Abstract
We summarize new knowledge that has accrued in recent years on chronic kidney disease (CKD) in Indigenous Australians. CKD refers to all stages of preterminal kidney disease, including end‐stage kidney failure (ESKF), whether or not a person receives renal replacement therapy (RRT). Recently recorded rates of ESKF, RRT, non‐dialysis CKD hospitalizations and CKD attributed deaths were, respectively, more than sixfold, eightfold, eightfold and threefold those of non‐Indigenous Australians, with age adjustment, although all except the RRT rates are still under‐enumerated. However, the nationwide average Indigenous incidence rate of RRT appears to have stabilized. The median age of Indigenous people with ESKF was about 30 years less than for non‐Indigenous people, and 84% of them received RTT, while only half of non‐Indigenous people with ESKF did so. The first‐ever (2012) nationwide health survey data showed elevated levels of CKD markers in Indigenous people at the community level. For all CKD parameters, rates among Indigenous people themselves were strikingly correlated with increasing remoteness of residence and socio‐economic disadvantage, and there was a female predominance in remote areas. The burden of renal disease in Australian Indigenous people is seriously understated by Global Burden of Disease Mortality methodology, because it employs underlying cause of death only, and because deaths of people on RRT are frequently attributed to non‐renal causes.
These data give a much expanded view of CKD in Aboriginal people. Methodologic approaches must be remedied for a full appreciation of the burden, costs and outcomes of the disease, to direct appropriate policy development.
Keywords: chronic kidney disease, Indigenous Australian, kidney failure, renal replacement therapy
Summary at a Glance
Excellent review on the kidney health in the Aboriginal communities in Australia, describing the challenges and important priorities.
Introduction
High rates of chronic kidney disease (CKD) are well recognised in Aboriginal Australians. The suffering and expense associated with renal replacement therapy (RRT) remain a national concern, and numbers of people receiving dialysis treatment, as documented by the registry of the Australian and New Zealand Dialysis and Transplant Association (ANZDATA), continue to increase.1
The recent review of kidney disease among Aboriginal people, on the Australian Indigenous HealthInfoNet website2 provides excellent background for many comments in this manuscript.
The renal disease underlying these rates of renal failure has been described in scattered regional studies, mostly in indigenous people from remote and very remote areas, where rates are highest. Those studies showed that at early stage, renal disease is associated with albuminuria, which is followed by progressive loss of renal function. In the areas with the highest rates, there is a female predominance. Family clustering is often marked. A plethora of other associations include low birthweight, infections, the metabolic syndrome, cardiovascular disease and diabetes.3 A kidney biopsy series shows an excess of all common morphologic ‘disease’, with the sole defining entity in remote areas being glomerulomegaly and segmental glomerulosclerosis.4 Stereologic studies suggest an underlying nephron deficit,5 to which low birthweight probably contributes, while development of renal insufficiency in living related kidney donors hints that some component of that phenomenon is familial.6 Genetic studies thus far show associations of albuminuria with some ‘candidate gene’ alleles7, 8 but no major ‘smoking gun’. The condition does not resemble agricultural nephropathy or CKD of unknown aetiology seen in regions like Middle America and Sri Lanka.9, 10
Until recently, there has been limited understanding of the impact of preterminal CKD on hospitalizations and mortality and no systematic data collection on population‐based prevalence or incidence of CKD in the ambulatory environment. However, new studies and reports are transforming knowledge of CKD across its continuum.
Renal replacement therapy
The incidence and prevalence of RRT in Indigenous Australians remain very high, and indigenous people are substantially younger than non‐indigenous people at start of treatment. Overall, with age adjustment, incidence rates are about 8 to 9 times those of non‐Indigenous Australians.1, 11 There is vast variation in RRT rates by remoteness of residence (Fig. 1A), with even unadjusted incidence rates in remote and very remote regions the highest reported in any group in the world.1, 11, 12 Moreover, in remote areas, RRT incidence is higher in females than males, in contrast to the male excess of incident RRT in non‐Indigenous Australians.1 More than half of indigenous RRT patients come from remote/very remote regions, although only one fourth of the population live in those regions. In the context of Australia's individual states/territories, 77.5% of indigenous incident RRT patients have come from the larger more sparsely populated jurisdictions of the Northern Territory, Queensland and Western Australia, (Fig. 1B), which reflects in large part the proportions of their indigenous populations who live remotely.1 A recent review of RRT in the Northern Territory summarizes some of the challenges faced by Aboriginal patients and health systems in remote areas of Australia.13
Figure 1.
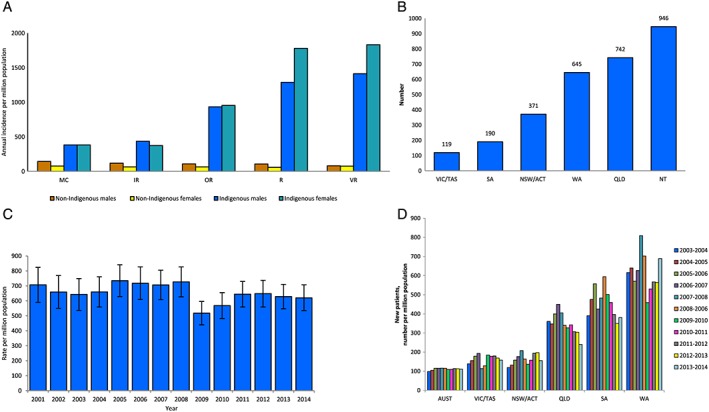
(A) Average annual incidence of renal replacement therapy among Australian adults by Indigenous status, sex and remoteness category, 2005–2008. Source: 11. Note: Rates are directly age‐standardized to the 2001 Australian population. (B) Incident renal replacement therapy among Indigenous Australians by state/territory, 2001–2014. Source: 1. (C) Age‐standardized incidence rates of renal replacement therapy among Indigenous Australians by year, 2001–2014. Source: Adapted from 1. Note: Rates are directly age‐standardized to the Australian population. (D) Incidence of renal replacement therapy among Indigenous Australians by 2 year interval and state/territory, 2003–2014. Source: Adapted from 1. Note: Rates are 2 year rolling averages.
For about two decades, starting in the 1980s, standardized incidence rates of RRT in indigenous people nationwide rose steadily, but they might now be falling (Fig. 1C). Data from individual states and territories seem to support such trends (Fig. 1D). Concurrently, death rates from natural causes are now falling in Australian Indigenous people.14
These welcome developments might reflect changing risk factor profiles, as well as more widespread recognition and treatment of preterminal kidney disease, along with other chronic diseases. However, with the increase in adult life expectancy and the size of the total population,14 absolute numbers of incident and prevalent patients on RRT will continue to rise, even if age‐standardized incidence rates stabilize or fall. This will increase the already formidable challenges for RRT service delivery.
There are systematic differences in modalities of RRT used in indigenous people compared with non‐indigenous people (Table 1) (Personal communication, SP McDonald, ANZDATA Registry, 2013). Rates of transplantation are substantially lower. Living donors are few, due to the high background prevalence of kidney disease and of risk of development of CKD in donors,6 and outcomes of deceased donor transplants are worse, with a high burden of comorbidities and infectious complications.15 There are important barriers arising from communication and other difficulties.16 Reduced access to transplantation is also seen among indigenous people in Canada and the USA.17
Table 1.
Treatment modality proportions among prevalent renal replacement therapy patients by Indigenous status and modality, 2013
| Centre haemodialysis | Home haemodialysis | Peritoneal dialysis | Transplant | |
|---|---|---|---|---|
| Non‐aboriginal Australians | 36% | 5% | 11% | 48% |
| Aboriginal Australians | 73% | 6% | 9% | 12% |
Source: (Personal communication, SP McDonald, ANZDATA Registry, 2013)
The predominant dialysis modality used is haemodialysis, performed in satellite and hospital units (Table 1). The proportion of indigenous patients receiving dialysis in a home setting (either peritoneal or haemodialysis) is much lower than for non‐indigenous patients. While peritoneal dialysis theoretically is attractive for patients living remotely, high rates of peritonitis, technique failure and mortality are reported.18
There are ongoing efforts to improve dialysis services on a number of fronts, including increasing provision of both temporary and permanent dialysis services at remote locations.
Total incidence of end‐stage kidney failure
A linkage study of ANZDATA registrations from 2003 to 2007 with death records, done in five Australian states/territories, exposed a much greater burden of end‐stage kidney failure (ESKF) than represented by people starting RRT (Fig. 2).19 Indigenous people with ESKF were on average about 30 years younger than non‐indigenous people with ESKF (55 vs 85 years), a much wider age gap than between those starting RRT. With age standardization, total rates of ESKF in Indigenous and non‐Indigenous Australians were 1142 and 183 per million, respectively, a 6.2‐fold elevation for indigenous people. Furthermore there were almost identical rates for indigenous males and females, unlike the male dominance of ESKF among non‐indigenous subjects (240 and 150 per million, respectively).
Figure 2.
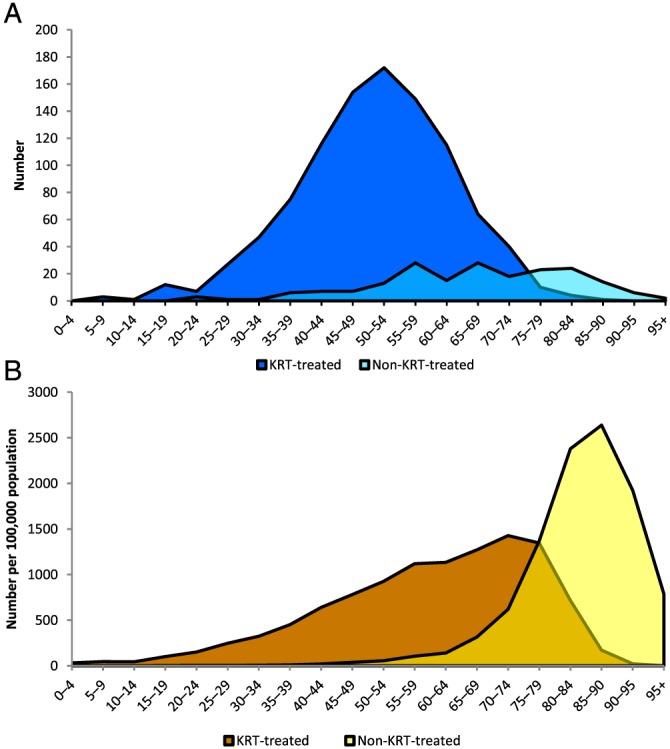
(A) End‐stage kidney failure among Indigenous Australians by treatment status and age‐group, 2003–2007. Source: 18 (Australian Institute of Health and Welfare (AIHW) analysis of Linked Australian and New Zealand Dialysis and Transplant Association (ANZDATA) Registry, AIHW National Mortality Database and National Death Index). Note: Only New South Wales, Queensland, Western Australia, South Australia and the Northern Territory are included. (B) End‐stage kidney failure among non‐Indigenous Australians by treatment status and age‐group, 2003–2007. Source: 18.
That study showed that almost half (49%) of non‐indigenous subjects with ESKF did not receive RRT (Fig. 2). Those who did not were largely older patients (age >70 years). However, among indigenous people with ESKF, 84% received RRT (Fig. 2). This is compatible with the much younger age at which indigenous people develop ESKF,19 and reflects success in provision of RRT to this population, despite its many challenges.
The capture of ESKF diagnoses in the death certificates of people who subsequently died, whether or not they had started RRT, was notably deficient, as discussed later.19, 20, 21
Hospitalizations associated with chronic kidney disease in indigenous people in Australia
Chronic kidney disease‐associated hospitalizations include those for centre‐based dialysis, because these procedures are considered same‐day hospital episodes of care in Australia. In 2012–2013, dialysis accounted for 48% of hospitalization episodes of Indigenous Australians nationwide.1, 20 Rates were increased 6.8‐fold for indigenous males and 10.8‐fold for indigenous females over their non‐indigenous counterparts. Dialysis accounted for 86% of the difference in frequency of hospitalizations between indigenous and non‐indigenous people.
There are steep gradients by remoteness for CKD‐related hospitalizations not involving dialysis, both when CKD is the principal diagnosis, and again, where CKD is an additional diagnosis (Fig. 3).22 Table 2 shows excessive rates in relation to non‐indigenous rates for all categories of indigenous CKD hospitalizations, while rates for dialysis, conflated by thrice‐weekly procedures, are hugely excessive.11, 12, 21 Again, very different burdens are borne by individual states and territories (Fig. 3), which has serious implications for their health budgets.20
Figure 3.
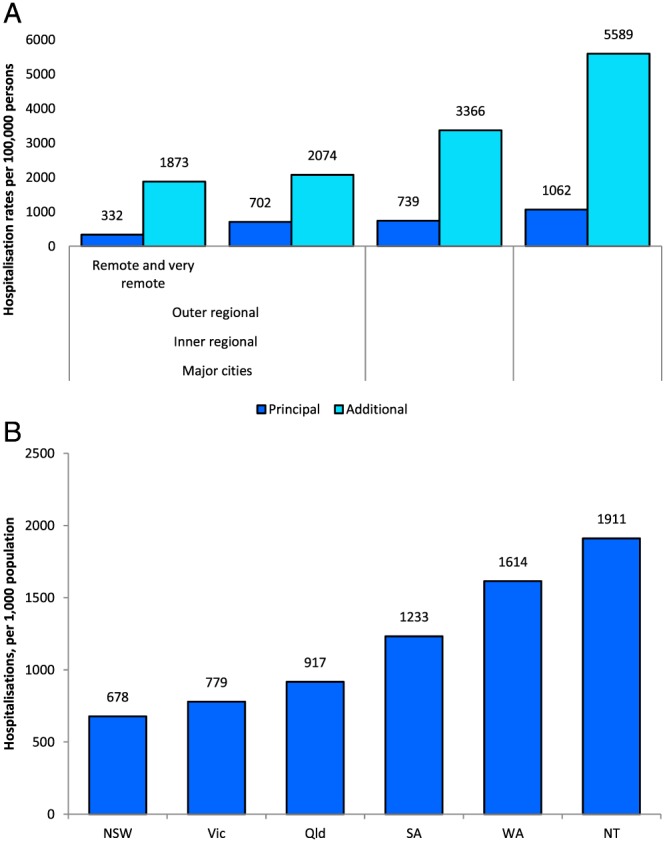
(A) Chronic kidney disease hospitalization rates among Indigenous Australians by diagnosis‐group (excluding regular dialysis) and remoteness category, 2010–2011. Source: 21. (B) Age‐standardized chronic kidney disease hospitalization rates among Indigenous Australians by state/territory, 2012–2013. Source: 19
Table 2.
Chronic kidney disease hospitalization rate ratios for Indigenous vs non‐Indigenous Australians by dialysis category and remoteness category, 2010–2011
| Major city | Inner regional | Outer regional | Remote/very remote | |
|---|---|---|---|---|
| For dialysis, same day | 8.0 | 7.2 | 24.1 | 58.8 |
| Not dialysis, CKD underlying | 2.2 | 4.5 | 5.5 | 8.1 |
| Not dialysis, CKD associated | 2.9 | 3.8 | 6.9 | 12.8 |
Source: 11,21.
CKD, chronic kidney disease.
However, these striking figures understate the impact of CKD on hospitalizations, with end‐stage kidney disease, for example, captured in the hospital ICD coding of fewer than half the subjects who died shortly thereafter with the condition.20
Deaths associated with chronic kidney disease
Chronic kidney disease‐related deaths can include deaths in people with ESKF, deaths in people who were on RRT and deaths with any other CKD diagnosis. The CKD diagnosis can be recorded as an underlying cause, but is more often listed as an associated cause.
Data from Australian Institute of Health and Welfare show that, in 2010–2012, in the five states/territories with adequate identification of indigenous status, CKD was recorded on 16% of death certificates of indigenous people, compared with 10% among non‐indigenous persons.12 Rates rose with age in both groups, and the disparity was greater for younger age‐groups (about a 10‐fold increase for those less than 65 years old, and twice as high for those age 75+ years). Overall, rates were 3.9 times higher in indigenous females and 2.6 times higher for indigenous males, a disparity which has not changed much between 1998 and 2012. Rates in indigenous people in Major Cities and Inner Regional areas, at 100 per 100 000 population, were twice as high as non‐indigenous rates, while rates in Outer Regional, Remote and Very Remote areas, at 218 per 100 000 population, were increased 4‐fold.
Even so, these data understate the contribution of CKD to death.19, 21, 23 Among people who died after starting RRT, fewer than half had a recorded ESKF contribution to their death (underlying or associated), while 12% had no recorded CKD contribution of any type.21 Deaths were more often attributed to cardiovascular disease, diabetes, chronic lung disease and the cause of ESKF like urinary obstruction or multiple myeloma.
The Global Burden of Disease's assessment of cause of mortality employs only the underlying cause of death recorded on death certificates.23 In the Australian context, where most indigenous people with ESKF receive RRT and where fewer than half the people dying after starting RRT have ESKF recorded anywhere in the death certificates, this approach vastly underestimates the burden of renal failure in the indigenous population. It minimalizes one of the most serious health issues in the indigenous population, invalidates comparisons with the burden of renal disease in other populations for whom RRT is not freely available, elevates the estimated burden of deaths because of the more immediate conditions to which dialysis patients ultimately succumb, inflates life expectancy in comparison with populations in whom a diagnosis of ESKF means imminent death and fails to highlight the enormous costs of RRT at which that extension of life expectancy in Australian Aboriginal people is achieved. Some of these issues will be addressed in the next cycle of Burden of Disease estimates in Australia.
Population‐based survey of chronic kidney disease
Biochemical testing of a nationwide random sample of indigenous adults was conducted for the first time in the 2012–2013 Australian Health Survey (AHS).24 Fig. 4A compares the distribution of CKD stages among indigenous and non‐indigenous adults. Rates of stages 1 and 2 CKD (largely albuminuria) were notably higher in Indigenous Australians, and proportions with stages 4 and 5 CKD were about 3‐fold higher, with a female excess in stage 5. Fig. 4B confirms higher prevalence CKD markers in indigenous than non‐indigenous adults, most marked in young and middle adult life.
Figure 4.
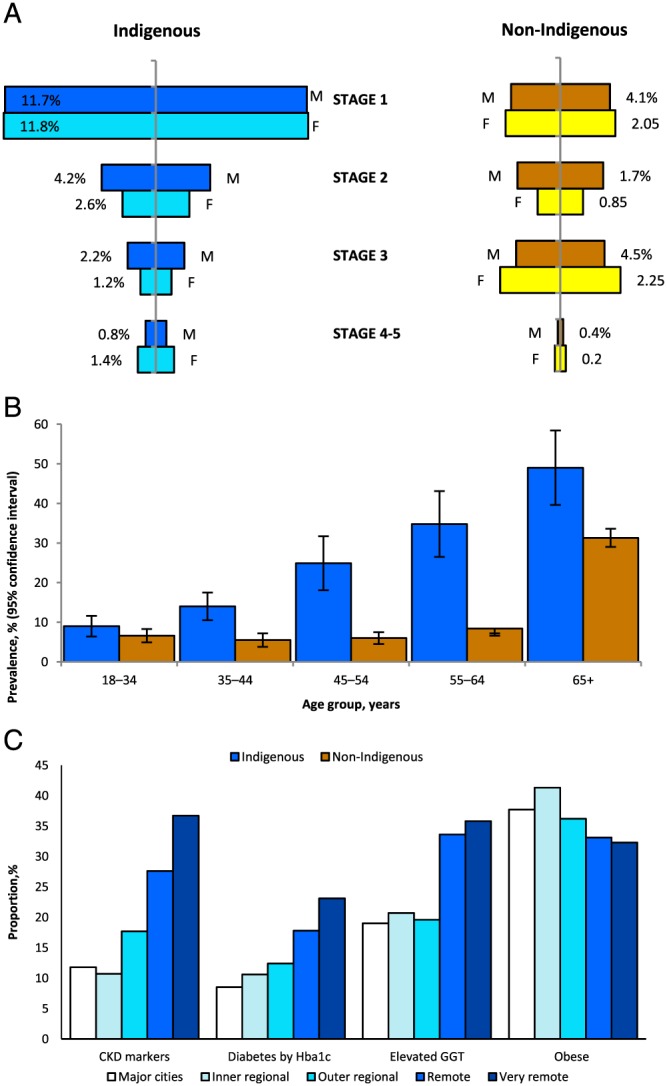
(A) Prevalence of chronic kidney disease among Australian adults by Indigenous status and disease stage, 2011–2012. Source: 23. (B) Proportions of chronic kidney disease among Australian adults by Indigenous status and age‐group, 2011–2012. Source: 23. (C) Chronic conditions among Indigenous Australian adults by chronic kidney disease marker/other selected condition and remoteness category, 2011–2012. Source: 23.
Figure 4C shows that CKD and chronic disease risk markers, like all other markers of more advanced CKD, increased with remoteness of residence. Notably, CKD rates exceeded diabetes rates, most especially in the highest risk areas, and remote areas had the lowest rates of obesity. These facts refute prevailing dogma that CKD largely results from diabetes and is tightly correlated with degree of adiposity. They are, however, consistent with biopsy studies4 and the demonstrated multifactorial aetiology of indigenous CKD.3 The gamma‐glutamyl transpeptidase data point to associations with liver disorders, which attract a default diagnosis of (sometimes non‐alcoholic) fatty liver. This association, which is insufficiently explored in the Australian context, is supported by other regional and international studies and is generally said to mark the metabolic syndrome and insulin resistance.25, 26 In that Australian Health Survey (AHS) survey, CKD in indigenous people was also associated with anaemia and with higher triglyceride levels, but not with high total or low‐density lipoprotein cholesterol levels. The AHS did not include measures of infection or inflammation.
More insights from regional studies
Additional studies confirm that albuminuria marks CKD and predicts loss of glomerular filtration rate, renal failure and non‐renal deaths over time.27, 28, 29, 30 Furthermore, it has been established that low birthweight is associated with albuminuria in adults up through 50 years of age and an approximate doubling of rates of all‐cause natural death.31 Progression is being studied in several of these cohorts.28 Community‐based profiles (albumin creatinine ratio (ACR) and blood pressure) have improved in one community over 10 years.32
Chronic kidney disease detection and management
The knowledge that renal disease in these environments is almost always part of a metabolic/hemodynamic/vascular disease syndrome4 supports the imperative to incorporate integrated chronic disease screening into the regular primary care health checks of Aboriginal people. Given the slow evolution of CKD to end‐stage, there should be plentiful opportunities to reduce the rate of progression of CKD. There is strong evidence that a systematic programme of screening and secondary prevention based in the community and structured around use of ACE inhibitors, blood pressure, glycemic and lipid control can lead to dramatic benefits.33 There have been demonstrable improvements in process measures and chronic disease markers in settings where such systematic approaches have been supported.34, 35 However, providing basic health services, let alone screening and treatment programmes, are difficult in remote environments where basic commodities are expensive and often unavailable; ongoing vigorous activity is needed to maintain the benefits of such programmes.36
Regular check‐ups of all adults, appropriate streams of management for diagnosed conditions and free medications for people in remote areas have now been supported for more than a decade by Medicare reimbursements from the Australian Federal Government.12, 37 Chronic disease care is increasingly recognised as the purview of health workers and nurses, working within primary care, rather than by doctors or providers operating in specialty silos. Anecdotally, involvement of community members in such programmes is an integral element of success. There has been no systematic qualitative study of attitudes among Aboriginal people to CKD. However, examination of interactions between clinicians and Aboriginal patients receiving dialysis treatment has shown pervasive problems with miscommunication and misunderstandings16, 38
Looking forward
Incidence rates of RRT in Indigenous Australians have stabilized. A repeat AHS in 2018 will reveal trends in the earlier stage of renal disease, as well as other conditions, while inclusion of children and adolescents in the biomarker component of that survey (omitted in 2012/2013) will shed light on the full spectrum of biomarkers with age. A unique identifier for each person, which is needed to link hospital episodes of care to individuals, is not yet on the horizon in Australia (stymied by privacy legislation), although the existing Medicare number is the obvious tool, but the Northern Territory and Western Australia have circumvented this problem using their own identifiers and linkage programmes. In other places, the widespread deployment of the integrated electronic medical record should partly remedy this deficiency. Furthermore, CKD registry and surveillance functions are developing in public renal services39 and in primary care.40 Refinements and continuous training in diagnostic coding for hospitalizations and death will improve surveillance from these sentinel sources.
We all look forward to better, cheaper dialysis modalities, wider application of transplantation and improved and extended renal supportive care services. Detection and treatment of earlier stages of CKD can always be improved. Improvements in health were documented in the Tiwi community over a recent 10 year period, most notably better growth (height attainment) in children, reductions in ACR and blood pressure in young men and rises in high‐density lipoprotein cholesterol levels in both sexes:32 these probably reflect improved birthweights over the last 50 years, as well as, arguably, as better living conditions. Notably, in that same community, a randomized controlled trial showed no benefit of long acting ACE inhibitors in delaying or preventing the combined endpoint of new onset albuminuria (ACR >3.4), or hypertension (BP > 140/90) or diabetes in people who were free of these conditions at the outset.41
Taking the broader view, 50 years of longitudinal data in the Tiwi population now frame the emergence of non‐communicable chronic diseases in the context of the epidemiologic and health transitions, low birthweights, dramatic reductions in infant mortality and increased adult lifespan.42 In such a scenario, the current high‐risk adult health profile is the expression of the exacerbated risk of low birthweight infants for later chronic disease, as proposed Barker and colleagues;43 furthermore, as competing causes of mortality are reduced by better management of other conditions like cardiovascular disease and chronic lung disease, renal disease has more opportunity to follow its more indolent course to renal failure. The challenge is to understand these dynamics, anticipate, screen and treat, applying the best standards of health care, maintain a strong focus of promoting maternal and child health, and to support initiatives to reduce socio‐economic disadvantage in all its dimensions. As birthweights, living conditions and opportunities improve, their chronic disease profiles should ultimately more closely resemble those of non‐Indigenous Australians. These concepts are surely generalizable to other populations undergoing rapid transition.44
Acknowledgements
This report draws heavily on data from the Australian Institute of Health and Welfare (AIHW), the Australian and New Zealand Dialysis and Transplant Association (ANZDATA) and the Australian Bureau of Statistics (ABS).
This work has been supported by funds from the National Health and Medical Research Council (NHMRC) of Australia (Project grant #921134, Project grant #193316, Program grant #320860, Australia Fellowship award #511081, and the current NHMRC CKD Centre for Research Excellence # APP1079502). It has also been supported by funds from Kidney Health Australia, the Colonial Foundation of Australia, Rio Tinto and AMGEN Australia.
Hoy, W. E. , Mott, S. A. , and Mc Donald, S. P. (2016) An expanded nationwide view of chronic kidney disease in Aboriginal Australians. Nephrology, 21: 916–922. doi: 10.1111/nep.12798.
References
- 1. ANZDATA Registry . 38th Report, chapter 12: Indigenous people and end stage kidney disease. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2016. [Cited 2 Feb 2016.] Available from URL: http://www.anzdata.org.au
- 2. Australian Indigenous HealthInfoNet . Chronic conditions: Review of kidney disease among indigenous people. Last update Sept 2015. [Cited 3 Sept 2015.] Available from URL: http://www.healthinfonet.ecu.edu.au/chronic‐conditions
- 3. Hoy WE, Kondalsamy Chennakesavan S, McDonald SP et al. Chronic kidney disease in Aboriginal Australians In: El Nahas M. (ed). Kidney Disease in Ethnic Minorities and the Developing World. New York, U.S: Taylor & Francis, 2005; 305–333. [Google Scholar]
- 4. Hoy WE, Samuel T, Mott SA et al. Renal biopsy rates and findings among Indigenous Australians: A nationwide review. Kidney Int. 2012; 82: 1321–31. [DOI] [PubMed] [Google Scholar]
- 5. Hoy WE, Hughson MD, Singh GR, Douglas‐Denton R, Bertram JF. Reduced nephron number and glomerulomegaly in Australian Aborigines: A group at high risk for renal disease and hypertension. Kidney Int. 2006; 70: 104–110. [DOI] [PubMed] [Google Scholar]
- 6. Rogers NM, Lawton PD, Jose MD. Indigenous Australians and living kidney donation [letter]. N. Engl. J. Med. 2009; 361: 1513–6. [DOI] [PubMed] [Google Scholar]
- 7. McDonald SP, Hoy WE, Maguire GP, Duarte N, Wilcken DEL, Wang XI. The p53Pro72Arg polymorphism is associated with albuminuria among Aboriginal Australians. J. Am. Soc. Nephrol. 2002; 13: 677–83. [DOI] [PubMed] [Google Scholar]
- 8. Duffy DL, Hoy WE, Hayhurst B et al Familial aggregation of albuminuria, renal insufficiency and arterial hypertension in an Aboriginal Australian community and the contribution of variants in ACE and TP53. Under revision for BMC Nephrology, March, 2016. [DOI] [PMC free article] [PubMed]
- 9. Johnson RJ, Sánchez‐Lozada LG. Chronic kidney disease: Mesoamerican nephropathy—new clues to the cause. Nat. Rev. Nephrol. 2013; 9: 560–1. [DOI] [PubMed] [Google Scholar]
- 10. Martin‐Cleary C, Ortiz A. CKD hotspots around the world: where, why and what the lessons are. A CKJ review series. Clin. Kidney J. 2014; 7: 519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Australian Institute of Health and Welfare . Chronic kidney disease in Aboriginal and Torres Strait Islander people 2011. Cat. no. PHE 151 Canberra: AIHW, 2011.
- 12. Australian Institute of Health and Welfare . Cardiovascular disease, diabetes and chronic kidney disease–Australian facts: Aboriginal and Torres Strait Islander people. Cardiovascular, diabetes and chronic kidney disease series no. 5. Cat. no. CDK 5. Canberra: AIHW, 2015.
- 13. Hoy WE. Kidney disease in Aboriginal Australians: A perspective from the Northern Territory. Clin. Kidney J. 2014; 7: 524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Australian Institute of Health and Welfare . Australia's Health 2014. Australia's health series no. 14. Cat. no. AUS 178. Canberra: AIHW, 2014.
- 15. Rogers NM, Lawton PD, Jose MD. Kidney transplant outcomes in the indigenous population in the Northern Territory of Australia. Transplantation 2006; 82: 882–6. [DOI] [PubMed] [Google Scholar]
- 16. Devitt J, Cass A, Cunningham J, Preece C, Anderson K, Snelling P. Study protocol–Improving access to kidney transplants (IMPAKT): A detailed account of a qualitative study investigating barriers to transplant for Australian Indigenous people with end‐stage kidney disease. BMC Health Serv. Res. 2008; 8: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeates KE, Cass A, Sequist TD et al. Indigenous people in Australia, Canada, New Zealand and the United States are less likely to receive renal transplantation. Kidney Int. 2009; 76 (6): 659–64. [DOI] [PubMed] [Google Scholar]
- 18. Lim WH, Boudville N, McDonald SP, Gorham G, Johnson DW, Jose M. Remote indigenous peritoneal dialysis patients have higher risk of peritonitis, technique failure, all‐cause and peritonitis‐related mortality. Nephrol. Dial. Transplant. 2011; 26 (10): 3366–72. [DOI] [PubMed] [Google Scholar]
- 19. Australian Institute of Health and Welfare . End stage kidney disease in Australia. Total incidence, 2003‐2007. Cat no. PHE 143, Canberra: AIHW, 2011.
- 20. Australian Indigenous HealthInfoNet . Overview of Australian indigenous health status, 2014. Hospitalisations. Australian Indigenous HealthInfoNet, 2015. [Cited 3 Sept 2015.] Available from URL: http://www.healthinfonet.ecu.edu.au/health‐facts/overviews/hospitalisation
- 21. Australian Institute of Health and Welfare . Assessment of the coding of ESKD in deaths and hospitalisation data. A working paper using linked hospitalisation and deaths data from Western Australia and New South Wales. Cat. no. PHE 182. Canberra: AIHW, 2014.
- 22. Australian Institute of Health and Welfare . Chronic kidney disease: Regional variation in Australia. Cat. no. PHE 172. Canberra: AIHW, 2013.
- 23. Australian Institute of Health and Welfare . Australian burden of disease study: Fatal burden of disease in Aboriginal and Torres Strait Islander people 2010. Australian Burden of Disease Study series 2. Cat no BOD 2. Canberra: AIHW, 2015.
- 24. Australian Bureau of Statistics . Australian Aboriginal and Torres Strait Islander Health Survey: Biomedical Results, 2012–2013. Cat no 4727.0.55.003. Canberra: Australian Bureau of Statistics, 2014. [Cited 3 Sept 2015.] Available from URL: http://www.abs.gov.au/ausstats/abs@.nsf/mf/4727.0.55.003
- 25. Nagel G, Zitt E, Peter R, Pompella A, Concin H, Lhotta K. Body mass index and metabolic factors predict glomerular filtration rate and albuminuria over 20 years in a high‐risk population. BMC Nephrol. 2013; 14: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Targher G, Chonchol MB, Byrne CD. CKD and non‐alcoholic fatty liver disease. Am. J. Kidney Dis. 2044; 64 (4): 638–52. [DOI] [PubMed] [Google Scholar]
- 27. Wang Z, Hoy WE. The predictive value of albuminuria for renal and nonrenal natural death over 14 years follow up in a remote Aboriginal community. Clin. Kidney J. 2012; 5: 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maple‐Brown LJ, Hughes JT, Ritte R et al Progression of kidney disease in Indigenous Australians: The eGFR follow‐up study. Submitted to AJKD Sept 2015. [DOI] [PMC free article] [PubMed]
- 29. Ritte R, Luke J, Nelson C et al Clinical outcomes associated with albuminuria in central Australia: A cohort study. Under revision with BMC Nephrology, Sept 2015. [DOI] [PMC free article] [PubMed]
- 30. Hoy WE, Swanson CE, Mott SA. Progression of albuminuria (ACR) and estimated glomerular filtration rate (EGFR) in a high risk Aboriginal group over 10–14 years. Nephrology 2014; 19 (s4): 40.24191893 [Google Scholar]
- 31. Hoy WE, Scott JA, Nicol JL. Further demonstration of associations of low birthweight with adult death and renal failure in a remote Aboriginal community. Nephrology 2015; 20 (s3): 50. [Google Scholar]
- 32. Wang Z, Scott J, Hoy WE. Trends in health status and chronic disease risk factors over 10–14 years in a remote Australian community: a matched pair study. Aust. N. Z. J. Public Health 2014; 38 (1): 73–7. [DOI] [PubMed] [Google Scholar]
- 33. Hoy WE, Wang Z, Baker PR, Kelly AM. Secondary prevention of renal and cardiovascular disease: Results of a renal and cardiovascular treatment program in an Australian Aboriginal community. J. Am. Soc. Nephrol. 2003; 14: S178–85. [DOI] [PubMed] [Google Scholar]
- 34. Hoy WE, Davey RL, Sharma S, Hoy PW, Smith JM, Kondalsamy‐Chennakesavan S. Chronic disease profiles in remote Aboriginal settings, and implications for health services planning. Aust. N. Z. J. Public Health 2010; 34 (1): 11–8. [DOI] [PubMed] [Google Scholar]
- 35. Hoy WE, Swanson CE, Hope A, Smith J, Masters C. Evidence for improved patient management through electronic patient records at a Central Australian Aboriginal Health Service. Aust. N. Z. J. Public Health 2014; 28 (2): 154–9. [DOI] [PubMed] [Google Scholar]
- 36. Hoy WE, Kondalsamy‐Chennakesavan SN, Nicol JL. Clinical outcomes associated with changes in a chronic disease treatment program in an Australian Aboriginal community. Med. J. Aust. 2005; 183: 305–9. [DOI] [PubMed] [Google Scholar]
- 37. Hoy WE. Chronic disease care in remote Aboriginal Australia has been transformed. Personal View. BMJ 2013; 347: f6127. [DOI] [PubMed] [Google Scholar]
- 38. Cass A, Lowell A, Christie M et al. Sharing the true stories: Improving communication between Aboriginal patients and healthcare workers. Med. J. Aust. 2002; 176: 466–70. [DOI] [PubMed] [Google Scholar]
- 39. Venuthurupalli SK, Hoy WE, Healy HG et al. Invited review: CKD.QLD: Chronic kidney disease surveillance and research in Queensland, Australia. Nephrol Dial Transp. 2012; 27 (Suppl 3): iii139–iii45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lawton PD, Cunningham J, Hadlow N, Zhao Y, Jose MD. Chronic kidney disease in the Top End of the Northern Territory of Australia, 2002–2011: A retrospective cohort study using existing laboratory data. BMC Nephrol. 2015; 16: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoy WE, Mott SA, McLeod B et al. Conduct and outcomes of an RCT of prevention of albuminuria, hypertension and diabetes in Aborigines. Nephrology 2014; 19 (s4): 39. [Google Scholar]
- 42. Hoy WE, Mott SA, Swanson C, Bloomfield H, Sharma S, McLeod BM. The rise of chronic kidney disease in remote living aboriginal people in the context of the epidemiologic and health transition. Nephrology 2011; 16 (s1): 61–2. [Google Scholar]
- 43. Barker DJP. Mothers, Babies and Disease in Later Life. London: BMJ Publishing Group, 1994. [Google Scholar]
- 44. Radhakrishnan J, Remuzzi G, Saran R et al. Taming the chronic kidney disease epidemic: A global view of surveillance efforts. Kidney Int. 2014; 86 (2): 246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


