Abstract
BACKGROUND
The impact of cancer on socioeconomic outcomes is attracting attention as the number of survivors of cancer in young age continues to rise. This study examines economic independence in a national cohort of survivors of cancer at a young age in Norway.
METHODS
Through the linkage of several national registries, the study cohort comprised 1,212,013 individuals born in Norway during 1965 through 1985, of which 5440 had received a cancer diagnosis before age 25 years. Follow‐up was through 2007, and the main outcomes were receipt of governmental financial assistance, employment, income, and occupation. Analytic methods included Cox proportional hazard regression, log‐binomial regression, and quantile regression models.
RESULTS
Individuals in the cancer survivor group had an increased probability of receiving governmental financial assistance (men: hazard ratio [HR], 1.4; 95% confidence interval [CI], 1.3‐1.5; women: HR, 1.5; 95% CI, 1.3‐1.6) and of not being employed (men: HR, 1.4; 95% CI, 1.2‐1.7; women: HR, 1.4; 95% CI, 1.2‐1.6) compared with those in the noncancer group. Income discrepancies were particularly pronounced for survivors of central nervous system tumors. There was no difference in representation in higher skilled occupations.
CONCLUSIONS
Survivors of cancer at a young age in Norway had an increased risk of being economically dependent and unemployed. This was evident in several tumor groups and was most pronounced in female survivors. There were only small differences in income or representation in higher skilled occupations for most employed survivors compared with the noncancer group. The current results are important for understanding the impact of a cancer diagnosis at a young age on subsequent job market outcomes. Cancer 2016;122:3873–3882. © 2016 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Keywords: adolescent, cancer, childhood, cohort studies, employment, income, occupations, socioeconomic factors, survivors, young adult
Short abstract
Cancer during childhood, adolescence, and young adulthood can influence subsequent economic and occupational outcomes. In a national cohort study from Norway, increased economic dependence and unemployment is demonstrated; however, overall, there are no substantial differences in income or occupational fields among survivors of cancer diagnosed before age 25 years when compared with cancer‐free references except for survivors of central nervous system tumors.
INTRODUCTION
During the last 4 decades, the treatment of cancer in young individuals has improved substantially and has led to an expanding number of survivors in the adult population.1 As many as two‐thirds of these cancer survivors suffer from a variety of late effects and chronic conditions, possibly affecting their ability to fully participate in the job market.1, 2
Previous studies have demonstrated that subgroups of childhood cancer survivors (CCS) (typically ages birth to 14 years, and sometimes up to age 20 years, at diagnosis) have an increased risk of being unemployed, earning low incomes, and receiving social security benefits compared with their siblings or the general population.3, 4, 5 However, there are important discrepancies regarding the vocational and financial outcomes of cancer survivors who are diagnosed at a young age in Europe and the United States. In a meta‐analysis, the unemployment risk among CCS in the United States is found to be tripled, but the results from European studies diverged.3, 4, 6, 7 Reports from the Childhood Cancer Survivor Study in the United States indicate that CCS have reduced personal incomes and a higher representation in lower skilled jobs compared with their siblings as well as an increased risk of unemployment, particularly for survivors of brain and bone tumors and in those diagnosed before age 4 years.7, 8 Previous Nordic studies of cancer survivors revealed only modest reductions in income compared with siblings or the cancer‐free general population, but those studies are limited because they included only selected cancer diagnoses, or cancers diagnosed at all ages, or childhood cancers only.4, 6, 9 A recent Swiss study indicated that CCS have lower monthly incomes compared with their siblings, but it did not assess occupation or compensatory financial assistance (FA) measures and was questionnaire based; therefore, it may have been subject to response bias.10 The enrollment in governmental supplemental security income and disability insurance programs is increased for CCS in both Norway and the United States.5, 11 The total impact of a cancer diagnosis during the vulnerable developmental period of childhood, adolescence, and young adulthood on later economic independence largely has been unexplored.
Survivors of cancers diagnosed in adolescence and young adulthood (typically ages 15‐29 years, and sometimes up to age 39 years, at cancer diagnosis) are faced with particular survivorship challenges, because their diagnoses and treatments occur in a different psychosocial and biologic context from those in survivors who are diagnosed at younger or older ages.12 However, most research in this population originates from publications in which adolescents and young adults (AYAs) are only a small percentage of the much larger adult survivor group, and comparison groups often are limited.
The developmental period of adolescence and young adulthood poses unique challenges, and important information regarding economic and work‐related matters in AYA cancer survivors is currently lacking.12, 13, 14 This is a time when individuals seek independence from their parents and develop autonomy through relationship building with peers and through making important, often permanent decisions regarding higher education and pursuits of a prospective career. It is also a time of continuous physiologic development, including maturation of the prefrontal cortex, which is important for executive functions and the implementation of goal‐oriented behaviors.14, 15 Disruptions to this brittle process may lead to unfavorable decision making that could have long‐lasting implications. Having to cope with a life‐threatening cancer diagnosis during adolescence and young adulthood is a potent intrusion during this already challenging phase of life, and AYAs with cancer report financial difficulties, disruptions in social relationships, and employment challenges, especially when they are diagnosed during early adolescence and young adulthood.15, 16 Therefore, it is of utmost importance to study the work‐related outcomes in this group and to develop targeted interventions, including vocational rehabilitation programs, for future AYA cancer survivors.
AYA cancer survivors are difficult to track in a clinic‐based setting because of a combination of high mobility (relocating to study/work), treatment and follow‐up at both pediatric and adult cancer departments, and often a reduced interest and/or lack of opportunity to participate in follow‐up care programs.13 Thus there is a need for large, population‐based studies with national coverage, including noncancer comparisons, to address the full impact of economic late effects in CCS and AYA cancer survivor populations. Such studies may aid in the development of risk‐based follow‐up strategies (including interventions) during the transition into and passage through adulthood. The objective of the current study was investigate economic independence by studying employment, occupation, income, and the need of governmental FA in a large, population‐based Norwegian cohort of cancer survivors who were diagnosed before age 25 years.
MATERIALS AND METHODS
The study cohort included all individuals born alive in Norway during 1965 through 1985 identified through the national registry. Patients with cancer were identified through the Cancer Registry of Norway (CRN) (exclusions were made if a cancer diagnosis was made by autopsy only or if the basis for the cancer diagnosis was uncertain). Follow‐up was through 2007, at which time the cohort members had reached ages 22 to 42 years. The term “cancer survivor” in our study comprises all individuals who had a cancer diagnosis before age 25 years.
Data Sources
We linked several national registries in Norway using the unique 11‐digit personal identification number assigned to every resident. The national registry contains updated demographic information on all residents in Norway.17 Reporting newly diagnosed cancer cases to the CRN has been compulsory for all clinicians and pathologists since 1951. The quality and completeness of CRN data have been identified as high, in line with other Western European cancer registries.18, 19 The CRN provides information on cancer cases, including date of diagnosis, cancer site, and tumor morphology.19 Information on demographics, FA, income, employment, and disability pensions (DPs) was provided by Statistics Norway.20, 21 Data on education were provided by the Norwegian National Education Database.22
Study Outcomes
Governmental FA is a benefit available to all legal residents of Norway. It is intended as a temporary measure when all other means of self‐support have been exhausted and is independent of prior work history and other social security benefits.23 The local Labor and Welfare Service (NAV) office makes a monthly individual assessment on the amount needed for necessary subsistence costs.
For information on income and employment in 2007, only “work‐related income” was used, which includes income from work only (employed or self‐employed), and not income from social security benefits (including DPs). This includes any degree of work‐related income. Thus all individuals with some form of employment are included in the income analyses, and those with no income are excluded. For the analysis on employment, we defined unemployment as not having registered a work‐related income in 2007. To qualify for a DP in Norway, an individual's earning capacity has to be permanently reduced by at least 50% because of illness and/or injury, and vocational rehabilitation measures have to be completed.
To analyze fields of occupation, we used the occupational codes according to the International Standard Classification of Occupations (ISCO‐88), which reflect the skill level required for the occupation category.24 Occupation was classified into 4 categories; “unskilled” (ISCO group 9), “semiskilled blue collar” (ISCO groups 6‐8; agriculture, craft, machine operators), “semiskilled white collar” (ISCO groups 4 and 5; clerks, service, and sales), and “skilled” (ISCO groups 1‐3; managers, professionals, and technicians), in accordance with recent European Union classification standards.25
Statistical Analyses
For the outcome of governmental FA, an extended Cox regression model was applied to the whole cohort, with age at cancer diagnosis as the time‐dependent variable, yielding hazard ratios (HRs) with 95% confidence intervals (CIs). This method was chosen to fully take advantage of the prospective nature of the data and to account for the changes in hazard rates during the course of follow‐up. The follow‐up for this analysis started at age 18 years (parents are obliged by law to sustain their children until that age) and ended at the date of the first occasion an individual received FA, censoring at the date of death, emigration, or December 31, 2007, whichever occurred first.
To analyze income and employment, a cross‐sectional analysis for those who were alive and living in Norway in 2007 was performed using binomial logistic regression and quantile regression models, yielding relative risks (RRs) with 95% CIs and regression coefficients with P values, respectively, comparing the cancer survivors with the cancer‐free group. High income was defined as income >80th percentile of that for all individuals born in the same year and with the same sex, and low income was defined correspondingly as income <20th percentile. After testing for interaction, separate analyses of male and female survivors were conducted, conforming to recommendations for studies on labor market outcomes in cancer survivors.26
To study occupational fields, multinomial logistic regression was applied using “skilled” as the reference category, yielding RR ratios (RRR) with 95% CIs. Linear regression analysis of differences in income within the occupational categories was performed (after the exclusion of outliers).
Cancer survivors were further categorized into major cancer groups (leukemia, lymphoma, central nervous system [CNS] tumors, testicular tumors, malignant melanoma, bone and soft tissue sarcomas, cancers of the female genital tract [cervix/uterus/ovarian], and “other”). Survivors also were classified into CCS (those aged < 15 years at cancer diagnosis) and AYAs (ages 15‐24 years at cancer diagnosis). Analyses were adjusted for year of birth and for parental education (the highest education achieved by both parents) divided into 3 categories—lower education (<11 years), intermediate education (11‐14 years), and tertiary education (>14 years)—to account for differences in household socioeconomic status as a possible confounder.27 Marital status was included as a mediator in early analyses but was not included in the final model, because the estimates produced were similar, and adjusting for this variable could have introduced collider‐stratification bias.
We wanted to determine the impact of a cancer diagnosis on survivors who were healthy enough to work; thus, we excluded DP recipients from all analyses except for the analysis on FA, for which the results with and without DP recipients are presented.
SPSS version 21 (IBM SPSS, Armonk, NY) and STATA version 14 (StataCorp LP, College Station, Tex) were used for statistical analyses. The study was approved by the Norwegian Data Protection Authority and the Regional Committee for Medical and Health Research Ethics of Western Norway.
RESULTS
In total, 1,218,013 individuals were born in Norway during 1965 through 1985. Of these individuals, 5440 were diagnosed with cancer before age 25 years and were included in the FA analysis. After the exclusion of those who emigrated (n = 34,840; 1.3% of the cancer survivor group, 2.9% of the noncancer control group), died (n = 34,774), were lost to follow‐up (n = 719; all in the noncancer group), and were missing residential code (n = 1226; all in the noncancer group), 1146,444 individuals were alive and still living in Norway in 2007, including 3945 cancer survivors (2170 men and 1775 women). Approximately 40% of those survivors were diagnosed as children, and the remaining were diagnosed as AYAs (Table 1). The largest cancer site groups were CNS tumors (20%), leukemia (16%), testicular cancer (14%), and lymphoma (13%).
Table 1.
Characteristics of Cancer Survivors by Cancer Site Stratified by Sex, Age, and Period of Cancer Diagnosis
| No. (%) | |||||
|---|---|---|---|---|---|
| Age at Cancer Diagnosis | |||||
| 0‐14 Years | 15‐24 Years | ||||
| Cancer Sitea | Men | Women | Men | Women | Total |
| Leukemia | 343 (28.8) | 290 (30.7) | 128 (6.8) | 84 (5.9) | 845 (15.5) |
| Lymphoma | 122 (10.2) | 57 (6) | 313 (16.8) | 229 (16) | 721 (13.3) |
| CNS tumors | 321 (26.9) | 222 (23.5) | 272 (14.6) | 259 (18.1) | 1074 (19.7) |
| Testicular cancer | 43 (3.6) | 695 (37.2) | 738 (13.6) | ||
| Malignant melanoma | 14 (1.2) | 25 (2.6) | 117 (6.2) | 323 (22.5) | 479 (8.8) |
| Bone and soft tissue tumors | 110 (9.2) | 111 (11.7) | 169 (9.1) | 105 (7.3) | 495 (9.1) |
| Female genital tract tumors | 25 (2.6) | 143 (10) | 168 (3.1) | ||
| Other | 240 (20.1) | 216 (22.8) | 174 (9.3) | 290 (20.2) | 920 (16.9) |
| All cancer | 1193 (100) | 946 (100) | 1868 (100) | 1433 (100) | 5440 (100) |
Abbreviation: CNS, central nervous system.
Cancer sites are based on International Classification of Diseases (7th edition) site codes and on Manual of Tumor Nomenclature and Coding and International Classification of Diseases for Oncology (2nd edition) morphology codes.
FA
For all cancers combined, there was an increased risk of receiving governmental FA for both male and female survivors (men: HR, 1.4; 95% CI, 1.3‐1.5; women: HR, 1.5; 95% CI, 1.3‐1.6) (Table 2). Excluding individuals who were receiving a DP (n = 33,408) yielded smaller but nonetheless significantly increased risks (Table 2). In particular, survivors of leukemia (women), lymphoma, CNS tumors, testicular cancer (men), and bone and soft tissue sarcomas were at increased risk of receiving FA, whereas female survivors of malignant melanoma had a reduced risk (Table 2). The mean age at the first receipt of FA was 23.1 years for the noncancer controls and 22.6 years for the cancer survivors (P < .001). The mean age was similar when survivors were stratified by sex, age at diagnosis, and cancer type (Table 2). When we reran the models censoring the individuals who had received a cancer diagnosis after age 25 years, the results were similar.
Table 2.
Hazard Ratios and 95% Confidence Intervals for Receipt of Governmental Financial Assistance in Cancer Survivors, by Cancer Site and Age at Diagnosis, Compared With Cancer‐Free Individuals
| Men | Women | ||||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||||||
| Cancer Sitea | No. of FA Recipients | Model 1b | Model 2c | Mean Age at FA, y | Model 1b | Model 2c | Mean age at FA, y |
| Noncancer | 239,996 | Reference | Reference | 23.1 | Reference | Reference | 23.1 |
| Leukemia | 134 | 1.24 (0.95‐1.60) | 1.15 (0.86‐1.53) | 22.6 | 1.72 (1.34‐2.21) | 1.62 (1.23‐2.12) | 21.1 |
| Lymphoma | 181 | 1.43 (1.13‐1.80) | 1.37 (1.07‐1.75) | 22.4 | 2.12 (1.68‐2.70) | 2.02 (1.57‐2.62) | 21.9 |
| CNS tumors | 245 | 1.74 (1.20‐1.80) | 1.13 (0.85‐1.49) | 22.8 | 1.71 (1.40‐2.08) | 1.48 (1.15‐1.91) | 22.4 |
| Testicular cancer | 156 | 1.27 (1.05‐1.54) | 1.27 (1.04‐1.56) | 22.3 | |||
| Malignant melanoma | 73 | 1.14 (0.71‐1.83) | 1.20 (0.73‐1.95) | 24.2 | 0.63 (0.44‐0.90) | 0.65 (0.45‐0.95) | 23.1 |
| Bone/soft tissue tumors | 99 | 1.79 (1.36‐2.36) | 1.62 (1.18‐2.22) | 22.7 | 1.63 (1.20‐2.20) | 1.52 (1.08‐1.24) | 23.1 |
| Female genital tract tumors | 44 | 1.45 (0.99‐2.11) | 1.29 (0.84‐1.98) | 23.4 | |||
| Other | 168 | 1.36 (1.05‐1.75) | 1.24 (0.94‐1.65) | 23.5 | 1.28 (1.03‐1.60) | 1.28 (1.01‐1.63) | 22.6 |
| All cancer | 1100 | 1.38 (1.26‐1.51) | 1.27 (1.14‐1.41) | 22.7 | 1.45 (1.32‐1.60) | 1.36 (1.22‐1.52) | 22.4 |
| Age at cancer diagnosis, y | |||||||
| <15 | 310 | 1.16 (1.00‐1.36) | 0.98 (0.81‐1.18) | 22.8 | 1.23 (1.05‐1.45) | 1.09 (0.90‐1.32) | 22.1 |
| 15‐24 | 790 | 1.53 (1.36‐1.72) | 1.46 (1.29‐1.66) | 22.7 | 1.61 (1.43‐1.82) | 1.54 (1.35‐1.76) | 22.5 |
Abbreviations: CI, confidence interval; HR, hazard ratio; CNS, central nervous system; FA, financial assistance.
Cancer sites are based on International Classification of Diseases (7th edition) site codes and on Manual of Tumor Nomenclature and Coding and International Classification of Diseases for Oncology (2nd edition) morphology codes.
Those who were receiving disability pensions were included (adjusted for year of birth and parental education).
Those who were receiving disability pensions were excluded (adjusted for year of birth and parental education).
Employment
Cancer survivors had a 34% increased risk of not being employed (HR, 1.3; 95% CI, 1.2‐1.5) compared with cancer‐free individuals, and the results were similar separately for men and women (Table 3). There was a significantly increased risk of unemployment among survivors of lymphoma (women), CNS tumors (both sexes), testicular cancer, and bone and soft tissue cancer (men), regardless of age at diagnosis.
Table 3.
Relative Risks and 95% Confidence Intervals for Unemployment in Cancer Survivors, by Cancer Site and Age at Diagnosis, Compared With Cancer‐Free Individuals
| Men | Women | |||
|---|---|---|---|---|
| Cancer Sitea | No. Unemployed/Total No. (% Not Employed)b | RR (95% CI) | No. Unemployed/Total No. (% Not Employed)b | RR (95% CI) |
| Noncancer | 25,009/570,080 (4.4) | 1.00 (Ref) | 33,982/543,580 (6.3) | 1.00 (Ref) |
| Leukemia | 6/221 (2.7) | 0.63 (0.29‐1.39) | 17/204 (8.3) | 1.42 (0.90‐2.23) |
| Lymphoma | 17/334 (5.1) | 1.19 (0.76‐1.90) | 23/222 (10.4) | 1.72 (1.18‐2.51) |
| CNS tumors | 20/286 (7) | 1.60 (1.06‐2.44) | 36/281 (12.8) | 2.11 (1.56‐2.86) |
| Testicular cancer | 43/644 (6.7) | 1.59 (1.19‐2.12) | ||
| Malignant melanoma | 5/106 (4.7) | 1.19 (0.50‐2.80) | 13/303 (4.3) | 0.75 (0.44‐1.27) |
| Bone/soft tissue tumors | 12/152 (7.9) | 1.84 (1.07‐3.16) | 10/122 (8.2) | 1.33 (0.73‐2.41) |
| Female genital tract tumors | 12/130 (9.2) | 1.49 (0.87‐2.53) | ||
| Other | 18/270 (6.7) | 1.64 (1.05‐2.55) | 23/356 (6.5) | 0.97 (0.64‐1.49) |
| All cancers | 121/2013 (6) | 1.42 (1.20‐1.69) | 134/1618 (8.3) | 1.36 (1.16‐1.61) |
| Age at cancer diagnosis, y | ||||
| <15 | 40/621 (6.4) | 1.38 (1.12‐1.71) | 47/517 (9.1) | 1.53 (1.16‐2.01) |
| 15‐24 | 81/1392 (5.8) | 1.51 (1.12‐2.04) | 87/1101 (7.9) | 1.30 (1.06‐1.59) |
Abbreviations: CI, confidence interval; CNS, central nervous system; Ref, reference category; RR, relative risk.
Cancer sites are based on International Classification of Diseases (7th edition) site codes and on Manual of Tumor Nomenclature and Coding and International Classification of Diseases for Oncology (2nd edition) morphology codes.
Those who were receiving disability pensions were excluded (adjusted for year of birth and parental education).
Income
The median income for the male CCS in the cohort was 366,369 Norwegian kroner (NOK) (equivalent to $62,283 US dollars [USD], according to the annual conversion rate of 0.17 in 200728). For men in the noncancer comparison group, the median income was 379,794 NOK ($64,565 USD). For female cancer survivors, the median income was 259,088 NOK ($44,045 USD) compared with 272,077 NOK ($46,253 USD) in the cancer‐free female comparison group (Table 4). In general, the cancer survivors had lower median incomes than individuals in the noncancer comparison group (Fig. 1), although the difference was not statistically significant (men, P = .07; women, P = .28). Survivors of CNS tumors had significantly reduced incomes across all quantiles (data not shown) and an increased risk of being in the low‐income category versus cancer‐free controls with the same birth year and sex (men: RR, 1.3; 95% CI, 1.1‐1.6; women: RR, 1.4; 95% CI, 1.1‐1.7) (Table 4). In the female survivor group, there was also an increased risk of low income for survivors of lymphoma (HR, 1.4; 95% CI, 1.1‐1.7) and an increased probability of high income (and higher annual salaries across all quantiles) for survivors of malignant melanoma (HR, 1.3; 95% CI, 1.1‐1.6). The median income was significantly reduced for male survivors who were diagnosed in childhood (<15 years of age) and, to a lesser degree, for AYA cancer survivors (Table 4). Adjustment for parental education did not change the estimates significantly.
Table 4.
Work‐Related Income in Cancer Survivors, by Cancer Site and Age at Diagnosis, Compared With Cancer‐Free Individuals
| RR (95% CI) | |||||
|---|---|---|---|---|---|
| Variable | No. of Individualsa | Median Income, NOKb | P c | Low Incomed | High Incomee |
| Men | |||||
| Noncancer | 543,788 | 379,794 | Ref | 1.00 (Ref) | 1.00 (Ref) |
| All cancer | 1884 | 366,369 | .07 | 1.06 (0.98‐1.16) | 0.92 (0.84‐1.01) |
| Cancer sitef | |||||
| Leukemia | 215 | 350,265 | .72 | 1.11 (0.86‐1.43) | 0.83 (0.62‐1.12) |
| Lymphoma | 317 | 368,100 | .34 | 1.08 (0.88‐1.34) | 1.00 (0.80‐1.25) |
| CNS tumors | 263 | 326,066 | .01 | 1.33 (1.09‐1.63) | 0.71 (0.53‐0.96) |
| Testicular cancer | 598 | 378,767 | .92 | 0.92 (0.77‐1.09) | 0.95 (0.80‐1.12) |
| Malignant melanoma | 99 | 397,139 | .38 | 0.83 (0.53‐1.30) | 1.08 (0.75‐1.56) |
| Bone/soft tissue tumors | 140 | 372,795 | .40 | 1.18 (0.88‐1.59) | 0.94 (0.67‐1.32) |
| Other | 252 | 362,391 | .35 | 1.07 (0.84‐1.36) | 1.02 (0.80‐1.31) |
| Age at cancer diagnosis. y | |||||
| <15 | 580 | 339,523 | < .01 | 1.19 (1.03‐1.38) | 0.85 (0.71‐1.02) |
| 15‐24 | 1304 | 378,934 | .64 | 1.01 (0.90‐1.13) | 0.95 (0.85‐1.06) |
| Women | |||||
| Noncancer | 508,288 | 272,077 | Ref | 1.00 (Ref) | 1.00 (Ref) |
| All cancer | 1479 | 259,088 | .28 | 1.20 (1.09‐1.31) | 0.94 (0.85‐1.05) |
| Cancer sitef | |||||
| Leukemia | 186 | 242,797 | .96 | 1.07 (0.81‐1.41) | 0.75 (0.53‐1‐05) |
| Lymphoma | 197 | 249,060 | .47 | 1.38 (1.10‐1.74) | 0.74 (0.53‐1,03) |
| CNS tumors | 244 | 222,414 | .03 | 1.38 (1.12‐1.69) | 0.81 (0.61‐1.08) |
| Malignant melanoma | 290 | 300,786 | .03 | 0.93 (0.73‐1.19) | 1.34 (1.12‐1.61) |
| Bone/soft tissue tumors | 112 | 261,811 | .90 | 1.35 (0.99‐1.83) | 0.85 (0.56‐1.28) |
| Female genital tract tumors | 118 | 276,719 | .86 | 1.27 (0.94‐1.73) | 0.95 (0.66‐1.39) |
| Other | 332 | 254,212 | .05 | 1.18 (0.96‐1.45) | 0.95 (0.75‐1.20) |
| Age at cancer diagnosis, y | |||||
| <15 | 469 | 243,655 | .17 | 1.18 (1.00‐1.39) | 0.76 (0.62‐0.94) |
| 15‐24 | 1010 | 268,911 | .80 | 1.21 (1.08‐1.35) | 1.03 (0.91‐1.16) |
Abbreviations: CI, confidence interval; CNS, central nervous system; NOK = Norwegian kroner; Ref, reference category; RR, relative risk.
Those who were receiving disability pensions were excluded.
The conversion rate in 2007 was 1 NOK = $0.17 US dollar.
P values are for differences in median income with cancer‐free individuals as the reference group (adjusted for year of birth and parental education).
Low income was defined as less than the 20th percentile of work‐related income in 2007 by year of birth and sex (adjusted for parental education).
High income was defined as greater than the 80th percentile of work‐related income in 2007 by year of birth and sex (adjusted for parental education).
Cancer sites are based on International Classification of Diseases (7th edition) site codes and on Manual of Tumor Nomenclature and Coding and International Classification of Diseases for Oncology (2nd edition) morphology codes.
Figure 1.
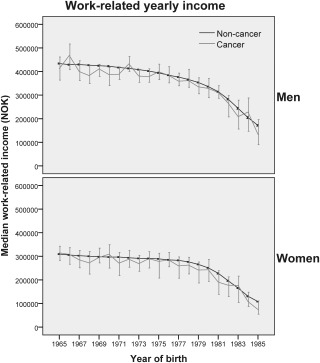
The median annual work‐related income in 2007 (in Norwegian kroner [NOK]; conversion rate, 1 NOK = $0.17 US dollars), with 95% confidence intervals, is illustrated according to birth year stratified by sex and cancer.
Occupation
The largest occupational category for both cancer survivors and cancer‐free controls was “skilled” (legislators, managers, professionals, technicians, and associate professionals), amounting to 49% and 48% in the 2 groups, respectively. By using this occupational category as reference, the cancer survivors were less likely to be in the employment category “semiskilled blue collar” (both sexes) (Table 5). An analysis by cancer site revealed a particularly low probability for this occupational category in the survivors of bone and soft tissue cancer (men) and malignant melanoma (men). There was no significant difference in occupational categories for survivors of CNS tumors (data not shown) compared with the cancer‐free reference group. Analyzing within‐group differences in income for the 4 occupational categories, the median annual salary was significantly reduced for male cancer survivors in the “skilled” occupational category (a reduction of 16,447 NOK [$2796 USD]), but no differences were observed for women or for either sex in the other 3 categories (Table 5).
Table 5.
Relative Risk Ratios for Occupational Category and Income Differences in Cancer Survivors Compared With Cancer‐Free Individuals
| No. (% Employed in Occupational Category) | RRR for Occupational Category (95% CI)a | P for Income Differencesb | ||||
|---|---|---|---|---|---|---|
| Employment Category/ISCO Group | Noncancer Controls | Cancer Survivors | Men | Women | Men | Women |
| Unskilled/ISCO 9 | 35,508 (3.9) | 109 (3.7) | 0.73 (0.54‐0.97) | 0.84 (0.60‐1.18) | .68 | .92 |
| Semiskilled blue collar/ISCO 6‐8 | 167,814 (18.3) | 531 (18) | 0.88 (0.78‐0.99) | 0.59 (0.40‐0.87) | .65 | .25 |
| Semiskilled white collar/ISCO 4 and 5 | 276,584 (30.2) | 879 (29.7) | 0.94 (0.82‐1.08) | 0.95 (0.84‐1.08) | .86 | .50 |
| Skilled/ISCO 1‐3 | 437,331 (47.7) | 1438 (49) | 1.00 (Ref) | 1.00 (Ref) | .02 | .08 |
Abbreviations: CI, confidence interval; ISCO, International Standard Classification of Occupation; Ref, reference category; RRR, relative risk ratio.
Analyses were adjusted for year of birth and parental education.
P values are for income differences within occupational categories for cancer survivors compared with noncancer controls (adjusted for year of birth and parental education).
DISCUSSION
We observed that CCS and AYA cancer survivors were at an increased risk of being financially dependent, as demonstrated by a 4‐fold to 5‐fold increased risk of receiving FA from the government as well as an increased risk of not being employed. Unfavorable outcomes were particularly prevalent in survivors of CNS tumors, lymphoma, and bone/soft tissue sarcomas; whereas survivors of malignant melanoma in general fared better. For the cancer survivors holding jobs, incomes were only slightly reduced compared with those in the cancer‐free reference group. The occupational fields were similar in the cancer survivors and the cancer‐free group, although cancer survivors were represented less in manual labor occupations. Furthermore, the median incomes within the occupational categories were largely comparable.
The increased risk of receiving FA was more pronounced in female cancer survivors and in survivors of certain cancer sites. The threshold to apply for governmental financial support may be lower for cancer survivors, and applications may be more easily approved for individuals who have a history of a cancer diagnosis. Receipt of FA also could be because of a desire not to work full‐time, or the inability to work full‐time, thus requiring supplemental income sources.29, 30 However, it is important to keep in mind that FA is a temporary measure and does not suggest long‐term financial dependency. Nonetheless, the increased use of financial assistance suggests that the economic flexibility of young cancer survivors is not optimal in Norway. Because FA is an uncertain and temporary compensatory measure, other measures probably would be more appropriate for this group if their long‐term health and welfare is to be secured.
Our finding of increased unemployment for CCS and AYA cancer survivors correlates well with some previous publications on this topic.3, 6, 7, 9, 31 Studies from the United States have indicated that poor physical health, and particularly neurocognitive deficits, is strongly associated with unemployment, and US studies have demonstrated an overall 3‐fold increased risk of unemployment in CCS.3, 29 Certain important differences were observed when we compared our results with those from a Swedish study in which there was no significant association between a previous cancer diagnosis (at age < 16 years) and not being employed.4 The different results may be because we also included AYA cancer survivors in our study. In addition, we had no information regarding students, and the Swedish study excluded individuals who were aged <25 years at follow‐up, leaving out a group particularly vulnerable to unemployment.32
Only a few studies have investigated income inequalities and occupational differences between survivors of cancer diagnosed at a young age and the general population.4, 6, 9 In our study, we examined differences not only in the median income but the whole range of income quantiles. For the most part, our results were reassuring, although cancer survivors did have slightly lower earnings compared with individuals in the noncancer group. This may be a matter of survivors choosing to work reduced hours, but it may also reflect reduced working capacity because of chronic medical conditions in the survivor group. Unfortunately, data were not available on hours worked per week; however, because of strict work discrimination laws in Norway, the most likely explanation for the reduced income among the cancer survivors is reduced working hours. We observed reduced representation in manual labor occupations in the cancer group and only slight within‐group differences in income. This is in contrast to US studies, in which CCS (ages 0‐19 years at diagnosis) were more often employed in lower skilled jobs than their siblings and had lower personal incomes within the different occupational categories.8, 29 Those studies also indicated that neurocognitive limitations, mainly as a result of cranial irradiation, are associated with employment in lower skilled jobs. This discrepancy between previous US studies and the current analysis from Norway may reflect the separation of health insurance and employment within the Norwegian system and that education is available free of charge in Norway, therefore a history of cancer does not restrict access to higher education or higher skilled occupations.
In this study, we particularly observed indications of economic dependency in survivors of lymphoma (especially women), CNS tumors, and bone and soft tissue sarcomas. Multiple publications have reported that survivors of CNS tumors suffer from adverse medical late effects, especially those who received CNS irradiation during childhood, and fall behind during education and in job market participation.2, 4, 33, 34, 35, 36 Lymphoma survivors (especially Hodgkin lymphoma) reportedly also are at increased risk of adverse long‐term outcomes, particularly heart failure (because of the widespread use of irradiation until the mid‐1990s) as well as secondary malignancies.37 These late effects are likely to influence the outcomes measured in the current study. Regarding survivors of bone cancer, although surgical techniques have improved dramatically since the 1970s, musculoskeletal morbidity is still increased, which may explain the poor work‐related outcomes in this group.2, 38 The fortunate economic outcomes of melanoma survivors in our study are probably linked to pre‐existing socioeconomic status before cancer diagnosis, because previous research has demonstrated that increased incidence is associated with higher socioeconomic class.39 In addition, melanomas in children and AYAs most frequently present as localized lesions, are treated only by surgery, and have an excellent prognosis.40
An altered association to working life may negatively affect an individual's integrity, life satisfaction, and social relationships. For individuals who are diagnosed with cancer during adolescence and young adulthood, when primary developmental tasks such as identity development, seeking independence from parents, and exploring educational and occupational paths, this may be particularly pronounced. The return to (or maintenance of) school or work for CCS and AYAs is vital if a cancer survivor is to become independent and self‐sustained as an adult.31 It has been demonstrated that vocational training and job assistance measures are associated with an increased odds of employment after cancer in AYAs.41 Therefore, identifying subgroups of CCS and AYA cancer survivors who are at risk of low job market participation is important to implement vocational rehabilitation services early for individuals in the more vulnerable survivor groups (lymphoma, CNS tumors, and bone/soft tissue sarcomas). Accounting for future education and employment within the treatment setting has also been identified as a key issue by AYA survivors along with the availability of counseling and FA.12
Strengths of this national cohort study include coverage of an entire population, without selection or recall bias, as well as minimal loss to follow‐up. Our inclusion of AYA cancer survivors provides important information on the socioeconomic outcomes in this survivor group, which is currently lacking. Challenges unique to AYA cancer survivors include increased mobility (and, consequently, increased loss to follow‐up), not wanting to partake in follow‐up care or studies, and failure to take responsibility for their own health care.13 Limitations of the study include the lack of individual treatment data, which could allow for more precise correlation of different treatment exposures with economic outcomes, as well as the lack of information on the study participants' work preferences or hours worked per week. Information on student status also was not available, and this may have an effect on our outcomes, particularly for the youngest members of our cohort. Previous research has indicated that CCS have a delay in educational accomplishments, although the final educational achievements (at least in Europe) seem to be comparable to those in controls.35, 36 In our cohort, 18% did not have an occupational code. Within the Norwegian registry system, this means that they could be students, self‐employed, or unemployed.
Knowledge of the possible disadvantageous effects after cancer in childhood, adolescence, or young adulthood on labor market outcomes and subsequent income is vital if authorities are to appropriately target subgroups in need of counseling and interventions. Norway is an egalitarian society with public health care and strongly enforced antidiscrimination laws. Public welfare interventions, such as work reintegration programs and more permanent compensation for reductions in earnings directed specifically toward CCS, may be warranted.
FUNDING SUPPORT
Maria W. Gunnes was supported by the Western Norway Regional Health Authority (grant 911612) and data linkage by the Norwegian Cancer Society.
CONFLICT OF INTEREST DISCLOSURES
Dag Moster reports grants from the Norwegian Cancer Society and from the Western Norway Regional Health Authority during the conduct of the study.
AUTHOR CONTRIBUTIONS
Maria W. Gunnes: Responsible for the overall article content; contributed to the conception, design, analysis, and interpretation of the data; and drafted and revised previous and final versions of the article. Rolv Terje Lie: Contributed to the design, analysis, and interpretation of the data; revised the article, including final approval; and was accountable for all aspects of the work. Tone Bjørge: Contributed to the study conception and design; revised the article, including final approval, and was accountable for all aspects of the work. Astri Syse: Contributed to analysis and interpretation of the data; revised the article, including final approval; and was accountable for all aspects of the work. Ellen Ruud: Contributed to the study conception and design; revised the article, including final approval; and was accountable for all aspects of the work. Finn Wesenberg: Contributed to the study conception and design, revised the article, including final approval; and was accountable for all aspects of the work. Dag Moster: Contributed to the design, analysis, and interpretation of the data; revised the article, including, final approval; and was accountable for all aspects of the work.
REFERENCES
- 1. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. [DOI] [PubMed] [Google Scholar]
- 3. de Boer AG, Verbeek JH, van Dijk FJ. Adult survivors of childhood cancer and unemployment: a metaanalysis. Cancer. 2006;107:1–11. [DOI] [PubMed] [Google Scholar]
- 4. Boman KK, Lindblad F, Hjern A. Long‐term outcomes of childhood cancer survivors in Sweden: a population‐based study of education, employment, and income. Cancer. 2010;116:1385–1391. [DOI] [PubMed] [Google Scholar]
- 5. Ghaderi S, Engeland A, Moster D, et al. Increased uptake of social security benefits among long‐term survivors of cancer in childhood, adolescence and young adulthood: a Norwegian population‐based cohort study. Br J Cancer. 2013;108:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johannesen TB, Langmark F, Wesenberg F, Lote K. Prevalence of Norwegian patients diagnosed with childhood cancer, their working ability and need of health insurance benefits. Acta Oncol. 2007;46:60–66. [DOI] [PubMed] [Google Scholar]
- 7. Pang JW, Friedman DL, Whitton JA, et al. Employment status among adult survivors in the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2008;50:104–110. [DOI] [PubMed] [Google Scholar]
- 8. Kirchhoff AC, Krull KR, Ness KK, et al. Occupational outcomes of adult childhood cancer survivors: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117:3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Syse, Astri Tretli , Steinar Kravdal O. Cancer's impact on employment and earnings—a population‐based study from Norway. J Cancer Surviv. 2008;2:149–158. [DOI] [PubMed] [Google Scholar]
- 10. Wengenroth L, Sommer G, Schindler M, et al. Income in adult survivors of childhood cancer [serial online]. PLoS One. 2016;11:e0155546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirchhoff AC, Parsons HM, Kuhlthau KA, et al. Supplemental security income and Social Security disability insurance coverage among long‐term childhood cancer survivors [serial online]. J Natl Cancer Inst. 2015;107:djv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zebrack B, Bleyer A, Albritton K, et al. Assessing the health care needs of adolescent and young adult cancer patients and survivors. Cancer. 2006;107:2915–2923. [DOI] [PubMed] [Google Scholar]
- 13. Tonorezos ES, Oeffinger KC. Research challenges in adolescent and young adult cancer survivor research. Cancer. 2011;117:2295–2300. [DOI] [PubMed] [Google Scholar]
- 14. Zebrack B, Isaacson S. Psychosocial care of adolescent and young adult patients with cancer and survivors. J Clin Oncol. 2012;30:1221–1226. [DOI] [PubMed] [Google Scholar]
- 15. Prasad PK, Hardy KK, Zhang N, et al. Psychosocial and neurocognitive outcomes in adult survivors of adolescent and early young adult cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2015;33:2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Warner EL, Kent EE, Trevino KM, et al. Social well‐being among adolescents and young adults with cancer: a systematic review. Cancer. 2016;122:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Norwegian Tax Administration . National Registry. Available at: http://www.skatteetaten.no/en/Person/National-Registry/This-is-the-National-Registry/. Accessed April 12, 2016.
- 18. Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999‐2007: results of EUROCARE‐5—a population‐based study. Lancet Oncol. 2014;15:35–47. [DOI] [PubMed] [Google Scholar]
- 19. Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45:1218–1231. [DOI] [PubMed] [Google Scholar]
- 20. Statistics Norway . FD‐Trygd List of Variables. Available at: https://www.ssb.no/en/sosiale-forhold-og-kriminalitet/artikler-og-publikasjoner/fd-trygd. Accessed April 12, 2016.
- 21. Statistics Norway . Income and Wealth Statistics for Households, 2014. Available at: https://www.ssb.no/en/inntekt-og-forbruk/statistikker/ifhus/aar/2015-0000-0000?fane=om#content. Accessed April 12, 2016.
- 22. Statistics Norway . About the National Education Database. Available at: http://www.ssb.no/a/english/mikrodata/datasamling/nudb/nudb_20130607-en.html. Accessed April 12, 2016.
- 23. Labour and Welfare Administration . Financial Assistance. Available at: https://www.nav.no/en/Home/Benefits+and+services/Relatert+informasjon/Financial+assistance+%28social+assistance%29.282014.cms. Accessed April 12, 2016.
- 24. International Labour Organization . International Standard Classification of Occupation (ISCO)‐88, 2004. Available at: http://www.ilo.org/public/english/bureau/stat/isco/isco88/. Accessed April 12, 2016.
- 25. Eurofond . Coding and Classification Standards, 2010. Available at: http://www.eurofound.europa.eu/surveys/ewcs/2005/classification. Accessed April 12, 2016.
- 26. Steiner JF, Cavender T, Main DS, Bradley CJ. Assessing the impact of cancer on work outcomes: what are the research needs? Cancer. 2004;101:1703–1711. [DOI] [PubMed] [Google Scholar]
- 27. Gupta S, Wilejto M, Pole JD, Guttmann A, Sung L. Low socioeconomic status is associated with worse survival in children with cancer: a systematic review [serial online]. PLoS One. 2014;9:e89482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Norway Central Bank . Exchange Rate for US Dollar. Available at: http://www.norges-bank.no/en/Statistics/exchange_rates/currency/USD/. Accessed April 12, 2016.
- 29. Kirchhoff AC, Krull KR, Ness KK, et al. Physical, mental, and neurocognitive status and employment outcomes in the Childhood Cancer Survivor Study cohort. Cancer Epidemiol Biomarkers Prev. 2011;20:1838–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dowling E, Yabroff KR, Mariotto A, et al. Burden of illness in adult survivors of childhood cancers: Findings from a population‐based national sample. Cancer. 2010;116:3712–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parsons HM, Harlan LC, Lynch CF, et al. Impact of cancer on work and education among adolescent and young adult cancer survivors. J Clin Oncol. 2012;30:2393–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eurostat . Harmonised Unemployment Rate by Sex. Available at: http://ec.europa.eu/eurostat/tgm/table.do?tab=table&tableSelection=1&labeling=labels&footnotes=yes&layout=time,geo,cat&language=en&pcode=teilm021&plugin=0. Accessed April 12, 2016.
- 33. Armstrong GT, Liu Q, Yasui Y, et al. Long‐term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghaderi S, Engeland A, Gunnes MW, et al. Educational attainment among long‐term survivors of cancer in childhood and adolescence: a Norwegian population‐based cohort study. J Cancer Surviv. 2016;10:87–95. [DOI] [PubMed] [Google Scholar]
- 36. CE Kuehni, MP Strippoli, CS Rueegg, et al. Educational achievement in Swiss childhood cancer survivors compared with the general population. Cancer. 2012;118:1439–1449. [DOI] [PubMed] [Google Scholar]
- 37. Dorffel W, Ruhl U, Luders H, et al. Treatment of children and adolescents with Hodgkin lymphoma without radiotherapy for patients in complete remission after chemotherapy: final results of the multinational trial GPOH‐HD95. J Clin Oncol. 2013;31:1562–1568. [DOI] [PubMed] [Google Scholar]
- 38. Isakoff MS, Bielack SS, Meltzer P, et al. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh SD, Ajani UA, Johnson CJ, et al. Association of cutaneous melanoma incidence with area‐based socioeconomic indicators—United States, 2004‐2006. J Am Acad Dermatol. 2011;65:58(suppl).e1–e12. [DOI] [PubMed] [Google Scholar]
- 40. Kirkwood J, Jukic D, Averbook B, Sender L. Melanoma in pediatric, adolescent and young adult patients. Semin Oncol. 2009;36:419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strauser D, Feuerstein M, Chan F, et al. Vocational services associated with competitive employment in 18‐25 year old cancer survivors. J Cancer Surviv. 2010;4:179–186. [DOI] [PubMed] [Google Scholar]


