarkers of inflammation. High levels of distress (a marker of poorly regulated emotion) are associated with deregulated stress hormones and elevated inflammation.31,32 Both depression and tobacco use are associated with increased levels of pro-inflammatory cytokines C-reactive protein, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α).33–36 One study found smokers with depression to have significantly higher levels of these pro-inflammatory cytokines compared with smokers without depression,2 and inflammation has been directly implicated in the pathogenesis of depression illness.37,38 That is, pro-inflammatory processes can directly contribute to development of depressed mood. Regular and more intensive physical activity has been shown to reduce levels of pro-inflammatory cytokines.39 Our exploratory hypothesis was that at the end of treatment, levels of pro-inflammatory cytokines would be lower for exercise versus the control group and that participants smoking would have elevated levels compared with those abstinent.
Methods
The study was approved by the Mayo Clinic Institutional Review Board and registered with clinicaltrials.gov (NCT01860924). Data were collected from September 2013 to April 2015.
Participants
A sample size of 30 was deemed sufficient to determine the intervention’s feasibility with respect to adherence to the exercise treatment protocol.40–42 The study was not powered to detect significant differences in smoking abstinence rates, but we sought to obtain estimates of the intervention effect toward planning a definitive trial. A doubling of the abstinence rate for the intervention versus control condition at end of treatment was considered to be of clinical significance and warrant proceeding to an efficacy trial.43
Participants were recruited by provider referrals and flyers posted in the clinic and radio and newspaper advertisements. Initial screening was completed by telephone. Eligible women were asked to complete an in-person screening assessment. After obtaining written informed consent, the study coordinator administered the Mini-International Neuropsychiatric Interview,44 to rule out bipolar and thought disorders. Participants then completed a urine pregnancy test and provided height and weight.
If eligible, individuals completed a baseline self-report questionnaire and exercise testing.45 Potential participants received the Modular Signal Recorder (MSR)46 accelerometer to wear for 4 days and return in a prepaid envelope. If the MSR was returned, the participant was enrolled.
Eligibility criteria were female, aged 18–55 years, smoked at least 10 cigarettes/day for at least the past year, willing to make a quit attempt, currently depressed defined by a clinical cutoff score of at least 16 on the 10-item Center for Epidemiological Studies Depression Scale corresponding to moderate–severe depression,47 not currently meeting the American College of Sports Medicine (ACSM) guidelines of moderate intensity exercise for at least 30 minutes on at least 5 days/week or vigorous exercise for at least 20 minutes on at least 3 days/week,48 willing and able to participate in all aspects of the study, provide written informed consent, and if using antidepressant medication no changes in dose or type of medication during the past 3 months. Exclusionary criteria were positive pregnancy test (urine dipstick test), currently breastfeeding or planning to become pregnant during the nicotine patch study phase, physical limitations to participate in vigorous intensity exercise,49 current use (past 3 months) of smokeless tobacco products or stop smoking medications/behavioral treatments, any medical condition precluding nicotine patch use, current or lifetime DSM-IV diagnosis of bipolar disorder, schizophrenia/other major thought disorder, and another person from the same household had enrolled. There was no upper cutoff for body mass index (BMI) if no other cardiovascular risk factors were present if BMI was at least 30.
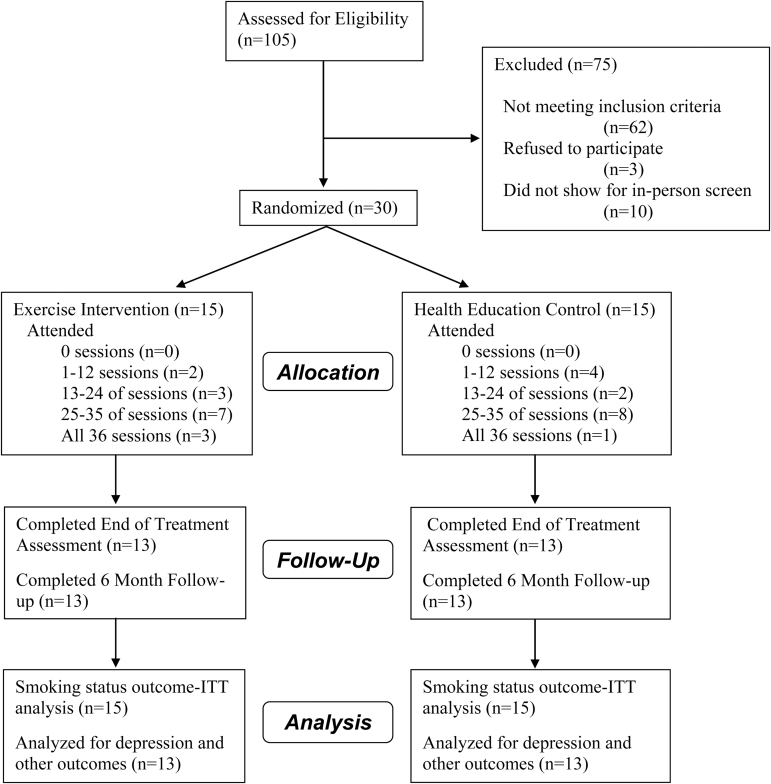
One hundred five individuals were screened, of which 30 (29%) were enrolled (Figure 1). Sixty-two of those not enrolled did not meet the study eligibility criteria, and the primary reason was a Center for Epidemiological Studies Depression Scale score less than 16 (38/62, 61%). Others were medical exclusions (n = 8), smoked infrequently (n = 6), recent change in antidepressant medication (n = 2), age (n = 5), too physically active (n = 1), or distance from exercise facility (n = 2). Women not enrolled were given referral resources.
Figure 1.
Participant flow.
Procedures
We used a randomized, two-group design with assessments completed at baseline, after 12 weeks of treatment, and at 6 months post-target quit date. Participants were stratified according to current depression severity (baseline Patient Health Questionnaire [PHQ-9]50 score: mild/moderate vs. severe) and antidepressant medication use (yes/no) and randomly assigned to the exercise intervention group (EX, n = 15) or to the health education contact control group (HE, n = 15). The conditions were matched for wellness coach contact time and duration of treatment. Allocation to treatment conditions was unknown to the study staff or investigators prior to assignment, and participants completed baseline assessments prior to being informed of their allocation to treatment condition. A study coordinator blinded to allocation group conducted all follow-ups in-person. Participants received $25 for completing the baseline assessment and $50 after completing each follow-up. All participants received a free 6-month YMCA membership (HE participants received this after the final assessment). No incentives were offered for treatment adherence.
For the biomarker analysis, participants were asked for their written informed consent to provide a blood sample during the baseline visit and again at Week 12. A participant’s decision to participate in this aspect of the study did not affect enrollment in the pilot trial. All 30 enrolled women provided consent to participate. They received an additional $25 for providing a blood sample at baseline and $50 for providing the Week 12 sample.
Interventions
For both conditions, the 12-week program comprised three 30–40 minute individual-based sessions per week delivered by wellness coaches.51–53 At one session each week for 12 weeks, participants received 15–20 minutes of smoking cessation counseling delivered by their HE or EX wellness coach.
Smoking Cessation Counseling and Pharmacotherapy
The evidence-based cessation counseling54 was identical for both conditions, except that for HE participants the use of exercise was not discussed as a strategy for managing depression, craving, or withdrawal symptoms. At Weeks 2 and 6, participants were mailed a 4-week supply of nicotine patches. The target quit date was the first session of Week 3. At this visit, participants received instructions on using the patch. Patch dosing consisted of 21mg/24h for 4 weeks, 14mg/24h for 2 weeks, then 7mg/24h for 2 weeks.30 The dosing was tailored and based on cigarette consumption, prior experience with the patch, and degree of dependence. The recommended cutoff was at least 15 cigarettes/day for the 21mg patch and 10–14 cigarettes/day for the 14mg patch.30 Each week during the treatment phase, the coach assessed participants for side effects and adverse events associated with patch therapy, depressive symptoms, and suicidality.
Health Education
Lectures, handouts, films, and discussions covered various women’s health and lifestyle issues, as used in previous trials.12,19 Attendance was recorded and missed appointments were rescheduled.
Exercise Intervention
The intervention was identical on exercise duration and intensity to that of Marcus et al.19 The intervention manual (see Supplementary Material) incorporated language based on a study of consumer preferences for exercise interventions conducted among depressed adults.54 In addition, we incorporated feedback on the treatment protocol obtained from phone interviews with 12 community women smokers who had received treatment for depression.
All EX sessions were held at the YMCA, with the exception of four sessions that were conducted at a worksite fitness center. Participants engaged in exercise during each session and were encouraged to attend the YMCA on other days and/or to exercise at home. Attendance was documented and missed appointments were rescheduled.
Participants exercised on cardiovascular equipment of their choice and received supervision, reinforcement, and counseling from the coach. Sessions comprised of a 5-minute warm up, 20–30 minutes of aerobic activity, and a 5-minute cool down with stretching.19 Exercise was gradually progressed from moderate to vigorous intensity by adding 2–4 minutes of vigorous exercise weekly.48 Participants started out their first week with 3 days of 20 minutes of moderate and 4 minutes of vigorous intensity exercise. By Week 12, participants were to complete 3 days of 30 minutes of vigorous intensity exercise. The wellness coach recorded the number of minutes the participants spent engaging in vigorous and moderate exercise at each session. Target heart rates (using heart rate reserve) were determined from the baseline Max VO2 exercise test.45 Participants were instructed to work at a Rating of Perceived Exertion55 using a scale of 0–10 with 0–2 rest to easy; 3–4 moderate to sort of hard; 5–8 hard to really hard; 9–10 really, really, hard to maximal: just like my hardest race. Moderate intensity corresponded with a score of 3–4 and vigorous intensity corresponded with a score of 5–845; Rating of Perceived Exertion was monitored.
To reduce the time, the coach delivered the exercise counseling while the participant was engaged in exercise.56 The counseling included social cognitive theory–based57 assessment and problem-solving of exercise barriers, reinforcement (shaping) of exercise, and methods to enhance exercise self-efficacy—including guidance on exercise technique, intensity, and positive feedback51,58,59 delivered using a motivational interviewing counseling style.
At the first session, participants were given a Kinetic Activity Monitor60 that was to be worn on the waist to provide feedback and reinforcement for activity and calories spent.61 However, nearly all EX participants chose not to wear or use the device, or lost it, and thus data on Kinetic Activity Monitor use were not collected or analyzed.
Wellness Coaches, Training, and Treatment Fidelity
The interventions were delivered by female ACSM-certified wellness coaches with a master’s degree in clinical psychology (HE) or bachelors’ degree in health education (EX). Coaches received 6 hours of training on the treatment protocols. Coaches used a written treatment manual containing an outline/script and checklist of critical topics to be covered.62 All sessions were audiotaped and reviewed with the coaches during weekly meetings to reinforce treatment fidelity, provide feedback, and conduct additional training. Fifteen percent of the sessions were randomly selected to be checked for the proportion of intended topics that were delivered.63 Coach adherence to the HE and EX manuals was 90% and 95%, respectively, indicating high fidelity.
Biomarker Methodology
Laboratory analysis of inflammatory markers was conducted at the Mayo Clinic Translational Neuroscience Laboratory (Tye). Peripheral blood samples (5mL) were collected from participants at the Clinical Research Unit and centrifuged immediately at 2500rpm for 10 minutes. Serum was stored at −80°C for analysis. Serum levels of pro-inflammatory cytokines IL-6, tumor necrosis factor-alpha, and C-reactive protein serum levels were determined using commercially available enzyme-linked immunosorbent assays in accordance with manufacture instructions (Life Technologies, NY).
Measures
Baseline Characteristics
A baseline questionnaire documented age, race/ethnicity, marital status, and education as well as Fagerström Test for Cigarette Dependence score.64
Feasibility
Data related to participant recruitment were collected, including the number of potential participants screened, and the number excluded for each of the specific inclusion/exclusion criteria. Study retention was based on the proportion of enrolled women completing the 6-month follow-up assessment. Treatment adherence was based on the proportion of 36 sessions completed. In EX, duration of vigorous intensity exercise (of 30 minutes) was recorded at each session and was summarized for the last session participants attended. In addition, self-reported NRT adherence was measured as the proportion of participants compliant with the recommended 8 weeks of patch use.
Treatment Acceptability
At Week 12, participants completed the 10-item validated Consultation and Relational Empathy measure to assess satisfaction with the coach.65 Each item was rated on a five-point scale ranging from poor to excellent (range 10–50). At 6 months, participants were asked if they would recommend the program to depressed women interested in quitting smoking (options: definitely would, probably would, unsure, probably would not, and definitely would not).
Smoking Status
Seven-day point-prevalence, self-reported cigarette smoking status was obtained at Week 12 and at 6-month follow-up.30 A saliva sample was collected at each time point for cotinine analysis.66 We used a semiquantitative, NicAlert salivary cotinine test strips, a valid and reliable method for verifying smoking status.67 Participants are classified as smoking if the result noted is 1–6 (≥10ng/mL cotinine) and abstinent if the reading is a 0 (<10ng/mL cotinine). We assessed use of NRT because use would elevate the cotinine concentrations. At each time point, participants who self-reported no cigarette smoking (not even a puff) in the last 7 days confirmed with a cotinine test strip were classified as nonsmokers.66,68
Cardiorespiratory Fitness
Changes in cardiorespiratory fitness by treatment group served as a manipulation check. At baseline and Week 12, all participants underwent a symptom limited incremental treadmill test using the Bruce protocol.45 Participants were encouraged to continue the exercise protocol to maximal exertion, confirmed by a Rating of Perceived Exertion of at least 17 on the Borg (6–20) scale or a respiratory exchange ratio of at least 1.10. Oxygen consumed (VO2), carbon dioxide produced (VCO2), and minute ventilation (VE) were measured by mouth piece and pneumotachograph (MedGraphics, St. Paul, MN) throughout exercise.69,70 The respiratory exchange ratio was calculated as VCO2/VO2. Manual volume calibration was performed with a 3L syringe, and gas calibration was performed with manufacturer-recommended gases of known concentration. All calibration procedures were accomplished immediately prior to each test. Data were averaged over the last 30 seconds of each stage. Max VO2 was defined as the mean of the last 30 seconds of the exercise test.
Physical Activity
At baseline and the week after the last (Week 12) intervention session participants were asked to wear the MSR (model 145) accelerometer, a miniature universal data logger validated for objective physical activity assessment.46 Participants were asked to wear the device on a belt placed on their lower back during waking hours; 4 days of at least 10 hours of wear was required for a valid assessment. Data were analyzed irrespective of weekend/weekday consideration. Participants returned the device using a postage-paid envelope.
MSR counts of total physical activity and sedentary time were summarized. The accelerometer units (AUs) are designed to capture non-exercise activity thermogenesis or very low level intensity exercise. While AUs still record walking and running activities, these record the movement associated with activities such as cooking or fidgeting in a chair. An average day’s total physical activity is about 10 000 AU.46 Sedentary time was defined as time when movement captured (AUs) was less than the (AUs) walking at 0.5 miles per hour and was reported as average sedentary time in minutes per day.
Body Mass Index
Height and weight were recorded at baseline and at Week 12 using a calibrated scale.
Depressive Symptoms
The PHQ-9 was used to assess depressive symptoms at baseline and at Week 12. This brief self-report measure has been extensively validated50,71 and has excellent test–retest reliability (r = .96) over a 1-week period among samples of untreated patients.72,73
Nonstudy Treatments
At Week 12 and 6-month follow-up, the study coordinator assessed participant use of concomitant stop smoking medication, antidepressant medication, and other depression treatment.
Statistical Methods
To assess the adequacy of the randomization, demographics were compared between treatment groups using the chi-square test for categorical variables or the two-sample t test/rank-sum test for continuous variables. The percentage of enrolled participants who completed the 6-month follow-up assessment (ie, study retention) was compared between treatment conditions using the chi-square test (Fisher’s exact test). The mean number of treatment sessions attended was compared across treatment conditions using a two-sample t test (rank-sum). Indices of treatment acceptability were compared across treatment conditions using the chi-square test for program recommendation and the two-sample t test (rank-sum) for Consultation and Relational Empathy scores. The effect of treatment group on PHQ-9 score, BMI, Max VO2, and MSR total physical activity and average sedentary time at Week 12 was evaluated using analysis of covariance with the baseline score/value as a covariate.
The biochemically confirmed 7-day point prevalence smoking abstinence rate at Week 12 and 6-month follow-up was summarized for each group (point estimate and 95% confidence interval) and compared between treatment groups using a chi-square test (Fisher’s exact test). Using an intent-to-treat approach,74 missing data were classified as smoking. Analyses also controlled for the stratification variables (PHQ-9 depression severity, antidepressant medication use).
A two-way analysis of variance was used to determine the impact of the exercise intervention and participants’ Week 12 smoking status on C-reactive protein, tumor necrosis factor-alpha, and IL-6 levels. Outliers more than 2 SD from the mean were excluded and significance was set as p < .05.
Results
Participants
Tables 1 and 2 show the baseline characteristics by study condition. Participants were primarily White, college-educated, middle-aged women with obesity and about half were taking antidepressant medication. Baseline characteristics were comparable across treatment groups.
Table 1.
Participant Baseline Characteristics by Treatment Group (n = 30)
| Health education control (n = 15) | Exercise intervention (n = 15) | p valuea | |
|---|---|---|---|
| Age | 38.0 ± 11.0 | 37.0 ± 10.0 | .77 |
| Range | 21–53 | 25–53 | |
| Married/lives with partner | 7 (47) | 5 (34) | .72 |
| White | 14 (93) | 13 (87) | .54 |
| Education | .72 | ||
| High school | 1 (7) | 2 (13) | |
| College | 12 (80) | 12 (80) | |
| Graduate school | 2 (13) | 1 (7) | |
| Employed | 13 (87) | 9 (60) | .10 |
| Time to first cigarette 5 or less minutes (FTCD) | 6 (40) | 7 (47) | .84 |
| FTCD total score | 4.2 ± 2.0 | 4.7 ± 2.2 | .55 |
| Range | 1–7 | 0–8 | |
| Taking antidepressant medication | 8 (53) | 9 (60) | .71 |
| Current psychiatric diagnosis | |||
| (eg, anxiety or depressive disorder) | 5 (33) | 6 (40) | .70 |
FTCD = Fagerström Test for Cigarette Dependence. Possible scores range from 0 to 10.
aTwo-sample t test or chi-square test as appropriate. Data are reported as n (%) or mean ± SD as appropriate.
Table 2.
Baseline and End of Treatment (Week 12) Outcomes by Treatment Group
| Baselinea | Week 12 | ||||
|---|---|---|---|---|---|
| Measure | Health education control | Exercise intervention | Health education control | Exercise intervention | p valueb |
| PHQ-9 score (depression) | 11.6 ± 4.0 | 11.7 ± 5.4 | 7.0 ± 5.1 | 7.4 ± 4.5 | .90 |
| Range | 3–18 | 4–22 | 0–17 | 3–17 | |
| Body mass index | 30.0 ± 9.0 | 31.0 ± 7.0 | 30.0 ± 9.0 | 31.0 ± 8.0 | .77 |
| Range | 19–45 | 21–42 | 19–48 | 21–44 | |
| Max VO2 c | 25.0 ± 5.0 | 24.0 ± 5.0 | 24.0 ± 4.0 | 29.0 ± 6.0 | .002 |
| Range | 17–37 | 13–33 | 20–32 | 20–39 | |
| Overall physical activityd | 11 452 ± 1687 | 12 133 ± 3581 | 10 924 ± 2057 | 11 459 ± 4545 | .94 |
| Range | 9192–13 016 | 7784–20 304 | 8013–15 127 | 7338–23 664 | |
| Sedentary timed
Range |
327.2 ± 92.6 202–469 |
319.6 ± 131.6 146–513 |
286.6 ± 96.7 154–477 |
257.4 ± 114.0 92–435 |
.56 |
PHQ-9 = Patient Health Questionnaire. For each treatment group n = 15 at baseline and n = 13 at Week 12. Data are reported as mean ± SD.
a p values at least .05 for all variables when comparing health education and exercise interventions on the baseline assessment using a two sample t test.
bAnalysis of covariance assessing change in measures. For these analyses, the week 12 assessment was the dependent variable and treatment group and the baseline assessment were the independent variables.
cVolume of oxygen consumed in milliliters of oxygen per kilogram of body weight per minute.
dData generated from Modular Signal Recorder accelerometer.
Feasibility
Figure 1 summarizes treatment completion and follow-up information. Treatment adherence was high in both groups. HE participants completed a mean (SD) = 24.0 (10.0) sessions (range 5–36) and EX participants completed a mean of 26.0 (10.0) sessions (range 5–36), p = .55. The average proportion of sessions attended was 66% for HE participants and 72% for EX. All exercise participants engaged in some vigorous exercise at every session. At the final session attended, participants averaged 22 minutes (SD = 10, range 3–30) of vigorous exercise (of 30 minutes recommended by Week 12). When excluding two participants who only attended five sessions, the remaining participants, attending between 18 and 36 sessions, achieved an average of 25 minutes of vigorous activity (SD = 6, range 12–30) at their final session.
NRT adherence was low in both groups with only 13% (2/15) of HE participants and 27% (4/15) of women in EX completing the recommended 8 weeks of nicotine patch. Reasons for nonadherence were relapse to smoking, skin irritation, patch falling off, or participants not feeling that they needed it.
Study retention was good, with 87% (13/15) of participants in both groups completing the 6-month assessment. Saliva samples were collected for all participants in both treatment conditions who self-reported smoking abstinence at Week 12 and at 6-month follow-up.
Treatment Acceptability
Satisfaction with the counseling provided by the coach was high for both treatment groups (mean Consultation and Relational Empathy score = 38.0±4.0 [range 30–40] for HE vs. 39.0±3.0 [range 30–41] for EX, p = .52). All participants in HE and 92% of participants in EX indicated they would “definitely” recommend the program to another female smoker with depression, p = .31.
Smoking Status
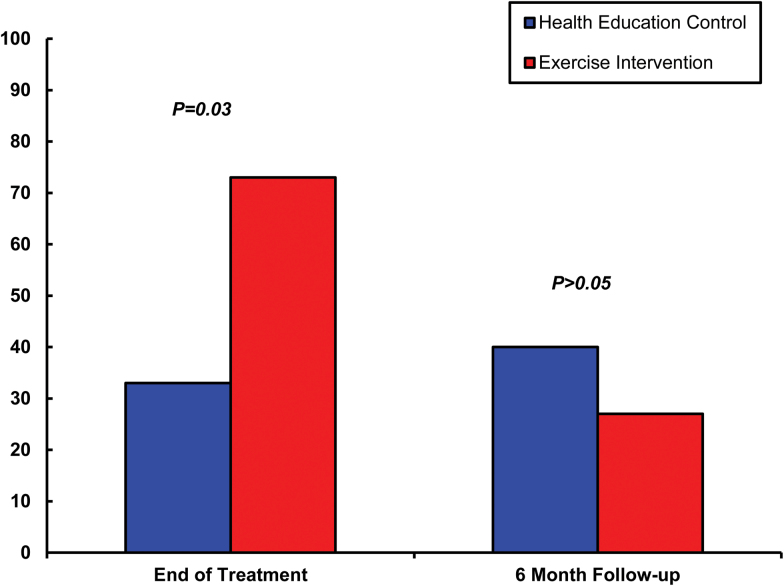
Based on intent-to-treat analysis, as expected, the EX condition was associated with significantly higher biochemically verified smoking abstinence rates (73% [11/15]) compared to HE (33% [5/15]) at Week 12; χ 2 = 4.821, df = 1, p = .028 (Figure 2). No statistically significant differences between groups were detected at 6-month follow-up (27% [4/15] for EX vs. 40% [6/15] for HE); χ 2 = .600, df = 1, p = .439. When adjusted for PHQ-9 score and antidepressant medication use, p = .035 at Week 12 and p = .48 at 6-month follow-up.
Figure 2.
Percentage of participants with biochemically confirmed, 7-day point prevalence smoking abstinence at the end of 12 weeks of treatment and 6 months follow-up by treatment group.
No participants reported engaging in nonstudy depression or smoking cessation treatments or changes in their medical or psychological depression treatment at Week 12. At 6 months, two participants in each group reported change in their depression treatment.
Cardiorespiratory Fitness
After adjusting for baseline assessment Max VO2 was, as expected, greater for EX than HE participants at Week 12 (p = .002; see Table 2).
BMI, Physical Activity, and Depression
None of the additional outcome measures were significantly different for EX compared with HE Week 12 (Table 2).
Inflammation Biomarkers
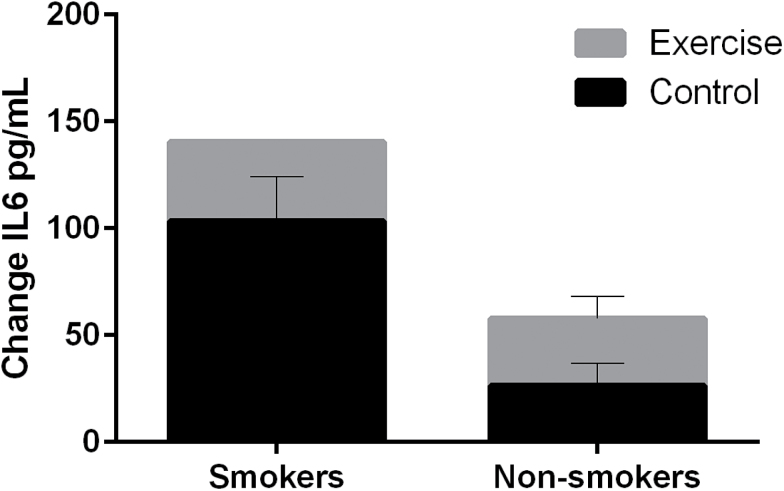
No significant group or interaction effect was observed for C-reactive protein, tumor necrosis factor-alpha, or IL-6 for intervention and smoking status pre- and postintervention (data not shown). When individual differences in biomarkers were compared (Week 12 − baseline level), a significant group effect was observed for IL-6 dependent on smoking status. As illustrated in Figure 3, IL-6 levels increased significantly more (F [1, 9] = 5.631; p = .04) for smokers compared with those who quit at Week 12. A small portion of subjects had levels below detectable limits (no difference between groups).
Figure 3.
Change in levels of interleukin-6 (IL-6) from baseline to end of treatment by study group.
Discussion
Vigorous intensity supervised exercise is feasible and enhances short-term smoking cessation among depressed female smokers. This study addressed an important gap in the field. Little previous work evaluated exercise interventions for smoking cessation among depressed smokers or those with comorbid psychiatric disorders.18 Observed increases in cardiorespiratory fitness for EX participants compared with HE confirmed the study conditions were implemented as intended. Strengths of the study are developing the intervention with advice from community women, use of an experimental design with a credible active contact control group, and inclusion of pharmacotherapy for both groups. The interventions were well specified in treatment manuals and delivered with high fidelity. Also, the exercise intervention was implemented in a community YMCA setting, enhancing external validity.
The exercise intervention appeared to benefit women only as long as it was active. YMCA data indicated that only two women in the exercise condition attended that facility after Week 12; of these, one exercised twice, another exercised 47 times. Thus, a key challenge for the field is to discover innovative and cost-effective strategies to bolster long-term adherence, while considering that supervised exercise is associated with better outcomes in studies of both depression75 and smoking cessation (see also Ussher et al.18 for review).76,77 A key difference between our study and another21 using vigorous exercise delivered at a YMCA community setting was the much greater frequency of supervised sessions (36 vs. 4).
The reasons why the intervention did not differentially impact depressive symptoms are unclear especially given that exercise adherence was high and increase in cardiorespiratory fitness was achieved. However, over half of the sample was already being treated for depression with an antidepressant medication, limiting potential impact of exercise on depression severity.
A novel finding indicates that IL-6 levels were significantly elevated at Week 12, relative to baseline, for those women who were smoking compared with those abstinent. This preliminary evidence for increases in IL-6 could be explored further in larger samples as a potential biological mediator of treatment efficacy.78,79 Although an interaction effect for smoking status by treatment was not observed, given the small sample size and with only one individual in EX who reported smoking at Week 12, it is not possible to draw any clear conclusions at this stage. Moreover, we did not assess the relationship between IL-6 levels and NRT use due to the low compliance to nicotine patch in this study sample. Future study designs could disentangle the medication component from the exercise treatment to examine associations of each with pro-inflammatory cytokines.
This study has a number of shortcomings that should be noted. Like most pilot trials, the study findings are limited by the small sample size; however, considering the statistically significant results in some of our key variables, the findings are encouraging. Moreover, characteristics of our sample: women only, primarily Caucasian race, and more severe depressive symptomatology, limit the generalizability. We inadvertently missed a substantial number of women who would have been eligible to participate if we used a less conservative Center for Epidemiological Studies Depression Scale-10 cutoff score of 10 recommended for general population samples. Two-thirds (38/62) of those screened but not eligible had a score of at least 10 but not as high as 16. Thus, in our definitive study which we plan, increasing the range of depressive symptoms among enrolled women and inclusion of a racially diverse sample could substantially extend the reach of the exercise intervention.80 We did not assess other tobacco or nicotine product use, eg, electronic cigarette use, and more comprehensive measures should be included in subsequent trials.
Despite these limitations, there is potential benefit of sustained, supportive, supervised exercise intervention for smoking cessation in depressed women, and this study points to new directions for future research. Our next step is to conduct a larger scale efficacy trial, broadening the racial/ethnic diversity and severity of depressive symptoms among participants, and including both men and women to allow for examination of potential sex differences in treatment response. To enhance intervention reach, innovative but less intensive methods for promoting long-term exercise adherence will be tested. A possible strategy would be to utilize fitness trainers at the YMCA to supervise and reinforce participants for attendance. The use of technology to reinforce exercise in the natural environment and during YMCA-based sessions also holds promise such as, digital health coaching or text messaging via mobile phones.81,82 Anecdotally, the Kinetic Activity Monitor device provided to participants was not acceptable or convenient because it was worn on the waist. Newly available accelerometers/pedometers are worn on the wrist and may be more practical. These also have the capability of connecting users and could be utilized for coach feedback and support. Moreover, because nicotine patch compliance was not optimal, alternative pharmacological adjuncts such as varenicline need consideration in subsequent trials.
Supplementary Material
Supplementary Material can be found online at http://www.ntr.oxfordjournals.org
Funding
This study was supported by CTSA grant number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Funding for this study was also provided by a Mayo Clinic NIH-relief award, and a small grant award from the Department of Psychiatry and Psychology.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
From Mayo Clinic, we acknowledge Julie Hathoway, Debi Judy, Marcelo Hanza, Christina Smith, Keagan McPherson, and Devika Basu for assistance with implementation of the study; Gabriel Koepp and Graham Moore for analysis of the accelerometer data; and Katheryn Wininger, J. Blair Price, and Shari Sutor for assistance with biospecimen sample processing and data analysis; and the Center for Clinical and Translational Science Clinical Research Unit staff for assistance with exercise testing and other measures. We also acknowledge the YMCA staff for assistance with implementing the exercise intervention. We would also like to thank the women who participated in the study.
References
- 1. Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. [DOI] [PubMed] [Google Scholar]
- 2. Piper ME, Cook JW, Schlam TR, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12(6):647–657. doi:10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004;99(11):1462–1469. doi:10.1111/j.1360-0443.2004.00845.x. [DOI] [PubMed] [Google Scholar]
- 4. Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15(5):391–411. [DOI] [PubMed] [Google Scholar]
- 5. Schnoll RA, Patterson F, Lerman C. Treating tobacco dependence in women. J Womens Health (Larchmt). 2007;16(8):1211–1218. [DOI] [PubMed] [Google Scholar]
- 6. Jessup MA, Dibble SL, Cooper BA. Smoking and behavioral health of women. J Womens Health (Larchmt). 2012;21(7):783–791. doi:10.1089/jwh.2011.2886. [DOI] [PubMed] [Google Scholar]
- 7. Burgess DJ, Fu SS, Noorbaloochi S, et al. Employment, gender, and smoking cessation outcomes in low-income smokers using nicotine replacement therapy. Nicotine Tob Res. 2009;11(12):1439–1447. doi:10.1093/ntr/ntp158. [DOI] [PubMed] [Google Scholar]
- 8. Japuntich SJ, Leventhal AM, Piper ME, et al. Smoker characteristics and smoking-cessation milestones. Am J Prev Med. 2011;40(3):286–294. doi:10.1016/j.amepre.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sonne SC, Nunes EV, Jiang H, Tyson C, Rotrosen J, Reid MS. The relationship between depression and smoking cessation outcomes in treatment-seeking substance abusers. Am J Addict. 2010;19(2):111–118. doi:10.1111/j.1521-0391.2009.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Passey M, Bonevski B. The importance of tobacco research focusing on marginalized groups. Addiction. 2014;109(7):1049–1051. doi:10.1111/add.12548. [DOI] [PubMed] [Google Scholar]
- 11. Williams JM, Steinberg ML, Griffiths KG, Cooperman N. Smokers with behavioral health comorbidity should be designated a tobacco use disparity group. Am J Public Health. 2013;103(9):1549–1555. doi:10.2105/AJPH.2013.301232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vickers KS, Patten CA, Lewis BA, et al. Feasibility of an exercise counseling intervention for depressed women smokers. Nicotine Tob Res. 2009;11(8):985–995. doi:10.1093/ntr/ntp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernard P, Ninot G, Cyprien F, et al. Exercise and counseling for smoking cessation in smokers with depressive symptoms: a randomized controlled pilot trial. J Dual Diagn. 2015;11(3–4):205–216. doi:10.1080/15504263.2015.1113842. [DOI] [PubMed] [Google Scholar]
- 14. Brown RA, Abrantes AM, Strong DR, et al. Efficacy of sequential use of fluoxetine for smoking cessation in elevated depressive symptom smokers. Nicotine Tob Res. 2014;16(2):197–207. doi:10.1093/ntr/ntt134. [DOI] [PubMed] [Google Scholar]
- 15. Evins AE, Culhane MA, Alpert JE, et al. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. J Clin Psychopharmacol. 2008;28(6):660–666. doi:10.1097/JCP.0b013e31818ad7d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall SM, Tsoh JY, Prochaska JJ, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808–1814. doi:10.2105/AJPH.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MacPherson L, Tull MT, Matusiewicz AK, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psychol. 2010;78(1):55–61. doi:10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ussher MH, Taylor AH, Faulkner GE. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2014;8: CD002295. doi:10.1002/14651858.CD002295.pub5. [DOI] [PubMed] [Google Scholar]
- 19. Marcus BH, Albrecht AE, King TK, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Arch Intern Med. 1999;159(11):1229–1234. [DOI] [PubMed] [Google Scholar]
- 20. Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. doi:10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whiteley JA, Williams DM, Dunsiger S, et al. YMCA commit to quit: randomized trial outcomes. Am J Prev Med. 2012;43(3):256–262. doi:10.1016/j.amepre.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 22. Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28(1):1–8. [DOI] [PubMed] [Google Scholar]
- 23. Singh NA, Stavrinos TM, Scarbek Y, Galambos G, Liber C, Fiatarone Singh MA. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci. 2005;60(6):768–776. [DOI] [PubMed] [Google Scholar]
- 24. Perraton LG, Kumar S, Machotka Z. Exercise parameters in the treatment of clinical depression: a systematic review of randomized controlled trials. J Eval Clin Pract. 2010;16(3):597–604. doi:10.1111/j.1365-2753.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- 25. Galper DI, Trivedi MH, Barlow CE, Dunn AL, Kampert JB. Inverse association between physical inactivity and mental health in men and women. Med Sci Sports Exerc. 2006;38(1):173–178. [DOI] [PubMed] [Google Scholar]
- 26. Hollenberg M, Haight T, Tager IB. Depression decreases cardiorespiratory fitness in older women. J Clin Epidemiol. 2003;56(11):1111–1117. [DOI] [PubMed] [Google Scholar]
- 27. Lavoie KL, Fleet RP, Lespérance F, et al. Are exercise stress tests appropriate for assessing myocardial ischemia in patients with major depressive disorder? Am Heart J. 2004;148(4):621–627. [DOI] [PubMed] [Google Scholar]
- 28. Marcus BH, Lewis BA, Hogan J, et al. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine Tob Res. 2005;7(6):871–880. [DOI] [PubMed] [Google Scholar]
- 29. Williams DM. Increasing fitness is associated with fewer depressive symptoms during successful smoking abstinence among women. Int J Fit. 2008;4(1):39–44. [PMC free article] [PubMed] [Google Scholar]
- 30. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U. S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 31. Appleton AA, Buka SL, Loucks EB, Gilman SE, Kubzansky LD. Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychol. 2013;32(7):748–756. doi:10.1037/a0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walker AJ, Kim Y, Price JB, et al. Stress, inflammation, and cellular vulnerability during early stages of affective disorders: biomarker strategies and opportunities for prevention and intervention. Front Psychiatry. 2014;5:34. doi:10.3389/fpsy.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi:10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 34. Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci. 2011;15:576–584. doi:10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 35. Maes M, Yirmyia R, Noraberg J, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24(1):27–53. doi:10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 36. Yanbaeva DG, Dentener MA, Creutzberg EC, Wouters EF. Systemic inflammation in COPD: is genetic susceptibility a key factor? COPD. 2006;3(1):51–61. [DOI] [PubMed] [Google Scholar]
- 37. Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi:10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi:10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pedersen BK, Fischer CP. Beneficial health effects of exercise–the role of IL-6 as a myokine. Trends Pharmacol Sci. 2007;28(4):152–156. [DOI] [PubMed] [Google Scholar]
- 40. Campbell M, Fitzpatrick R, Haines A, et al. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000;321(7262):694–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Czajkowski SM, Powell LH, Adler N, et al. From ideas to efficacy: the ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol. 2015;34(10):971–982. doi:10.1037/hea0000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: getting started and moving on from stage I. Clin Psychol Sci Pract. 2001;8(2):133–142. doi:10.1093/clipsy.8.2.133. [Google Scholar]
- 43. Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990–996. doi:10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 44. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- 45. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription.8th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 46. McCrady-Spitzer SK, Manohar CU, Koepp GA, Levine JA. Low-cost and scalable classroom equipment to promote physical activity and improve education. J Phys Act Health. 2015;12(9):1259–1263. doi:10.1123/jpah.2014-0159. [DOI] [PubMed] [Google Scholar]
- 47. Cole JC, Rabin AS, Smith TL, Kaufman AS. Development and validation of a Rasch-derived CES-D short form. Psychol Assess. 2004;16(4): 360–372. doi:10.1037/1040-3590.16.4.360. [DOI] [PubMed] [Google Scholar]
- 48. Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi:10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 49. Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci. 1992;17(4):338–345. [PubMed] [Google Scholar]
- 50. Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32(9):509–515. doi:10.3928/0048-5713-20020901-06. [Google Scholar]
- 51. Annesi JJ, Gorjala S. Association of reduction in waist circumference with normalization of mood in obese women initiating exercise supported by the Coach Approach protocol. South Med J. 2010;103(6):517–521. doi:10.1097/SMJ.0b013e3181de0eb5. [DOI] [PubMed] [Google Scholar]
- 52. Chapman LS, Lesch N, Baun MP. The role of health and wellness coaching in worksite health promotion. Am J Health Promot. 2007;21(6):suppl. 1–10, iii. [DOI] [PubMed] [Google Scholar]
- 53. Mettler EA, Preston HR, Jenkins SM, et al. Motivational improvements for health behavior change from wellness coaching. Am J Health Behav. 2014;38(1):83–91. doi:10.5993/AJHB.38.1.9. [DOI] [PubMed] [Google Scholar]
- 54. McPherson K, Bronars C, Patten C, et al. Understanding word preference for description of exercise interventions as a means for enhancing recruitment and acceptability of exercise treatment among adults treated for depression. Ment Health Phys Act. 2014;7(2):73–77. doi:10.1016/j.mhpa.2014.05.001. [Google Scholar]
- 55. Borg GA. Perceived exertion. Exerc Sport Sci Rev. 1974;2:131–153. [PubMed] [Google Scholar]
- 56. Ussher M, Lewis S, Aveyard P, et al. Physical activity for smoking cessation in pregnancy: randomised controlled trial. BMJ. 2015;350:h2145. doi:10.1136/bmj.h2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–164. doi:10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 58. Marcus BH, Forsyth LH. Motivating People to be Physically Active.2nd ed Champaign, IL: Human Kinetics; 2009. [Google Scholar]
- 59. Patten CA, Armstrong CA, Martin JE, Sallis JF, Booth J. Behavioral control of exercise in adults: studies 7 and 8. Psychol Health. 2000;15(4): 571–581. doi:10.1080/08870440008402014. [Google Scholar]
- 60. Redfield MM, Anstrom KJ, Levine JA, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373(24):2314–2324. doi:10.1056/NEJMoa1510774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Prochaska JJ, Hall SM, Humfleet G, et al. Physical activity as a strategy for maintaining tobacco abstinence: a randomized trial. Prev. Med. 2008;47(2):215–220. doi:10.1016/j.ypmed.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443–451. doi:10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 63. Waltz J, Addis ME, Koerner K, Jacobson NS. Testing the integrity of a psychotherapy protocol: assessment of adherence and competence. J Consult Clin Psychol. 1993;61(4):620–630. [DOI] [PubMed] [Google Scholar]
- 64. Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerström test for cigarette dependence. Nicotine Tob Res. 2012;14(1):75–78. doi:10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 65. Mercer SW, McConnachie A, Maxwell M, Heaney D, Watt GC. Relevance and practical use of the Consultation and Relational Empathy (CARE) measure in general practice. Fam Pract. 2005;22(3):328–334. doi:10.1093/fampra/cmh730. [DOI] [PubMed] [Google Scholar]
- 66. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi:10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 67. Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, Walker N. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tob Res. 2008;10(4):607–612. doi:10.1080/14622200801978680. [DOI] [PubMed] [Google Scholar]
- 68. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 69. Keller-Ross ML, Johnson BD, Carter RE, et al. Improved ventilatory efficiency with locomotor muscle afferent inhibition is strongly associated with leg composition in heart failure. Int J Cardiol. 2015;202:159–166. doi:10.1016/j.ijcard.2015.08.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Olson TP, Joyner MJ, Eisenach JH, Curry TB, Johnson BD. Influence of locomotor muscle afferent inhibition on the ventilatory response to exercise in heart failure. Exp Physiol. 2014;99(2):414–426. doi:10.1113/expphysiol.2013.075937. [DOI] [PubMed] [Google Scholar]
- 71. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Löwe B, Kroenke K, Herzog W, Grafe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9). J Affect Disord. 2004;81(1):61–66. doi:10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 73. Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42(12):1194–1201. [DOI] [PubMed] [Google Scholar]
- 74. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100(3): 299–303. doi:10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- 75. Stanton R, Reaburn P. Exercise and the treatment of depression: a review of the exercise program variables. J Sci Med Sport. 2014;17(2):177–182. doi:10.1016/j.jsams.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 76. Maddison R, Roberts V, McRobbie H, et al. Exercise counseling to enhance smoking cessation outcomes: the Fit2Quit randomized controlled trial. Ann Behav Med. 2014;48(2):194–204. doi:10.1007/s12160-014-9588-9. [DOI] [PubMed] [Google Scholar]
- 77. Bize R, Willi C, Chiolero A, et al. Participation in a population-based physical activity programme as an aid for smoking cessation: a randomised trial. Tob Control. 2010;19(6):488–494. doi:10.1136/tc.2009.030288. [DOI] [PubMed] [Google Scholar]
- 78. Boecker H, Henriksen G, Sprenger T, et al. Positron emission tomography ligand activation studies in the sports sciences: measuring neurochemistry in vivo. Methods. 2008;45(4):307–318. doi:10.1016/j.ymeth.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 79. Boecker H, Sprenger T, Spilker ME, et al. The runner’s high: opioidergic mechanisms in the human brain. Cereb Cortex. 2008;18(11):2523–2531. doi:10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- 80. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi:10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 81. Muench F, van Stolk-Cooke K, Morgenstern J, Kuerbis AN, Markle K. Understanding messaging preferences to inform development of mobile goal-directed behavioral interventions. J Med Internet Res. 2014;16(2):e14. doi:10.2196/jmir.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fanning J, Mullen SP, McAuley E. Increasing physical activity with mobile devices: a meta-analysis. J Med Internet Res. 2012;14(6):e161. doi:10.2196/jmir.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.