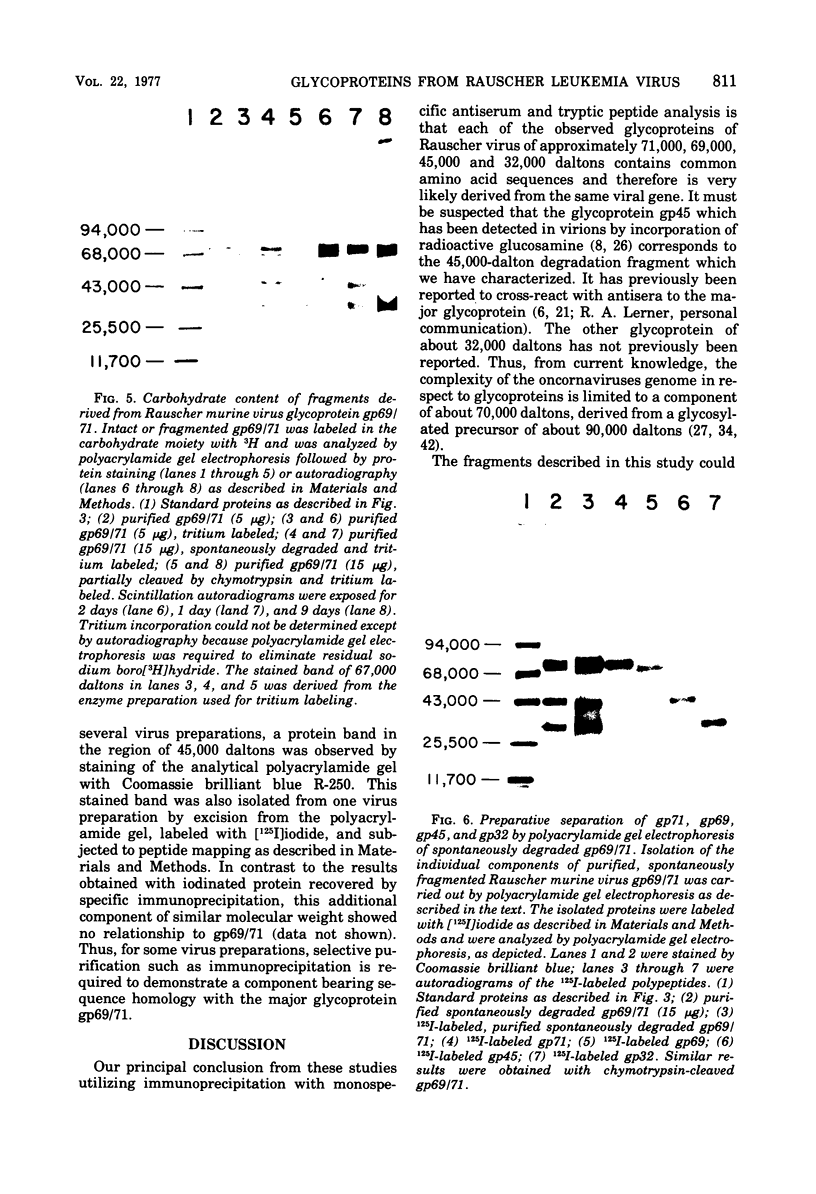
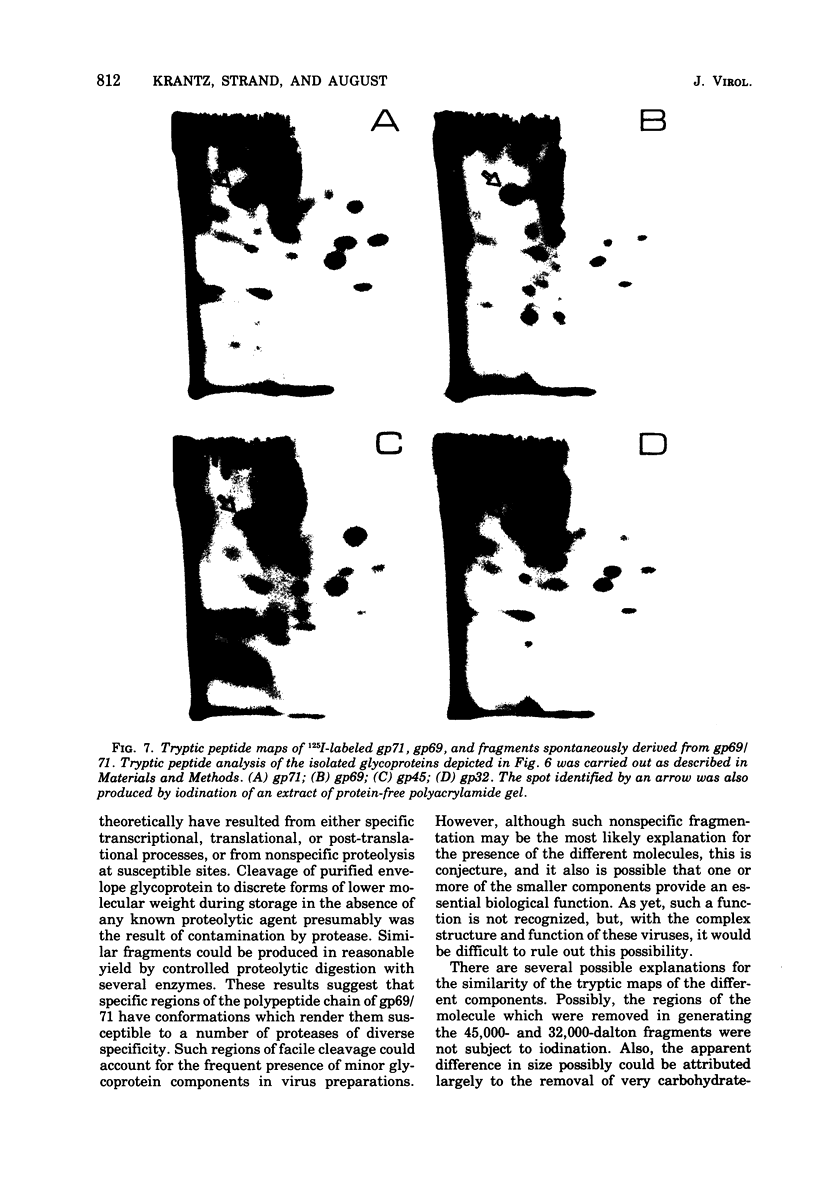
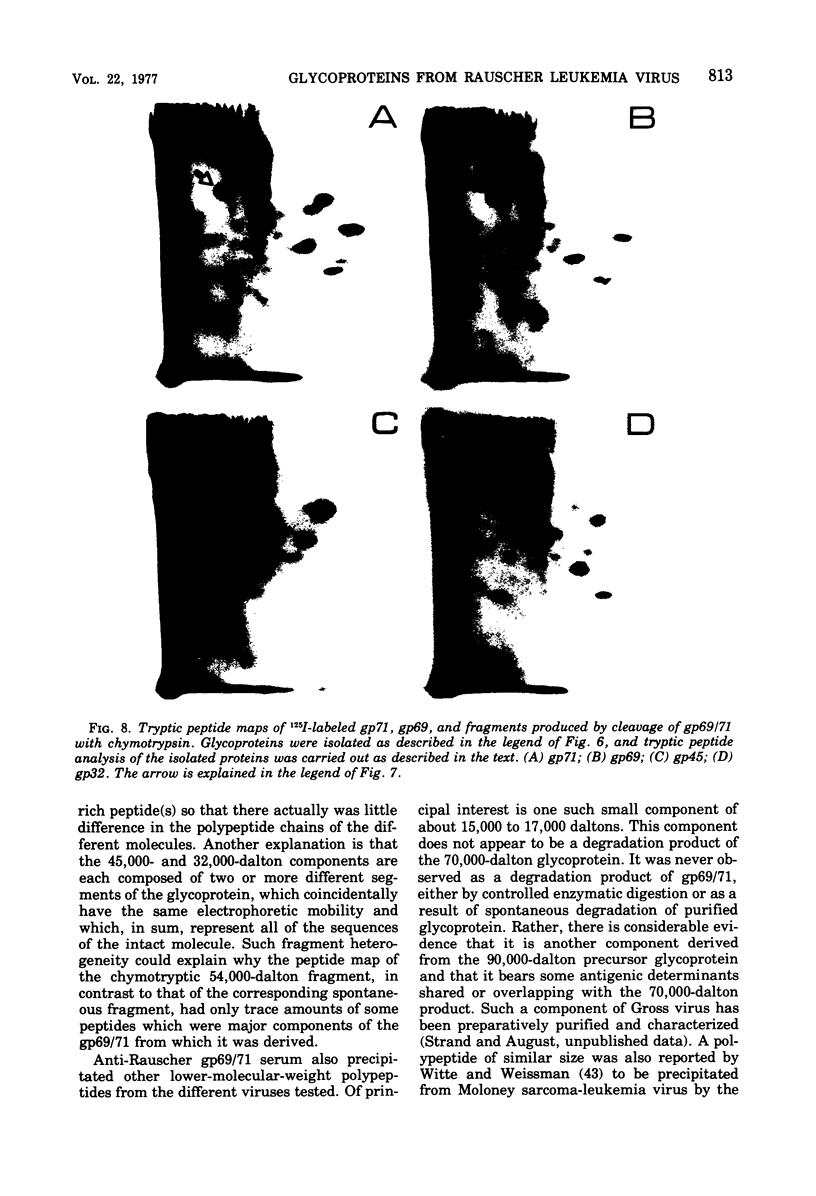
Abstract
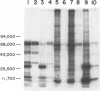
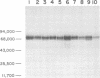
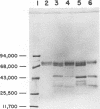
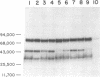
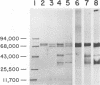
Analysis of the proteins of Rauscher murine oncornavirus by immunoprecipitation showed that antiserum to the purified envelope glycoprotein of approximately 69,000 and 71,000 daltons (gp69/71) reacted as well with a number of other components of several murine oncornaviruses of approximately 45,000, 32,000, and 15,000 daltons. Polypeptides of similar size were also produced by limited proteolysis of purified gp69/71; these degradation fragments were shown to contain carbohydrate by the incorporation of 3H from sodium boro[3H]hydride after neuraminidase and galactose oxidase treatment. Each of these glycoproteins was isolated by preparative polyacrylamide gel electrophoresis and was analyzed by tryptic peptide mapping. The major virion components of 69,000 and 71,000 daltons were nearly identical, as were the primary degradation fragments. Analysis of the immunological properties of the glycoproteins showed that the 71,000-, 69,000-, and 32,000-dalton glycoproteins behaved similarly with respect to type and group-specific antigenic determinants. In contrast, the 45,000-dalton glycoprotein lacked detectable interspecies and some of the group-specific reactivity. Components of about 45,000 and 32,000 daltons isolated directly from virions were also identified as constituents of the major envelope glycoprotein by immune precipitation and tryptic peptide mapping. These results indicate that all of the examined virion glycoproteins of approximately 71,000, 69,000, 45,000, and 32,000 daltons are derived from the same viral gene and that these lower-molecular-weight glycoproteins can readily be produced from the major envelope glycoprotein.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Independent segregation of loci for activation of biologically distinguishable RNA C-type viruses in mouse cells. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2055–2058. doi: 10.1073/pnas.70.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcement L. J., Karshin W. L., Naso R. B., Jamjoom G., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins: presence of p30 and envelope p15 sequences in precursor polypeptides. Virology. 1976 Feb;69(2):763–774. doi: 10.1016/0042-6822(76)90504-3. [DOI] [PubMed] [Google Scholar]
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bray D., Brownlee S. M. Peptide mapping of proteins from acrylamide gels. Anal Biochem. 1973 Sep;55(1):213–221. doi: 10.1016/0003-2697(73)90306-0. [DOI] [PubMed] [Google Scholar]
- Del Vellano B. C., Nave B., Croker B. P., Lerner R. A., Dixon F. J. The oncornavirus glycoprotein gp69/71: a constituent of the surface of normal and malignant thymocytes. J Exp Med. 1975 Jan 1;141(1):172–187. doi: 10.1084/jem.141.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Villano B. C., Lerner R. A. Relationship between the oncornavirus gene product gp70 and a major protein secretion of the mouse genital tract. Nature. 1976 Feb 12;259(5543):497–499. doi: 10.1038/259497a0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Sch5AAFER W., Bolognesi D. P. Neutralization of homologous and heterologous oncornaviruses by antisera against the p15(E) and gp71 polypeptides of Friend murine leukemia virus. Virology. 1976 May;71(1):169–184. doi: 10.1016/0042-6822(76)90103-3. [DOI] [PubMed] [Google Scholar]
- GROSS L. Development and serial cellfree passage of a highly potent strain of mouse leukemia virus. Proc Soc Exp Biol Med. 1957 Apr;94(4):767–771. doi: 10.3181/00379727-94-23080. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino S., Stephenson J. R., Aaronson S. A. Antigenic determinants of the 70,000 molecular weight glycoprotein of woolly monkey type C RNA virus. J Immunol. 1975 Oct;115(4):922–927. [PubMed] [Google Scholar]
- Hino S., Stephenson J. R., Aaronson S. A. Radiommunoassays for the 70,000-molecular-weight glycoproteins of endogenous mouse type C viruses: viral antigen expression in normal mouse tissues and sera. J Virol. 1976 Jun;18(3):933–941. doi: 10.1128/jvi.18.3.933-941.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsmann G., Moennig V., Pister L., Seifert E., Schäfer W. Properties of mouse leukemia viruses. VIII. The major viral glycoprotein of Friend leukemia virus. Seroimmunological, interfering and hemagglutinating capacities. Virology. 1974 Dec;62(2):307–318. doi: 10.1016/0042-6822(74)90394-8. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Denny T. P., Bolognesi D. P. Purification and serological characterization of the major envelope glycoprotein from AKR murine leukemia virus and its reactivity with autogenous immune sera from mice. J Virol. 1976 Mar;17(3):727–736. doi: 10.1128/jvi.17.3.727-736.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Hanna M. G., Jr, Roberson L. E., Kenney F. T. Autogenous immunity to endogenous RNA tumor virus. Identification of antibody reactivity to select viral antigens. J Exp Med. 1974 Jun 1;139(6):1568–1581. doi: 10.1084/jem.139.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Hardy W., Jr, Tress E., Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. V. Identification of a new murine viral protein, p15(E). J Virol. 1975 Jul;16(1):53–61. doi: 10.1128/jvi.16.1.53-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Pincus T., Yoshiki T., Strand M., August J. T., Boyse E. A., Mellors R. C. Biological expression of antigenic determinants of murine leukemia virus proteins gp69-71 and p30. J Virol. 1974 Nov;14(5):1274–1280. doi: 10.1128/jvi.14.5.1274-1280.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLONEY J. B. Biological studies on a lymphoid-leukemia virus extracted from sarcoma 37. I. Origin and introductory investigations. J Natl Cancer Inst. 1960 Apr;24:933–951. [PubMed] [Google Scholar]
- McLellan W. L., August J. T. Analysis of the envelope of Rauscher murine oncornavirus: in vitro labeling of glycopeptides. J Virol. 1976 Dec;20(3):627–636. doi: 10.1128/jvi.20.3.627-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moennig V., Frank H., Hunsmann G., Schneider I., Schafer W. Properties of mouse leukemia viruses. VII. The major viral glycoprotein of friend leukemia virus. Isolation and physicochemical properties. Virology. 1974 Sep;61(1):100–111. doi: 10.1016/0042-6822(74)90245-1. [DOI] [PubMed] [Google Scholar]
- Moroni C. Structural proteins of Rauscher leukemia virus and Harvey sarcoma virus. Virology. 1972 Jan;47(1):1–7. doi: 10.1016/0042-6822(72)90232-2. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins. Cell. 1975 Jan;4(1):31–36. doi: 10.1016/0092-8674(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Fleissner E., Sarkar N. H., Aoki T. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. II. Mammalian leukemia-sarcoma viruses. J Virol. 1972 Feb;9(2):359–366. doi: 10.1128/jvi.9.2.359-366.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAFTERY M. A., COLE R. D. Tryptic cleavage at cysteinyl peptide bonds. Biochem Biophys Res Commun. 1963 Mar 25;10:467–472. doi: 10.1016/0006-291x(63)90381-4. [DOI] [PubMed] [Google Scholar]
- RAUSCHER F. J. A virus-induced disease of mice characterized by erythrocytopoiesis and lymphoid leukemia. J Natl Cancer Inst. 1962 Sep;29:515–543. [PubMed] [Google Scholar]
- Schafer W., Hunsmann G., Moennig V., Noranha F., Bolognesi D. P., Green R. W., Hüper G. Polypeptides of mammalian oncornaviruses. II Characterization of murine leukemia virus polypeptide (p 15) bearing interspecies reactivity. Virology. 1975 Jan;63(1):48–59. doi: 10.1016/0042-6822(75)90369-4. [DOI] [PubMed] [Google Scholar]
- Schwarz H., Hunsmann G., Moenning V., Schäfer W. Properties of mouse leukemia viruses. XI. Immunoelectron microscopic studies on viral structural antigens on the cell surface. Virology. 1976 Jan;69(1):169–178. doi: 10.1016/0042-6822(76)90204-x. [DOI] [PubMed] [Google Scholar]
- Seifert E., Claviez M., Frank H., Hunsmann G., Schwarz H., Schäfer W. XII. Produktion grösserer Mengen von Friend-Virus durch eine permanente Zell-Suspensions-Kultur (Eveline-Suspensions-Zellen) Z Naturforsch C. 1975 Sep-Oct;30(5):698–700. [PubMed] [Google Scholar]
- Shapiro S. Z., Strand M., August J. T. High molecular weight precursor polypeptides to structural proteins of Rauscher murine leukemia virus. J Mol Biol. 1976 Nov 15;107(4):459–477. doi: 10.1016/s0022-2836(76)80078-2. [DOI] [PubMed] [Google Scholar]
- Slobin L. I., Singer S. J. The specific cleavage of immunoglobulin polypeptide chains at cysteinyl residues. J Biol Chem. 1968 Apr 25;243(8):1777–1786. [PubMed] [Google Scholar]
- Steeves R. A., Strand M., August J. T. Structural proteins of mammalian oncogenic RNA viruses: murine leukemia virus neutralization by antisera prepared against purified envelope glycoprotein. J Virol. 1974 Jul;14(1):187–189. doi: 10.1128/jvi.14.1.187-189.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of mammalian oncogenic RNA viruses: multiple antigenic determinants of the major internal protein and envelope glycoprotein. J Virol. 1974 Jan;13(1):171–180. doi: 10.1128/jvi.13.1.171-180.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of ribonucleic acid tumor viruses. Purification of envelope, core, and internal components. J Biol Chem. 1976 Jan 25;251(2):559–564. [PubMed] [Google Scholar]
- Strand M., Lilly F., August J. T. Host control of endogenous murine leukemia virus gene expression: concentrations of viral proteins in high and low leukemia mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3682–3686. doi: 10.1073/pnas.71.9.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., Wilsnack R., August J. T. Structural proteins of mammalian oncogenic RNA viruses: immunological characterization of the p15 polypeptide of Rauscher murine virus. J Virol. 1974 Dec;14(6):1575–1583. doi: 10.1128/jvi.14.6.1575-1583.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zaane D., Dekker-Michielsen J. A., Bloemers H. P. Virus-specific precursor polypeptides in cells infected with Rauscher leukemia virus: synthesis, identification, and processing. Virology. 1976 Nov;75(1):113–129. doi: 10.1016/0042-6822(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L., Kaplan H. S. Structural characteristics of some murine RNA tumor viruses studied by lactoperoxidase iodination. Proc Natl Acad Sci U S A. 1973 Jan;70(1):36–40. doi: 10.1073/pnas.70.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L. Oncornavirus budding: kinetics of formation and utilization of viral membrane glycoprotein. Virology. 1976 Feb;69(2):464–473. doi: 10.1016/0042-6822(76)90477-3. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]