Abstract
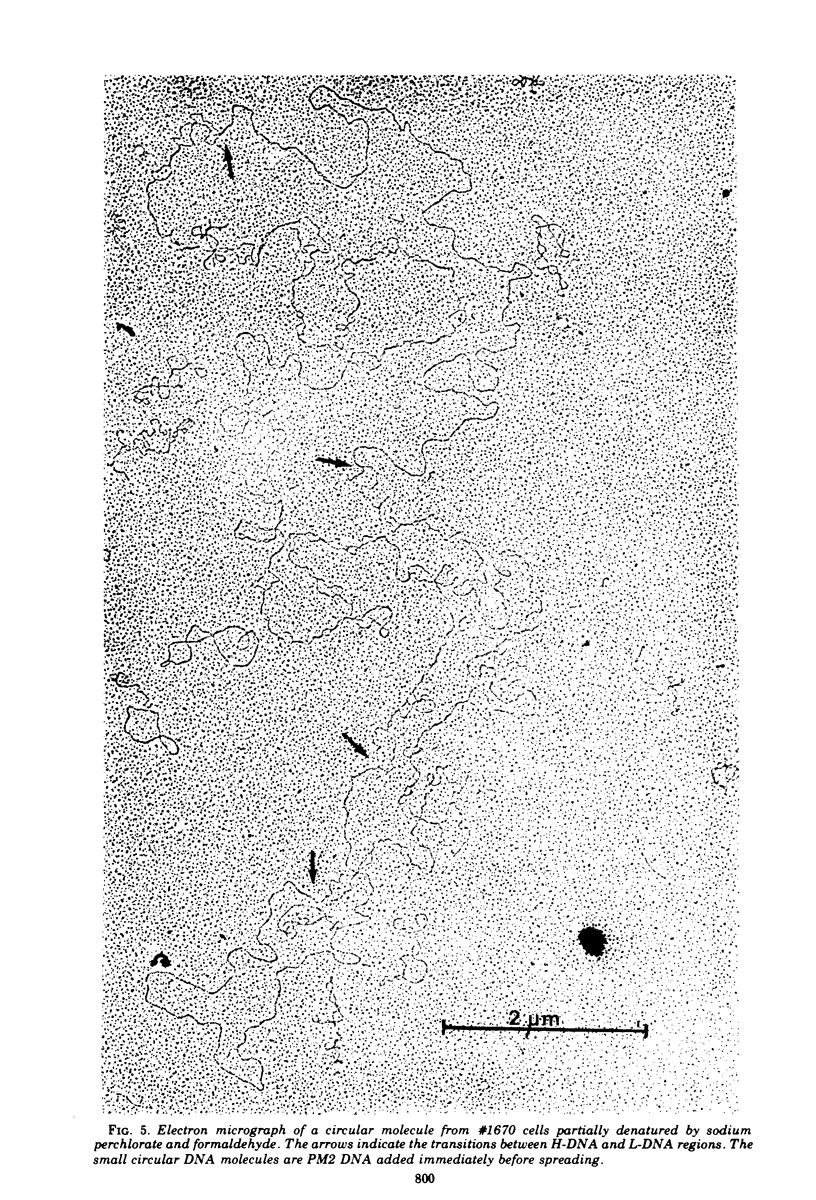
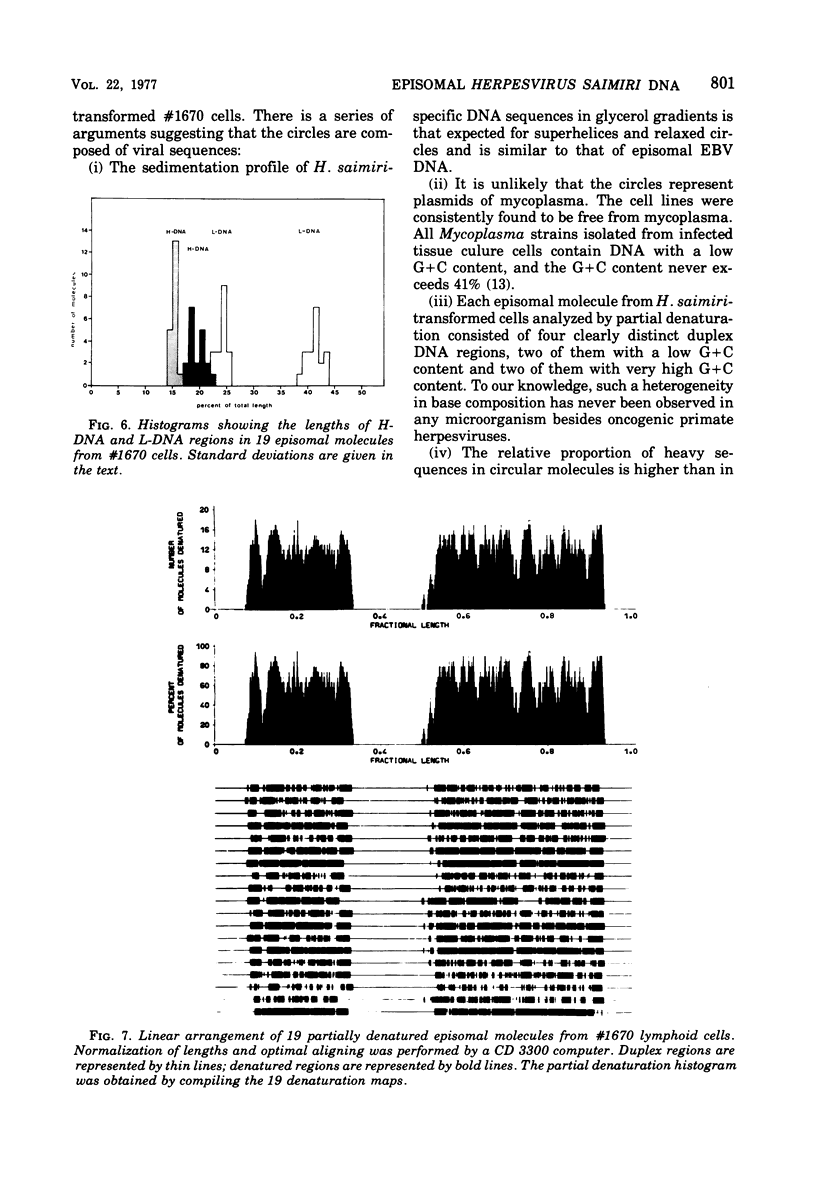
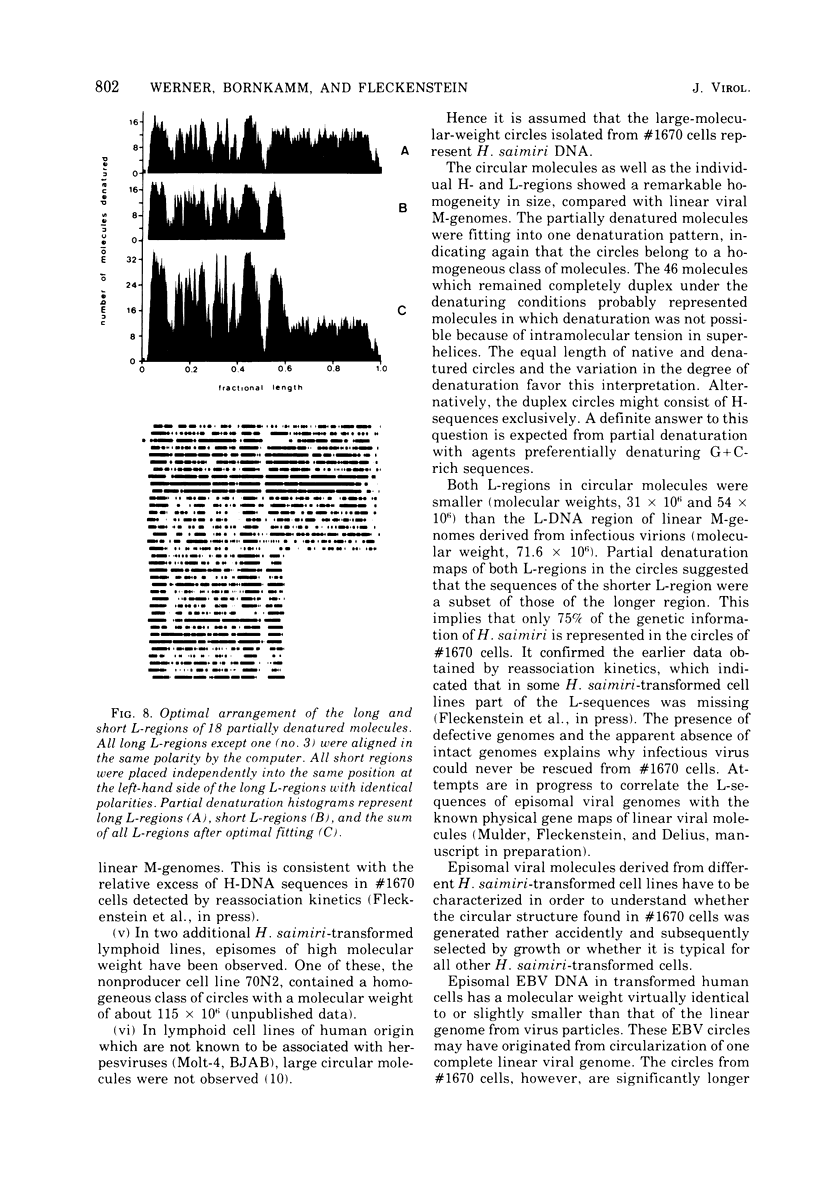
The lymphoid cell line #1670 has been derived from the infiltrated spleen of a tumor-bearing marmoset monkey infected with Herpesvirus saimiri. The cells contain both types of H. saimiri DNA, unique light (L-) DNA (36% cytosine plus guanine) and repetitive heavy (H-) DNA (71% cytosine plus guanine), without producing infectious virus. Viral DNA was found to persist in these cells as nonintegrated circular DNA molecules. Closed circular superhelical viral DNA molecules were isolated by three subsequent centrifugation steps: (i) isopycnic centrifugation in CsCl, (ii) sedimentation through glycerol gradients, and (iii) equilibrium centrifugation in CsCl-ethidium bromide. The isolated circles had a molecular weight of 131.5 ± 3.6 × 106. This is significantly higher than the molecular weight of linear DNA molecules isolated from purified H. saimiri virions (about 100 × 106). Partial denaturation mapping of circular molecules from #1670 lymphoid cells showed uniform arrangement of H- and L-DNA sequences in all circles. All denatured molecules contained two L-DNA regions (molecular weights of 54.0 ± 1.8 × 106 and 31.5 ± 1.3 × 106) and two H-DNA regions (molecular weight of 25.6 ± 1.9 × 106 and 20.0 ± 0.8 × 106) of constant length. Maps of both L-regions suggested that the sequences of the shorter L-DNA region were a subset of those of the longer region. The sequences of both L-regions had the same orientation. Circular molecules from H. saimiri-transformed lymphoid cell line #1670 appeared to represent defective genomes, containing only 75% of the genetic information present in L-DNA of H. saimiri virions.
Full text
PDF

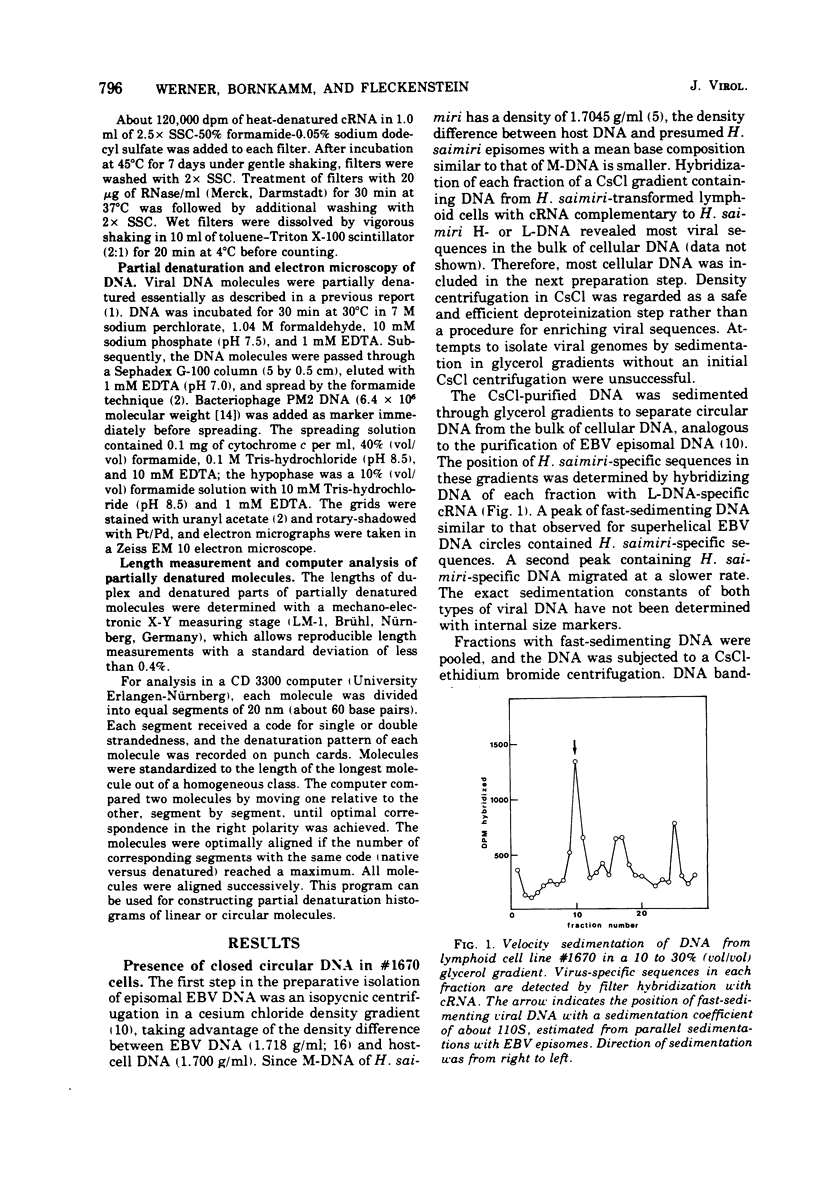
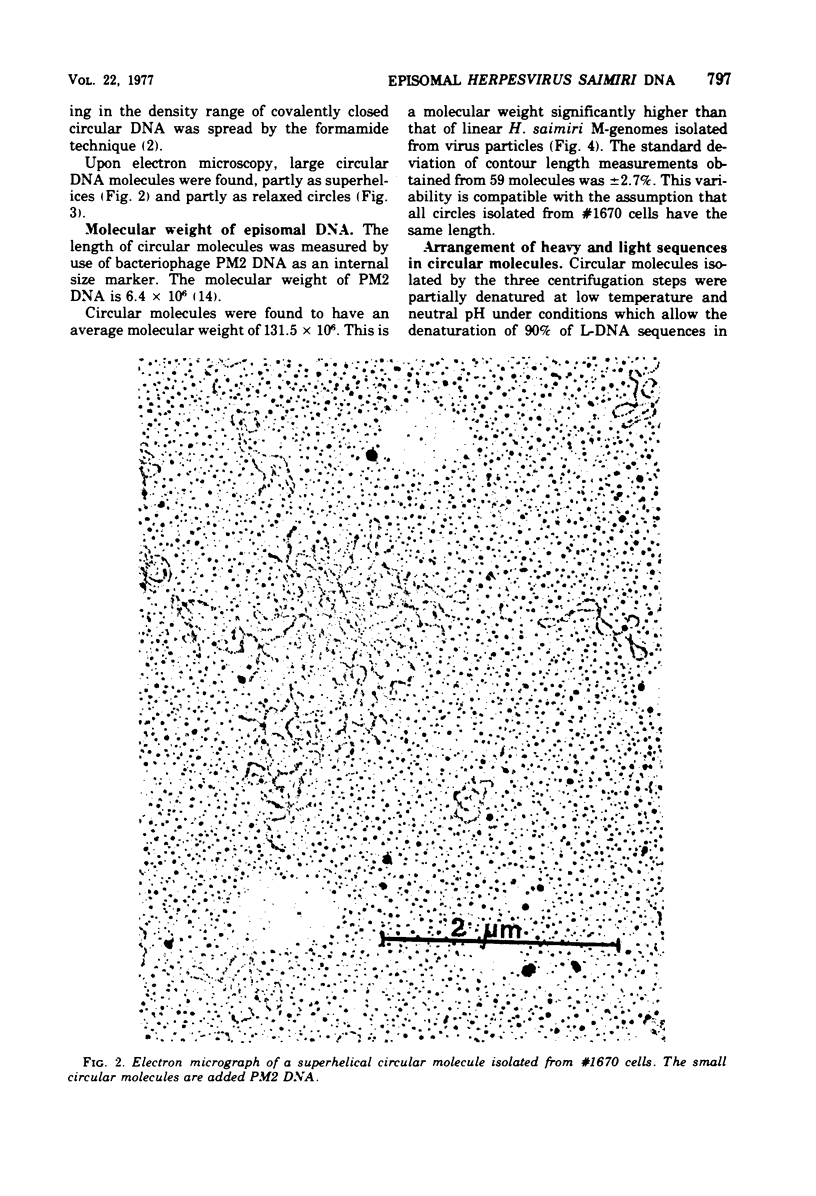
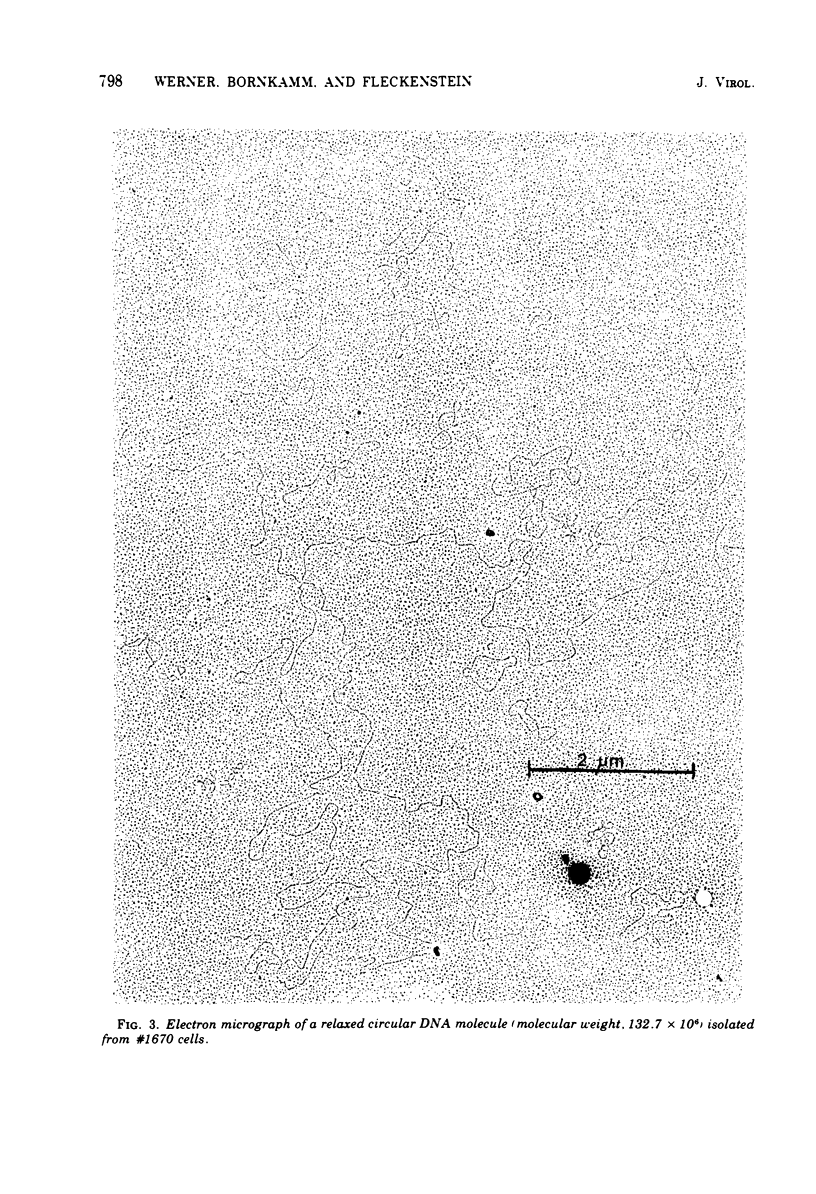
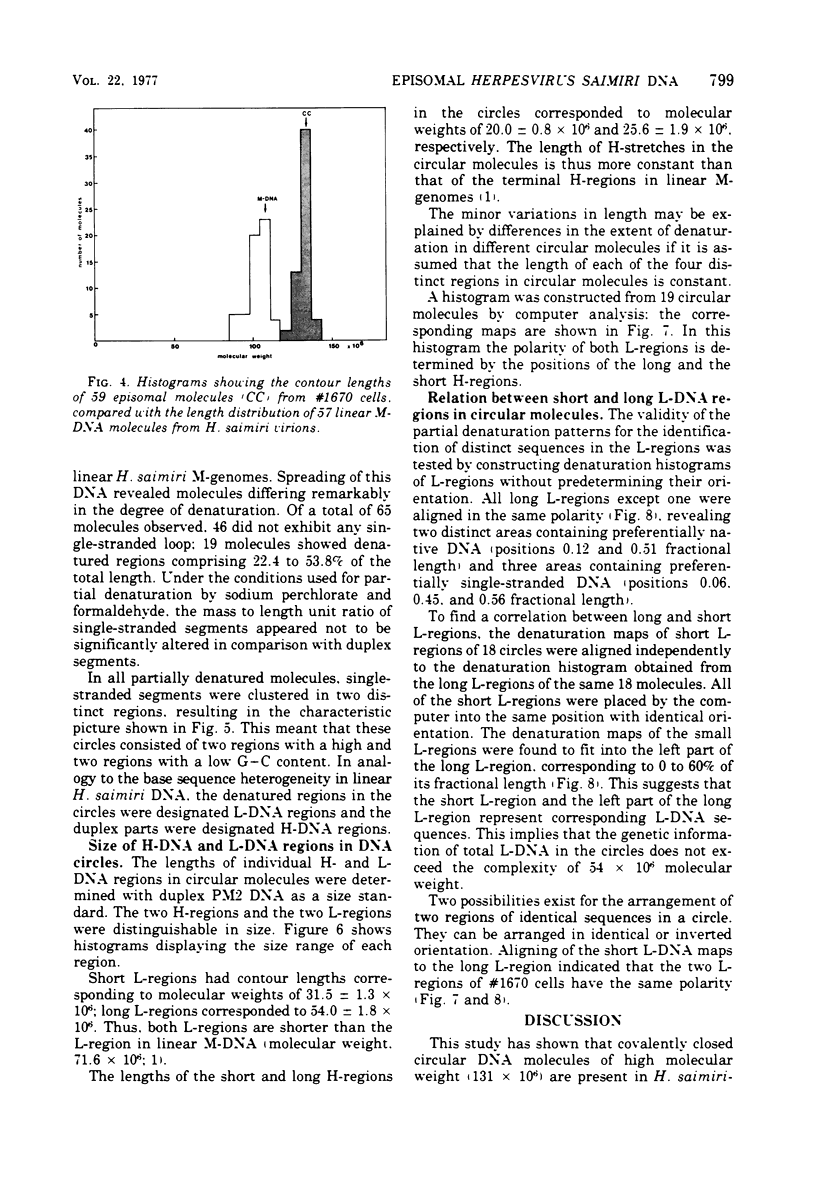

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk L. A., Wolfe L. G., Hoekstra J., Deinhardt F. Demonstration of Herpesvirus saimiri-associated antigens in peripheral lymphocytes from infected marmosets during in vitro cultivation. J Natl Cancer Inst. 1972 Feb;48(2):523–530. [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Ludwig H. Repetitive sequences in complete and defective genomes of Herpesvirus saimiri. J Virol. 1975 Feb;15(2):398–406. doi: 10.1128/jvi.15.2.398-406.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W. Structure and function of herpesvirus saimira DNA. IARC Sci Publ. 1975;(11 Pt 1):145–149. [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Werner F. J. The role of Herpesvirus saimiri genomes in oncogenic transformation of primate cells. Bibl Haematol. 1975 Oct;(43):308–312. doi: 10.1159/000399154. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Wolf H. Purification and properties of Herpesvirus saimiri DNA. Virology. 1974 Mar;58(1):55–64. doi: 10.1016/0042-6822(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Hunt R. D., Meléndez L. V., King N. W., Gilmore C. E., Daniel M. D., Williamson M. E., Jones T. C. Morphology of a disease with features of malignant lymphoma in marmosets and owl monkeys inoculated with Herpesvirus saimiri. J Natl Cancer Inst. 1970 Feb;44(2):447–465. [PubMed] [Google Scholar]
- Kaschka-Dierich C., Adams A., Lindahl T., Bornkamm G. W., Bjursell G., Klein G., Giovanella B. C., Singh S. Intracellular forms of Epstein-Barr virus DNA in human tumour cells in vivo. Nature. 1976 Mar 25;260(5549):302–306. doi: 10.1038/260302a0. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Meléndez L. V., Hunt R. D., Daniel M. D., García F. G., Fraser C. E. Herpesvirus saimiri. II. Experimentally induced malignant lymphoma in primates. Lab Anim Care. 1969 Jun;19(3):378–386. [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. S., O'Conor G. T., Lorenz D. E., Kirschstein R. L., Legallais F. Y., Tralka T. S. Lymphoid cell-culture line derived from lymph node of marmoset infected wtih Herpesvirus saimiri--preliminary report. J Natl Cancer Inst. 1971 May;46(5):1099–1109. [PubMed] [Google Scholar]
- Schulte-Holthausen H., zur Hausen H. Partial purification of the Epstein-Barr virus and some properties of its DNA. Virology. 1970 Mar;40(3):776–779. doi: 10.1016/0042-6822(70)90229-1. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Nonoyama M. Latent DNA of Epstein-Barr virus: separation from high-molecular-weight cell DNA in a neutral glycerol gradient. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4658–4661. doi: 10.1073/pnas.71.12.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen W. C., Neubauer R. H., Rabin H., Cicmanec J. L. Nonimmune rosette formation by lymphoma and leukemia cells from Herpesvirus saimiri-infected owl monkeys. J Natl Cancer Inst. 1973 Sep;51(3):967–975. doi: 10.1093/jnci/51.3.967. [DOI] [PubMed] [Google Scholar]





