Abstract
Multiple sclerosis (MS) is associated with inflammatory lesions in the brain and spinal cord. The detection of such inflammatory lesions using magnetic resonance imaging (MRI) is important in the consideration of the diagnosis and differential diagnoses of MS, as well as in the monitoring of disease activity and predicting treatment efficacy. Although there is strong evidence supporting the use of MRI for both the diagnosis and monitoring of disease activity, there is a lack of evidence regarding which MRI protocols to use, the frequency of examinations, and in what clinical situations to consider MRI examination. A national workshop to discuss these issues was held in Stockholm, Sweden, in August 2015, which resulted in a Swedish consensus statement regarding the use of MRI in the care of individuals with MS. The aim of this consensus statement is to provide practical advice for the use of MRI in this setting. The recommendations are based on a review of relevant literature and the clinical experience of workshop attendees. It is our hope that these recommendations will benefit individuals with MS and guide healthcare professionals responsible for their care.
Keywords: guidelines, magnetic resonance imaging, multiple sclerosis, recommendations
1. Introduction
Multiple sclerosis (MS) is an autoimmune, inflammatory, and degenerative disease of the central nervous system (CNS) associated with functional impairment and decreased quality of life.1 Recent national and regional studies of the incidence and prevalence of MS in Sweden report a prevalence of approximately 189 per 100 000 and an incidence of 6.0–10.2.2, 3, 4 The diagnosis of MS relies on the McDonald criteria, most recently revised in 2010.5 These criteria are based on the assessment of the presentation and history of clinical symptoms and imaging of the CNS with magnetic resonance imaging (MRI). In the case of primary progressive MS intrathecal IgG synthesis is also considered. The importance of MRI for the initial investigation of suspected MS, as well as for disease monitoring after a confirmed MS diagnosis, has increased due to advancements in the technology and availability of MRI, and in the scientific evidence supporting its use for MS.
Over the past few years, an increasing number of disease‐modifying treatments (DMTs) have become available that affects the MS disease in several meaningful ways. It has been shown that current DMTs can reduce focal inflammatory activity as well as the accumulation of disability6 and the rate of brain atrophy.7 The currently available DMTs differ in respect to treatment efficacy and associated risks, stressing the importance of clinical tools to individualize the choice of DMT and closely monitor the treatment efficacy.6 Examination with MRI exhibiting a higher sensitivity for inflammatory disease activity than clinical relapses alone,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 is routinely used in this regard and is an important addition to the care of individuals with MS.21
Despite the routine use of MRI in the care of patients with suspected or confirmed MS, there is a lack of scientific evidence defining its optimal use.21 The MRI protocols, timing, and frequency of investigations, as well as the positioning of patients in the MRI scanner, will in many cases differ between MS care centers. This may influence the reliability of clinical MRI data22 and thus the diagnostic accuracy as well as the ability to evaluate ongoing DMT.
There is also no complete international expert consensus available for these topics, although they are partially covered in the recent European consensus guidelines by MAGNIMS (Magnetic Resonance Imaging in MS).21, 23
Neurologists and neuroradiologists working in the field of MS in Sweden present a consensus agreement here regarding the use of MRI in MS. The aim was to establish consensus‐based recommendations for the use of MRI in the diagnosis and disease monitoring of MS in Sweden.
2. Methods
The Swedish Multiple Sclerosis Association24 (SMSS, a society for health care professionals working in the field of MS) and the Swedish Neuroradiological Society25 (SFNR, a society for neuroradiologists working in Sweden) held a national workshop meeting in Stockholm, Sweden, in August 2015. Attending neurologists and neuroradiologists working in the field of MS formed the first draft for these MRI guidelines based on professional experience and review of relevant literature. The draft was further iterated by email correspondence among the workshop attendees until reaching a consensus document in November 2015. This consensus document was sent out for review to the boards of the SMSS and SFNR and to representatives of the Neurology and Neuroradiology departments at all Swedish university hospitals: Karolinska University Hospital (Stockholm), Linköping University Hospital (Linköping), University Hospital of Umeå (Umeå), Sahlgrenska University Hospital (Gothenburg), Skåne University Hospital (Malmö/Lund), Uppsala University Hospital (Uppsala), and Örebro University Hospital (Örebro). Each university hospital consulted local or regional healthcare providers as appropriate and all referral parties provided feedback on the document.
After taking reviewer's opinions and suggestions into account, a consensus among the task force and referral parties was reached, and the guidelines document was considered effective as of February 14, 2016 and was published in Swedish on the SMSS website.26
The current guidelines for the use of MRI in MS mainly describe examination of the brain. Revisions of the guidelines will become necessary as the knowledge regarding the use of MRI in the care of individuals with MS evolves. Other important guidelines21, 23, 27, 28, 29, 30 for the current work have been taken into account and the MAGNIMS research group requires special mention.21, 23
3. Results
3.1. Clinical indications for MRI scans
There are a number of situations in which MRI investigation provides clinical benefit in the care of an individual with suspected or confirmed MS. The most prominent reasons are diagnostic examination, monitoring of treatment efficacy, and screening for progressive multifocal leukoencephalopathy (PML). The most important clinical situations in which MRI should be performed are summarized in Table 1.
Table 1.
Specific clinical situations when MRI investigation is warranted
| Situation | Reason for MRI examination | Aim |
|---|---|---|
| Investigation of clinically suspected MS | To find radiological signs that increase/decrease the probability of MS. To assess the level of disease activity |
|
| Monitoring of confirmed MS | To detect radiological disease progress. Identify reason for a change of treatment strategy |
|
| Monitoring of CIS or RIS | To detect radiological progression and to re‐evaluate fulfillment of MS diagnostic criteria. High degree of radiological progression may indicate initiation of DMT or change in DMT |
|
| Examination before and after change in DMT or treatment strategy | By examination before and after a change in DMT or treatment strategy, it is possible to correctly relate any potential new disease activity in regards to the DMT change |
|
| Clinical relapse or unforeseen clinical worsening, especially when the differential diagnosis of pseudo‐relapsea is difficult to rule out. | A clinical relapse in itself does not warrant an acute MRI and corticosteroid treatment of a clinically typical relapse should not be delayed to facilitate MRI examination. However, MRI can be valuable at the time of or after a relapse to assess the level of inflammatory activity as a ground for any re‐evaluation of the current DMT drug or treatment strategy |
|
| Monitoring for, or investigation of, suspected PML. | To find radiological signs indicative of PML when clinical suspicion of the disease or high clinical PML risk is present |
|
MRI, magnetic resonance imaging; MS, multiple sclerosis; RIS, radiologically isolated syndrome; DMT, disease‐modifying treatment; PML, progressive multifocal leukoencephalopathy.
New or worsened symptoms suggestive of, but not caused by MS disease activity (e.g., clinical worsening due to fever from infection).
3.2. Which parts of the CNS should be examined?
Multiple sclerosis disease activity can occur in any part of the CNS. For practical reasons, the imaging of the CNS is commonly divided into brain imaging and spinal cord imaging, with separate MRI protocols. The brain, encompassing the larger part of the total CNS parenchyma, should always be examined in routine MRI for diagnosis or follow‐up of MS. Examination with spinal cord MRI can provide additional information, but is generally not as informative as the brain examination in the typical case.31, 32, 33 However, in line with the recommendations of the recently published MAGNIMS consensus guidelines,21 we suggest that spinal cord MRI should be included in the diagnostic workup if the presenting symptoms indicate spinal cord involvement. Furthermore, spinal MRI can be helpful in the differential diagnosis when brain imaging is equivocal or inconclusive and when brain MRI findings suggest radiologically isolated syndrome (RIS).21, 34
3.3. Recommended MRI protocols for diagnosis and follow‐up
Recommended MRI brain imaging protocols for diagnostic evaluation and follow‐up of confirmed MS cases are specified in Table 2. These should be regarded as minimal protocols to provide adequate radiological information in most cases. The protocols can locally be extended with additional sequences, but the recommendations are intended as a minimum common denominator across Swedish hospitals. In situations where consideration of specific differential diagnoses is warranted, the protocols should be tailored to answer the relevant radiological questions at hand. The diagnostic protocol is more extensive, which allows for the consideration of radiological differential diagnoses required for diagnosing MS. The follow‐up protocol is less extensive because its main purpose is to assess the occurrence of new inflammatory disease activity.
Table 2.
Recommended MRI protocols for diagnostic and follow‐up examination
| Recommended MRI protocols | |
|---|---|
| Diagnostic protocol | Follow‐up protocol |
| 1. 3D T1 (Pre‐contrast) | 1. Administration of GBCA |
| 2. Hemorrhage sensitive sequence (i.e., SWI, GRE, or FFE) | 2. Axial T2 |
| 3. DWI | 3. 3D T2‐FLAIR |
| 4. Administration of GBCA | 4. 3D T1 (Post‐contrast) |
| 5. Axial T2 | |
| 6. 3D T2‐FLAIR | |
| 7. 3D T1 (Post‐contrast) | |
GBCA, gadolinium‐based contrast agent; MRI, magnetic resonance imaging.
At follow‐up examinations, previous examinations should be available to assess whether new inflammatory changes have occurred. It is important to keep serial MRI acquisitions as technically comparable as possible as even small interexamination protocol differences may make the MRI comparison more difficult. The same MR scanner as well as the same image parameters, including orientation of axial slices, should preferably be used in all follow‐up examinations of the same individual.
3.4. How often should MRI be performed in the follow‐up of MS?
Studies on MS disease activity, such as clinical drug trials, indicate that the number of new MRI lesions is approximately 4–12 times larger than the number of new clinical relapses during the same time period.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Such “clinically silent” MRI lesions stress the importance of monitoring patients with MRI even in the absence of clinical signs of disease activity. However, there is a lack of scientific evidence to support a specific interval between follow‐up examinations. The interindividual variation in disease activity between patients with MS makes it difficult to specify a general examination frequency suitable for all cases, as reflected in the recommendations given in other available guideline documents.21, 23, 28 While we acknowledge this and would like to emphasize that the MRI frequency and timing should be adapted to the clinical situation, it is our opinion that general recommendations of MRI frequency are still valuable in the clinical care of patients. The recommendations regarding MRI frequency presented here are based on clinical experience and extrapolation of study data on the level of disease activity seen in treated and untreated patients with MS in phase III studies.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20
As mentioned above, the degree of disease activity and frequency of new inflammatory lesions vary greatly among individuals with MS. Some information regarding the expected level of disease activity can be gained from the diagnostic baseline MRI, but to better establish the individual disease activity, a second MRI can be performed 3–6 months after baseline. However, if clinical or radiological signs of high disease activity are seen at the baseline examination, an earlier follow‐up MRI can be considered. A second follow‐up examination can be performed 6–12 months after the first follow‐up, or earlier if deemed clinically warranted. When the disease is in a stable state, annual examinations are recommended in order to provide an adequate level of monitoring for new disease activity (Fig. 1).
Figure 1.
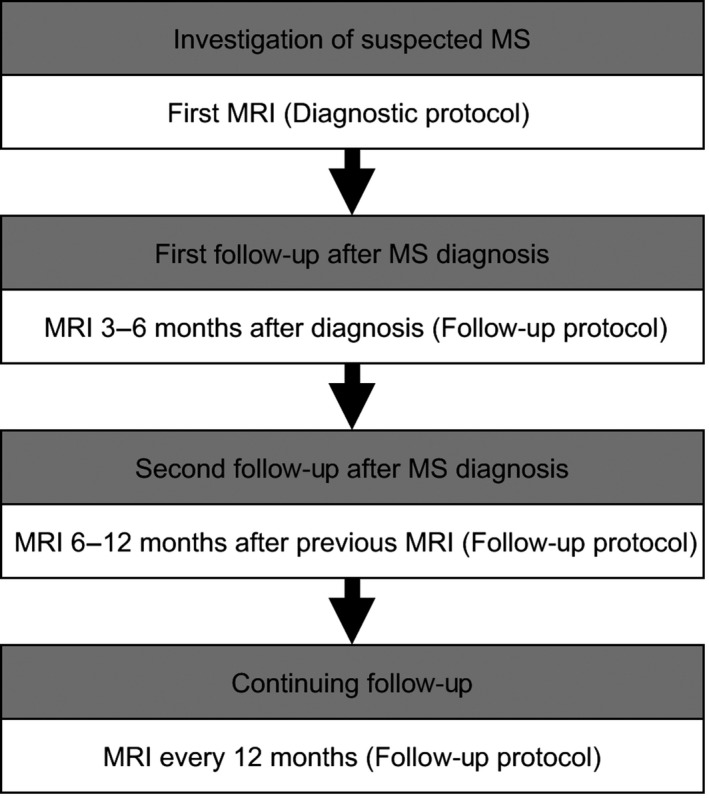
An example of timing of first, second, and continuing magnetic resonance imaging monitoring after multiple sclerosis diagnosis
3.5. In what situations should an increased frequency of MRI examinations be considered?
A change in treatment strategy may increase the risk of recurring disease activity.35 More frequent MRI monitoring is therefore recommended in relation to changes in treatment, including when discontinuing DMT in patients with long follow‐up without new MRI lesions or clinical relapses. A new MRI examination should be considered shortly before the change of treatment and again 3–6 months thereafter. The length of the interval between MRI examinations should reflect the estimated risk of recurring disease activity. This establishes a new baseline before changing the DMT, and the follow‐up examination is used to detect radiological signs of treatment failure from the new DMT drug or strategy.
A special situation occurs when switching from treatment with natalizumab to another DMT in a patient seropositive for JC virus. Early signs of PML can be difficult to detect36 and onset can occur even after natalizumab discontinuation.37 A baseline MRI should be performed within 1 month prior to switching therapy from natalizumab, and a follow‐up MRI should be performed approximately 3 months after natalizumab cessation. If clinical suspicion of PML arises, earlier follow‐up should be performed. Higher vigilance for PML is warranted in the case of ongoing treatment with natalizumab in a patient seropositive for JC virus. There is no available study data on which to reliably base recommendations regarding frequency of MRI monitoring in this case. Depending on the estimation of the PML risk in the individual patient, performing MRI every 3–6 months is a practical approach.38 For additional MRI follow‐ups specifically for PML monitoring, a reduced protocol can be used. This should, as a minimum, include 3D FLAIR and diffusion weighted imaging.39
3.6. In what situations can the frequency of MRI examinations be decreased?
Focal inflammatory activity in MS tends to decrease with age.40 A decrease in focal inflammatory activity, for this or other reasons, lessens the need for the frequent MRI monitoring recommended for patients with a more active disease. There is currently no evidence available to firmly establish at which age, disease duration, or clinical situations it is acceptable to decrease the frequency of MRI examinations. However, the consensus discussion identified a few situations when less frequent MRI monitoring can be considered (Table 3). To a large degree, these recommendations are based on clinical experience.
Table 3.
Examples of situations when decreased MRI monitoring can be considered
| Examples of situations when decreased frequency of MRI monitoring can be considered | Examples of situations when stopping the MRI monitoring can be considered |
|---|---|
| In the setting of MS disease that has been relapse free and radiologically stable during repeated follow‐up without change in therapy and with clinical variables suggesting a favorable disease course | In the setting of MS disease in a patient of higher age (>55–60 years old) that has been relapse free and radiologically stable during longer time of repeated follow‐up without DMT and with clinical variables suggesting a favorable disease course |
| In the setting of RIS or CIS that has been relapse free and radiologically stable during a repeated follow‐up of 3 to 5 years without DMT | In the setting of RIS or CIS that has been relapse free and radiologically stable during a longer repeated follow‐up without DMT |
MRI, magnetic resonance imaging; MS, multiple sclerosis; RIS, radiologically isolated syndrome; DMT, disease‐modifying treatment.
As a general recommendation, patients with inflammatory active MS should be recommended periodic MRI examinations. A decision to decrease the extent of MRI monitoring should be based on an individual risk versus benefit assessment. Factors that influence the activity, such as disease course, previous disease activity, DMT status, and age, should be considered. Table 3 gives examples on specific clinical situations where the workshop delegates reached consensus to recommend decreased MRI monitoring.
3.7. Administration of gadolinium‐based contrast agent
Detection of gadolinium‐based contrast agent (GBCA) enhancing lesions on T1‐weighted images suggests that focal inflammatory activity with disruption of the blood–brain barrier has occurred during the last few months.41 This is of great importance for demonstrating dissemination in time when MS diagnosis is considered. In the setting of disease monitoring of confirmed MS, the temporal information provided by detection of GBCA enhancement is less important. The finding of a GBCA enhancing lesion on T1‐weighted images is often accompanied by a corresponding lesion detectable on T2‐weighted images.42 Based on this, we assess the value of GBCA administration as lower in the setting of disease monitoring as compared to the diagnostic MRI. However, it cannot be ruled out that GBCA administration may increase sensitivity for new disease activity even at routine follow‐up MRI,42, 43 especially considering the interobserver variability of identifying new or enlarged T2 lesions.22
While short‐term use of GBCA generally appears to be safe in individuals with normal renal function,31, 44 some issues need to be mentioned. Administration of GBCA is associated with a small risk of anaphylaxis44 and development of nephrogenic systemic fibrosis (NSF). The latter has been associated especially with linear GBCA and renal dysfunction.45 To minimize risk of NSF, one of the more chemically stable macrocyclic GBCAs should be used.
Potential long‐term risks associated with repeated administration of GBCA have not been sufficiently investigated but increased signal intensity of the dentate nucleus and globus pallidus on T1‐weighted cerebral MRI scans has been reported after repeated administrations of both linear and macrocyclic GBCA and also in individuals without any major renal impairment.46, 47 The origin of this increased T1 signal has not been fully established, but histopathologic quantifications of gadolinium in brain tissues suggest deposition of gadolinium in brain tissue associated with repeated use.48 There is currently no evidence that such gadolinium deposition is harmful, but our opinion is that a cautionary approach is warranted, as gadolinium is toxic in its non‐chelated form.45 For this reason, we suggest that in patients free from relapses and MRI lesions for at least 5 years, MRI could be performed without administration of GBCA. However, if clinical or radiological signs of new inflammatory activity occur, or the patient's treatment strategy is changed, a new MRI including administration of GBCA should be considered.
3.8. What information should be included in the patient referral for MRI and the report from the neuroradiologist?
The information included in the patient referral for the MRI and the subsequent report written by the neuroradiologist is the cornerstones of the communication between the neurologist and the neuroradiologist. Table 4 includes examples of information that is of particular importance in this communication. Table 5 includes examples of templates that can be used for the radiological report.
Table 4.
Recommended information to be included in the referral and radiological report
| Diagnostic MRI | Routine follow‐up MRI | |
|---|---|---|
| Referral text | The main goal is to communicate the information that the neuroradiologist and the MRI staff will need to be able to correctly prioritize and plan the MRI examination, as well as to provide a context for the neuroradiologist to use as a base for the report.Important information in the referral includes:
|
The main goal is to communicate the current clinical situation and clinical signs of disease activity, if any. In the case of clinically stable disease, a brief summary is often adequate.Important information in the referral includes:
|
| Radiological report | The main goal is to communicate the radiological information that is needed to plan the future care of the patient and to decide whether or not an MS diagnosis should be made.Important information in the report includes:
|
The main goal is to communicate the radiological information that is needed to plan the future care of the patient.Important information in the report includes:
|
GBCA, gadolinium‐based contrast agent; MRI, magnetic resonance imaging; MS, multiple sclerosis; RIS, radiologically isolated syndrome; DMT, disease‐modifying treatment; PML, progressive multifocal leukoencephalopathy.
Table 5.
Examples of templates for the radiological report
| Examples of templates for radiological reports of MRI examination of a patient with MS | |
|---|---|
| Diagnostic MRI | Follow‐up MRI |
MRI of the brain with and without intravenous gadolinium‐based contrast agent administrationDate of earlier examination used for comparison (if applicable):Number of T2 lesions in the brain (0, 1–9, 10–20, >20):Brain region:
|
MRI of the brain with intravenous gadolinium‐based contrast agent administrationDate of earlier examination used for comparison:Number of T2 lesions in the brain (0, 1–9, 10–20, >20):Number of new or enlarged T2 lesions in the brain:Number of contrast‐enhancing lesions in the brain:
|
MRI, magnetic resonance imaging; MS, multiple sclerosis; RIS, radiologically isolated syndrome.
3.9. Future perspectives
The MAGNIMS collaboration has recently published suggestions for adjustments34 to the current McDonald criteria for the diagnosis of MS, last revised in 2010.5 Some important points in the suggested adjustments are the recommendations to:
add the optic nerve as another individual area to consider for the criteria of dissemination in space;
require three periventricular lesions for the dissemination in space to the periventricular space to be fulfilled;
expand the juxtacortical area to the juxtacortical/cortical area and consider cortical lesions in the fulfillment of the criteria for dissemination in space;
treat all lesions equally, regardless of if they are symptomatic or not;
use the same radiological criteria to diagnose RIS as is used for MS;
use the same radiological criteria for dissemination in space for primary progressive MS as for relapsing remitting MS.
Until further considerations have been made regarding the effect of these suggestions on the sensitivity and specificity of diagnosing MS, our consensus agreement will continue using the McDonald 2010 revision criteria. However, these suggestions warrant mentioning here as a perspective on possible future adjustments of the MS diagnostic criteria.
4. Conclusions
Examination with MRI is established as an important tool in the diagnostic workup and monitoring of disease activity in MS. Several clinically important questions regarding the use of MRI in MS currently have no clear evidence‐based answers. We used a consensus approach to provide practical recommendations for several such clinical issues. The Swedish consensus panel could agree upon a minimal set of MRI sequences for diagnostic as well as follow‐up examinations. The consensus panel recommends that patients with inflammatory active MS should be investigated with repeated MRI examinations. In patients with stable disease, MRI can be performed less frequently and omission of GBCA administration can be considered. The use of structured referrals and reports is recommended to facilitate communication between neurologists and neuroradiologists. Guidelines such as these have the potential to improve health care, and we hope that these recommendations will benefit individuals with MS as well as their medical caregivers.
Funding
Economical support to cover the expense for open access publication and proofreading was received from the Swedish Multiple Sclerosis Association.
Conflict of interest
Mattias Vågberg has received unconditional research grants and lecture honoraria from BiogenIdec AB and Neuro Sweden; has received travel grants from BiogenIdec AB, Novartis, and Baxter Medical AB; has received writing honoraria from Pharma Industry and BestPractice Multiple Sclerosis. Markus Axelsson has received compensation for lectures and/or advisory boards from Biogen, Genzyme, and Novartis. Richard Birgander has received lecture honoraria from BiogenIdec. Joachim Burman has received travel support and/or lecture honoraria from Almirall, Biogen, Genzyme a Sanofi Company, Hospira, and Merck Serono; has received unconditional research grants from Biogen and Merck Serono. Carmen Cananau has nothing to disclose. Yngve Forslin has nothing to disclose. Tobias Granberg has nothing to disclose. Martin Gunnarsson has served on the advisory board for Teva and received travel funding and/or speaker honoraria from BiogenIdec, Novartis, Merck Serono, and Bayer Schering Pharma. Anders von Heijne has received travel support and lecture honoraria from Roche and Biogen. Lars Jönsson has nothing to disclose. Virginija Danylaitė Karrenbauer has received unrestricted research grants from BiogenIdec AB, Novartis, and Neuro Sweden; has received lecture honoraria from Novartis; has received travel grants from BiogenIdec AB, Novartis, and Merc‐Serono. Elna‐Marie Larsson has received lecture honoraria from Bayer and Roche and research support from Bayer. Thomas Lindqvist has nothing to disclose. Jan Lycke has received travel support and/or lecture honoraria from Biogen, Novartis, Teva, Almirall and Sanofi Genzyme; has served on scientific advisory boards for Almirall, Teva, Biogen, Novartis and Sanofi Genzyme; and has received unconditional research grants from Biogen, Novartis, and Teva. Lucas Lönn has nothing to disclose. Eleni Mentesidou has nothing to disclose. Susanne Müller has nothing to disclose. Petra Nilsson has received travel support from Bayer Schering Pharma, Merck Serono, Biogen, and Genzyme a Sanofi Company; has received honoraria for lectures and advisory boards from Merck Serono and Genzyme a Sanofi Company, advisory boards for Novartis and Roche, lectures for Biogen; and has received unrestricted grants from Biogen. Fredrik Piehl has received unrestricted academic research grants from Biogen, Genzyme, and Novartis; has received travel support and/or compensation for lectures and/or participation in advisory boards from Biogen, Merckserono, Novartis, Genzyme, and Teva, which have been exclusively used for the support of research activities. Anders Svenningsson has served on the advisory board for Sanofi Genzyme and has received travel funding from Biogen Idec, Novartis, and Baxter Medical. Magnus Vrethem has received an unrestricted grant for research from Biogen and Novartis, and speaker honoraria from Biogen, Merck Serono, and Genzyme. Johan Wikström has nothing to disclose.
Acknowledgments
The authors would like to thank all the referral parties for their important input in shaping the current consensus document. We would also like to thank the Swedish Multiple Sclerosis Association for the financial support to facilitate proofreading and open access publication. We would like to thank Ellen Iacobaeus and Maria Kristoffersen Wiberg for critical reading of the manuscript.
Vågberg, M. , Axelsson, M. , Birgander, R. , Burman, J. , Cananau, C. , Forslin, Y. , Granberg, T. , Gunnarsson, M. , von Heijne, A. , Jönsson, L. , Karrenbauer, V. D. , Larsson, E.‐M. , Lindqvist, T. , Lycke, J. , Lönn, L. , Mentesidou, E. , Müller, S. , Nilsson, P. , Piehl, F. , Svenningsson, A. , Vrethem, M. and Wikström, J. (2017), Guidelines for the use of magnetic resonance imaging in diagnosing and monitoring the treatment of multiple sclerosis: recommendations of the Swedish Multiple Sclerosis Association and the Swedish Neuroradiological Society. Acta Neurologica Scandinavica, 135: 17–24. doi: 10.1111/ane.12667
References
- 1. Lobentanz IS, Asenbaum S, Vass K, et al. Factors influencing quality of life in multiple sclerosis patients: disability, depressive mood, fatigue and sleep quality. Acta Neurol Scand. 2004;110:6–13. [DOI] [PubMed] [Google Scholar]
- 2. Svenningsson A, Salzer J, Vagberg M, Sundstrom P, Svenningsson A. Increasing prevalence of multiple sclerosis in Vasterbotten County of Sweden. Acta Neurol Scand. 2015;132:389–394. [DOI] [PubMed] [Google Scholar]
- 3. Ahlgren C, Oden A, Lycke J. High nationwide prevalence of multiple sclerosis in Sweden. Mult Scler. 2011;17:901–908. [DOI] [PubMed] [Google Scholar]
- 4. Ahlgren C, Oden A, Lycke J. High nationwide incidence of multiple sclerosis in Sweden. PLoS ONE. 2014;9:e108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piehl F. A changing treatment landscape for multiple sclerosis: challenges and opportunities. J Intern Med. 2014;275:364–381. [DOI] [PubMed] [Google Scholar]
- 7. Vidal‐Jordana A, Sastre‐Garriga J, Rovira A, Montalban X. Treating relapsing‐remitting multiple sclerosis: therapy effects on brain atrophy. J Neurol. 2015;262:2617–2626. [DOI] [PubMed] [Google Scholar]
- 8. Calabresi PA, Kieseier BC, Arnold DL, et al. Pegylated interferon beta‐1a for relapsing‐remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double‐blind study. Lancet Neurol. 2014;13:657–665. [DOI] [PubMed] [Google Scholar]
- 9. Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing‐remitting multiple sclerosis (FREEDOMS II): a double‐blind, randomised, placebo‐controlled, phase 3 trial. Lancet Neurol. 2014;13:545–556. [DOI] [PubMed] [Google Scholar]
- 10. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first‐line treatment for patients with relapsing‐remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828. [DOI] [PubMed] [Google Scholar]
- 11. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease‐modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–1839. [DOI] [PubMed] [Google Scholar]
- 12. Comi G, Jeffery D, Kappos L, et al. Placebo‐controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med. 2012;366:1000–1009. [DOI] [PubMed] [Google Scholar]
- 13. Confavreux C, O'Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurol. 2014;13:247–256. [DOI] [PubMed] [Google Scholar]
- 14. Fox RJ, Miller DH, Phillips JT, et al. Placebo‐controlled phase 3 study of oral BG‐12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367:1087–1097. [DOI] [PubMed] [Google Scholar]
- 15. Gold R, Kappos L, Arnold DL, et al. Placebo‐controlled phase 3 study of oral BG‐12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–1107. [DOI] [PubMed] [Google Scholar]
- 16. Kappos L, Radue EW, O'Connor P, et al. A placebo‐controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. [DOI] [PubMed] [Google Scholar]
- 17. Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R. Three times weekly glatiramer acetate in relapsing‐remitting multiple sclerosis. Ann Neurol. 2013;73:705–713. [DOI] [PubMed] [Google Scholar]
- 18. O'Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–1303. [DOI] [PubMed] [Google Scholar]
- 19. Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo‐controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 20. Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta‐1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–923. [DOI] [PubMed] [Google Scholar]
- 21. Rovira A, Wattjes MP, Tintore M, et al. Evidence‐based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis‐clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11:471–482. [DOI] [PubMed] [Google Scholar]
- 22. Erbayat Altay E, Fisher E, Jones SE, Hara‐Cleaver C, Lee JC, Rudick RA. Reliability of classifying multiple sclerosis disease activity using magnetic resonance imaging in a multiple sclerosis clinic. JAMA Neurol. 2013;70:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wattjes MP, Rovira A, Miller D, et al. Evidence‐based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis – establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11:597–606. [DOI] [PubMed] [Google Scholar]
- 24. Webpage of The Swedish Multiple Sclerosis Association (http://www.mssallskapet.se/). Accessed April 20, 2016.
- 25. Webpage of The Swedish Neuroradiological Society (http://www.sfnr.org/). Accessed April 20, 2016.
- 26. Vågberg M, Birgander R, Burman J , et al. Användande av MR för diagnos och uppföljning av MS ‐ Rådgivande dokument utformat av Svenska MS‐sällskapets MR‐utskott i samarbete med Svensk Förening för Neuroradiologi (http://www.mssallskapet.se/Start_files/160214_Konsensusdokument_MR_1.pdf). Accessed April 20, 2016.
- 27. Cotton F, Kremer S, Hannoun S, Vukusic S, Dousset V, Imaging Working Group of the Observatoire Francais de la Sclerose en P . OFSEP, a nationwide cohort of people with multiple sclerosis: consensus minimal MRI protocol. J Neuroradiol. 2015;42:133–140. [DOI] [PubMed] [Google Scholar]
- 28. Filippi M, Rocca MA, Bastianello S, et al. Guidelines from The Italian Neurological and Neuroradiological Societies for the use of magnetic resonance imaging in daily life clinical practice of multiple sclerosis patients. Neurol Sci. 2013;34:2085–2093. [DOI] [PubMed] [Google Scholar]
- 29. Traboulsee A, Letourneau‐Guillon L, Freedman MS, et al. Canadian expert panel recommendations for MRI use in MS diagnosis and monitoring. Can J Neurol Sci. 2015;42:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Traboulsee A, Simon JH, Stone L, et al. Revised recommendations of the consortium of MS Centers Task Force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow‐up of multiple sclerosis. AJNR Am J Neuroradiol. 2016;37:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Silver NC, Good CD, Sormani MP, et al. A modified protocol to improve the detection of enhancing brain and spinal cord lesions in multiple sclerosis. J Neurol. 2001;248:215–224. [DOI] [PubMed] [Google Scholar]
- 32. Thorpe JW, Kidd D, Moseley IF, et al. Serial gadolinium‐enhanced MRI of the brain and spinal cord in early relapsing‐remitting multiple sclerosis. Neurology. 1996;46:373–378. [DOI] [PubMed] [Google Scholar]
- 33. Wiebe S, Lee DH, Karlik SJ, et al. Serial cranial and spinal cord magnetic resonance imaging in multiple sclerosis. Ann Neurol. 1992;32:643–650. [DOI] [PubMed] [Google Scholar]
- 34. Filippi M, Rocca MA, Ciccarelli O, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016;15:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79:950–958. [DOI] [PubMed] [Google Scholar]
- 36. Wattjes MP, Wijburg MT, Vennegoor A, et al. Diagnostic performance of brain MRI in pharmacovigilance of natalizumab‐treated MS patients. Mult Scler. 2016;22:1174–1183. [DOI] [PubMed] [Google Scholar]
- 37. Fine AJ, Sorbello A, Kortepeter C, Scarazzini L. Progressive multifocal leukoencephalopathy after natalizumab discontinuation. Ann Neurol. 2014;75:108–115. [DOI] [PubMed] [Google Scholar]
- 38. Plavina T, Subramanyam M, Bloomgren G, et al. Anti‐JC virus antibody levels in serum or plasma further define risk of natalizumab‐associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wattjes MP, Vennegoor A, Steenwijk MD, et al. MRI pattern in asymptomatic natalizumab‐associated PML. J Neurol Neurosurg Psychiatry. 2015;86:793–798. [DOI] [PubMed] [Google Scholar]
- 40. Tremlett H, Zhao Y, Joseph J, Devonshire V, Neurologists UC. Relapses in multiple sclerosis are age‐ and time‐dependent. J Neurol Neurosurg Psychiatry. 2008;79:1368–1374. [DOI] [PubMed] [Google Scholar]
- 41. He J, Grossman RI, Ge Y, Mannon LJ. Enhancing patterns in multiple sclerosis: evolution and persistence. AJNR Am J Neuroradiol. 2001;22:664–669. [PMC free article] [PubMed] [Google Scholar]
- 42. Rovaris M, Mastronardo G, Prandini F, Bastianello S, Comi G, Filippi M. Short‐term evolution of new multiple sclerosis lesions enhancing on standard and triple dose gadolinium‐enhanced brain MRI scans. J Neurol Sci. 1999;164:148–152. [DOI] [PubMed] [Google Scholar]
- 43. Miller DH, Barkhof F, Nauta JJ. Gadolinium enhancement increases the sensitivity of MRI in detecting disease activity in multiple sclerosis. Brain. 1993;116(Pt 5):1077–1094. [DOI] [PubMed] [Google Scholar]
- 44. Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol. 2011;196:W138–W143. [DOI] [PubMed] [Google Scholar]
- 45. Thomsen HS, Morcos SK, Almen T, et al. Nephrogenic systemic fibrosis and gadolinium‐based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2013;23:307–318. [DOI] [PubMed] [Google Scholar]
- 46. Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1‐weighted MR images: relationship with increasing cumulative dose of a gadolinium‐based contrast material. Radiology. 2014;270:834–841. [DOI] [PubMed] [Google Scholar]
- 47. Stojanov DA, Aracki‐Trenkic A, Vojinovic S, Benedeto‐Stojanov D, Ljubisavljevic S. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing‐remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium‐based contrast agent, gadobutrol. Eur Radiol. 2016;26:807–815. [DOI] [PubMed] [Google Scholar]
- 48. McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast‐enhanced MR imaging. Radiology. 2015;275:772–782. [DOI] [PubMed] [Google Scholar]


