Abstract
Background and objective
Epilepsy is associated with alterations in the structural framework of the cerebral network. The aim of this study was to measure the potential of global metrics of network architecture derived from resting state functional MRI to capture the impact of epilepsy on the developing brain.
Methods
Pediatric patients were retrospectively identified with: 1. Focal epilepsy; 2. Brain MRI at 3 Tesla, including resting state functional MRI; 3. Full scale IQ measured by a pediatric neuropsychologist. The cerebral cortex was parcellated into approximately 700 gray matter network nodes. The strength of a connection between two nodes was defined as the correlation between their resting BOLD signal time series. The following global network metrics were then calculated: clustering coefficient, transitivity, modularity, path length, and global efficiency. Epilepsy duration was used as an index for the cumulative impact of epilepsy on the brain.
Results
45 patients met criteria (age: 4–19 years). After accounting for age of epilepsy onset, epilepsy duration was inversely related to IQ (p: 0.01). Epilepsy duration predicted by a machine learning algorithm on the basis of the five global network metrics was highly correlated with actual epilepsy duration (r: 0.95; p: 0.0001). Specifically, modularity and to a lesser extent path length and global efficiency were independently associated with epilepsy duration.
Conclusions
We observed that a machine learning algorithm accurately predicted epilepsy duration based on global metrics of network architecture derived from resting state fMRI. These findings suggest that network metrics have the potential to form the basis for statistical models that translate quantitative imaging data into patient-level markers of cognitive deterioration.
Abbreviations: FSL, FMRIB Software Library; ICA, independent components analysis; IQ, intelligence quotient
Keywords: Network, Graph theory, Epilepsy, Brain, Intelligence
Highlights
-
•
Brain network architecture was measured using resting state functional MRI.
-
•
Global intelligence declined with increasing duration of epilepsy.
-
•
A machine learning algorithm accurately predicted the neurologic impact of epilepsy.
-
•
Network architecture was highly associated with the impact of epilepsy on the brain.
1. Introduction
Epilepsy has a substantial influence on the development and maintenance of cognitive functions. Although it is not clear whether such effects are mediated by ongoing seizure activity, anti-seizure medication or both, long-term epilepsy and poor seizure control have been consistently associated with poor cognitive outcomes (Elger et al., 2004, Hermann et al., 2002, Czochanska et al., 1994). These effects on intellectual function are exaggerated in children, which may reflect the fact that developmental physiology is primed to prioritize cerebral growth and reorganization (Bjornaes et al., 2001). While together these observations suggest that neural plasticity acts as a negative prognostic factor in children with epilepsy, these same characteristics likely contribute to their capacity for cognitive and neurologic recovery after successful epilepsy surgery (Spencer and Huh, 2008, Freitag and Tuxhorn, 2005). Despite the benefits of early intervention, however, surgery is frequently deferred, especially in imperfect candidates or in patients whose seizures have yet to meet the standard for intractability. Early markers of cognitive deterioration in these children would be of great value toward defining the optimal timing of surgical intervention.
As a result of advances in computational neuroscience, network organization of the brain is now accessible to systematic study. Although the field capitalizes on diverse techniques, one prominent approach leverages graph theory to characterize global topological features of the cerebral network (Hagmann et al., 2008). In this context, the brain is represented as a collection of nodes, or anatomical elements in the network, and their mutual connections as edges (Bullmore and Sporns, 2009, Guye et al., 2010, Xia and He, 2011). Graph theory-based analyses of networks constructed from functional imaging data have demonstrated that focal epilepsies are associated with global alterations in the cerebral network (Liao et al., 2010, Bernhardt et al., 2011, Vlooswijk et al., 2011, DeSalvo et al., 2014, Vaessen et al., 2013). More recently, it was observed that inter-individual differences in network efficiency, as quantified by graph theory, correlate with cognitive function in healthy populations of adults and children (Kim et al., 2016, Li et al., 2009, van den Heuvel et al., 2009). Together, these findings support the potential for topological features of the brain to provide markers of cognitive function in children. However, at any given time, the cognitive abilities of a child with epilepsy will reflect the intersection of his/her individual trajectory of brain development with maladaptive changes related to the cumulative impact of his/her disease. As yet, no data exist regarding the potential for network analyses to dissociate these processes to specifically capture those alterations that relate to epilepsy and its treatment.
The aim of this study was to measure the potential of global network metrics derived from resting state functional brain networks to capture the impact of epilepsy on the developing brain. Although there is no gold standard to measure these effects, the duration of a patient's disease has been shown to be a meaningful marker of the cumulative burden of epilepsy, particularly with regard to cognitive function (van Iterson et al., 2014). We therefore used the duration of each patient's epilepsy as an index for the overall impact of their disease on the brain.
2. Material and methods
2.1. Study population
This HIPAA-compliant, retrospective study was approved by the local institutional review board. Written informed consent was waived. Consecutive patients were identified from the medical record with the following inclusion criteria: 1. Pediatric age (less-than-or-equal-to 21 years), 2. a clinical diagnosis of focal epilepsy (Berg et al., 2010) by a pediatric epileptologist based on clinical history and seizure semiology, 3. available 3 Tesla MRI of the brain, including a resting state fMRI sequence, 4. Full scale intelligence quotient (IQ) according to an age-appropriate version of the Wechsler Intelligence Test administered by a pediatric neuropsychologist within 3 months of the MRI. Refinements to the above-defined population were planned based on the following exclusions: 1. prior brain surgery.
2.2. Neuropsychological assessment
Intelligence tests were performed by a single pediatric neuropsychologist (MC) with more than 25 year experience using an age-appropriate Wechsler Intelligence Scale test. In each patient, full scale IQ was determined by evaluation of 4 cognitive domains including verbal comprehension, perceptual/fluid reasoning, working memory, and processing speed.
2.3. MR imaging
All imaging was performed on a 3 Tesla magnet (Philips, Achieva Platform, Andover, Massachusetts) equipped with a 32-channel phased array head coil. For structural imaging, a T1-weighted, axial three-dimensional volume acquisition fast field echo was obtained with TR/TE: 7.2/2.9 ms, flip angle: 7°, inversion time: 1100 ms, voxel size: 0.9 × 0.9 × 0.9 mm3. Functional MRI data were acquired in the resting state using a single-shot echo planar acquisition depicting blood oxygenation level dependent contrast with TR/TE: 2000/30 ms, flip angle: 80°, voxel size: 3 × 3 × 3.75 mm3. Functional imaging was performed for 10 min, resulting in 300 volumes for each patient. Patients were instructed to lie quietly in the scanner with their eyes closed. All images were visually inspected for artifacts, including susceptibility and subject motion.
2.4. Image processing and analysis
2.4.1. Network node definition
Nodes in the network were defined for each patient according to parcellation of whole-brain gray matter on the structural images. The processing pipeline was implemented using MATLAB scripts (version 7.13, MathWorks, Inc.) in which adapter functions were embedded to execute FreeSurfer reconstruction (version 5.3.0; http://surfer.nmr.mgh.harvard.edu) and several FMRIB Software Library (FSL) suite tools (Smith et al., 2004). First, FreeSurfer reconstruction of cerebral cortical surfaces was performed on the T1 structural image. This processing stream includes motion correction, skull stripping, intensity normalization, segmentation of white matter and gray matter structures, parcellation of the gray matter and white matter boundary, and surface deformation following intensity gradients which optimally place the gray matter/white matter and gray matter/cerebrospinal fluid borders (Fischl et al., 2001, Fischl et al., 2004). The pial and gray white surfaces were visually inspected using the Freeview software for accurate placement.
Next, a self-developed MATLAB program was applied to the FreeSurfer output to further subdivide the 75 standard gray matter parcels according to their surface area. During this process, each parcel was iteratively divided into two new parcels of equal size until the surface area of each parcel (as defined on the FreeSurfer gray-white surface mesh) was less than a 350-mm2-threshold value. Each surface parcel was then converted into a volume mask of gray matter at that region to form a node on the network. The number of nodes in each patient's network ranged from 511 to 841 (mean: 684; standard deviation: 68).
2.4.2. Network edge definition
The first 5 volumes in each resting state functional data were removed to allow magnetization to reach equilibrium. Preprocessing and independent component analysis (ICA) of the functional data sets was performed using FSL MELODIC (Smith et al., 2004), consisting of motion correction, interleaved slice timing correction, brain extraction, spatial smoothing with a Gaussian kernel full width at half maximum of 5 mm, and high pass temporal filtering equivalent to 100 s (0.01 Hz). Noise related to motion and other physiologic nuisance was addressed according to an ICA technique (Thomas et al., 2002). All non-signal components were removed manually by an expert operator. Motion parameters measured during preprocessing were summarized for each patient as “translation” (the root mean square of the three translational parameters) and “rotation” (root mean square of three rotational parameters). FSL's FLIRT was then used to align the functional image volumes for each patient to that individual's structural T1 dataset using linear registration. Mean BOLD-signal time series were computed for each node. The strength of an edge between two nodes was defined as the absolute value of the Pearson correlation coefficient between their time series.
2.4.3. Construction of the brain functional network
Weighted, undirected graphs were constructed for each patient consisting of the pair-wise correlation between BOLD signal time series over all network nodes. Non-significant correlations were excluded based on Bonferroni adjusted p-values. To this end, the p-value of each pairwise correlation in the connection matrix was multiplied by total number of node pairs [(N2 − N)/2] and thresholded at 0.05.
2.4.4. Network metric calculation
We used MATLAB scripts available from the Brain Connectivity Toolbox (BCT, http://www.brain-connectivity-toolbox.net) to compute common metrics that summarize global network architecture including: Clustering coefficient: the fraction of a node's neighbors that are neighbors of each other. Clustering coefficient of a graph is the average clustering coefficient over all nodes in the network. Networks with high clustering coefficient are considered locally efficient networks (Rubinov and Sporns, 2010). Modularity: the degree to which the network tends to segregate into relatively independent modules, or subnetworks (Rubinov and Sporns, 2010, Newman and Girvan, 2004). Modularity reflects the capacity of a network to support functional sub-specialization. Transitivity: Transitivity is a variant of clustering coefficient, reflecting connectivity of a given region to its neighbors. Unlike clustering coefficient, nodes of lesser importance in the network (i.e. those with low degree) do not influence transitivity (Rubinov and Sporns, 2010, Newman, 2003). Modularity, clustering coefficient and transitivity are metrics that measure the brain's tendency to segregate into relatively independent, local neighborhoods. In other words, these measures reflect the ability of the brain to process specialized functions within highly interconnected functional sub networks. Characteristic path length: the path length between two nodes is the minimum number of edges that must be traversed to get from one node to the other. Characteristic path length is the average shortest path length between all pairs of nodes in the graph, indicating how easily information can be transferred across the network (Rubinov and Sporns, 2010). Global efficiency: related to characteristic path length, global efficiency is the average of the inverse of the shortest path length over the network. Compared to characteristic path length, global efficiency is less influenced by nodes that are relatively isolated from the network (ie. infinite path length) (Rubinov and Sporns, 2010, Latora and Marchiori, 2001). Characteristic path length and global efficiency are measures of global connectedness, providing an estimate of how easily information can be integrated across the network.
In order to account for differences in network size inherent to a pediatric cohort, we computed normalized network metrics as follows: for each patient, each metric was divided by the same metric computed in a random network of identical size (Rubinov and Sporns, 2011).
2.5. Statistical analyses
All statistical analyses were performed using R Language, version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). We used the duration of each patient's epilepsy (measured as the span of time from epilepsy onset to the date of MR imaging) as an index of the overall impact of their disease on the brain (van Iterson et al., 2014). Linear regression (alpha: 0.05) was used to assess the relationship between epilepsy duration and IQ (after accounting for age of epilepsy onset) in our patient cohort. Bonferroni was used to adjust for multiple comparisons.
In the primary analysis, we used a machine learning algorithm (Random Forest Approach) to predict epilepsy duration on the basis of the five global network metrics. To be specific, the algorithm was given access solely to the network metrics and no other variables during this step. This machine learning method was selected because it tests the predictive capacity of a “learned” statistical model on a subset of the cohort omitted during training. In other words, the ability of the model to predict epilepsy duration in each individual was tested in a previously unseen subset of patients. Machine learning approaches, therefore, represent an effective method by which metrics derived from quantitative imaging can be assessed with respect to their potential translation into clinically meaningful information at the level of a single patient (Paldino et al., 2014). Details regarding this particular technique have been previously described (Breiman, 2001).
In addition to prediction of epilepsy duration, the Random Forest algorithm also measures the independent contribution of individual variables to that prediction. This step is accomplished for each variable by comparing the prediction error over the cohort to that error which results when that variable has been removed from the model. Hence, the importance of each network metric toward accurate prediction of epilepsy duration was measured after adjusting for the contribution of all other variables.
For each variable deemed to be important to the predictive model, the relationships to epilepsy duration were further quantified in a univariate analysis using linear regression. To be specific, this step was not performed to verify the results of the above analysis; rather it was used to demonstrate graphically the direction of the relationships measured by the Random Forest algorithm.
It is important to note that a relationship between network metrics and global intelligence has been previously reported (Kim et al., 2016, Li et al., 2009). Hence, a “control model” was developed to confirm that the primary analysis measured the effects of epilepsy on the brain, rather than a transitive relationship between network metrics and epilepsy duration mediated by IQ. The control model included IQ as well as other potential confounders, including physiologic (gender, the use of anesthesia during imaging, and the number of network nodes) and nuisance variables (rotational and translational motion during MRI). To be specific, network metrics were not included in the control model. The control model was then used to predict epilepsy duration using an otherwise identical Random Forest algorithm. The relationship to true epilepsy duration was assessed using linear regression.
3. Results
3.1. Patients
Forty-seven patients were eligible for the study based on inclusion criteria. Two patients with cavernous malformations were excluded on the basis of susceptibility-related artifact obscuring the cortex. Characteristics of the forty-five patients (age range: 4–19 years) comprising the final study group are provided in Table 1. In 29 patients, MR imaging was performed under general anesthesia; 16 patients were imaged without sedation. Thirty-nine (87%) patients had identifiable structural abnormalities at MR imaging (Table 1). Time between neuropsychological assessment and neuroimaging ranged from 1 day to 3 months (median: 1 month).
Table 1.
Characteristics of the patient cohort.
| Patient characteristics | ||
|---|---|---|
| Sample size | 45 patients | |
| Gender | 26 males; 19 females | |
| Age | Mean (SD): 12.1 (4.7) years | |
| Age at epilepsy onset | Mean (SD): 5.1 (4.1) years | |
| Duration of epilepsy | Mean (SD): 7.1 (5.3) years | |
| Findings at MRI | Focal cortical dysplasia | 14 |
| Mesial temporal sclerosis | 7 | |
| Low-grade tumor | 5 | |
| Hypothalamic hamartoma | 4 | |
| Tuberous sclerosis complex | 3 | |
| Sturge-Weber syndrome | 2 | |
| Subependymal gray matter heterotopia | 1 | |
| Cavernous malformation | 1 | |
| Hypoxic ischemic injury | 1 | |
| Rasmussen's encephalitis | 1 |
3.2. Epilepsy duration and global intelligence
After accounting for age of epilepsy onset, epilepsy duration was inversely related to IQ (p: 0.039; Fig. 1).
Fig. 1.
Relationship between IQ and epilepsy duration. IQ was negatively associated with epilepsy duration after adjusting for the age of epilepsy onset (p: 0.01).
3.3. Network architecture and epilepsy duration
Epilepsy duration predicted by the machine learning algorithm solely on the basis of the five global network metrics was highly correlated with true epilepsy duration (r: 0.95; p: 0.0004; Fig. 2). After accounting for age and age of epilepsy onset, modularity, path length, and global efficiency were independently associated with epilepsy duration (Fig. 3). Notably, the use of anesthesia during imaging, IQ, and parameters summarizing translational and rotational motion did not impact the accurate prediction of epilepsy duration (Fig. 3). Similarly, prediction error was not related to the type of structural abnormality. Univariate relationships of the three important variables with epilepsy duration are provided in Fig. 4. Metric-predicted epilepsy duration closely paralleled true epilepsy duration in terms of its association with declining IQ (p: 0.036; Fig. 5).
Fig. 2.
Relationship between epilepsy duration predicted based solely on network metrics and true epilepsy duration (r: 0.95, p: 0.0001).
Fig. 3.
Importance of network metrics to the accurate prediction of epilepsy duration by the Random Forest algorithm. The independent contribution of each metric was estimated as the error of the machine learning algorithm's prediction of epilepsy duration compared to that error which results when that metric is negated. The greatest magnitude of negative importance defines the limit of noise. Hence, variables with importance greater in magnitude than the most negative variable are significantly associated with the outcome.
Fig. 4.
Univariate relationships between epilepsy duration and (a) modularity (p: 0.0015), (b) characteristic path length (p: 0.028) and (c) global efficiency (p: 0.036).
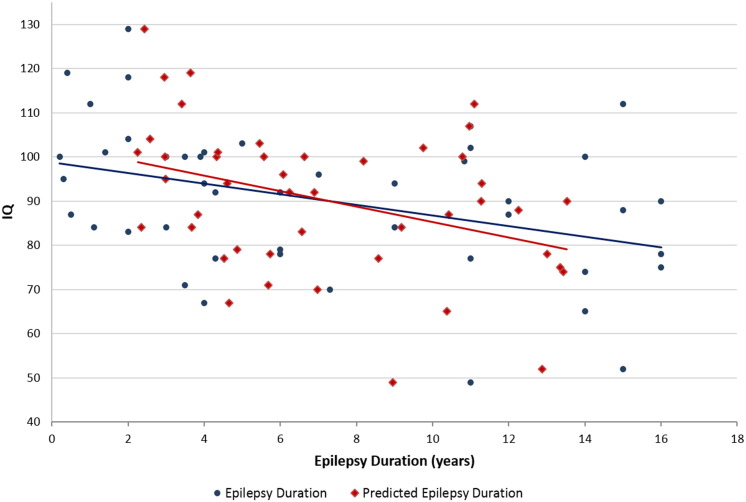
Fig. 5.
Relationship of true epilepsy duration (blue) and predicted epilepsy duration (red) to IQ (True duration: r: − 1.2, p: 0.0106, R2: 0.1422; Predicted duration: r: − 1.7, p: 0.0102, R2: 0.1439). Blue data in the graph is identical to Fig. 1.
Epilepsy duration predicted using the control model (based on IQ and other potential confounders) without access to network metrics was poorly correlated with true epilepsy duration (r: 0.04; p: 0.45; Fig. 6a). Similarly, no individual variable in the control model was significantly associated with epilepsy duration (Fig. 6b).
Fig. 6.
Epilepsy duration predicted without network metrics. (A) Random Forest predicted duration on the basis of the control model (IQ and other non-network variables) is a poor surrogate for true epilepsy duration (r: 0.04; p: 0.45; R2: 0.02). (B) No individual variable in the control model was significantly associated with epilepsy duration. Again, the greatest magnitude of negative importance defines the limit of noise. Hence, only variables with importance greater in magnitude than the most negative variable are significant.
4. Discussion
We report the following main findings in a cohort of children with focal epilepsy: 1. IQ declined with increasing duration of epilepsy. 2. Epilepsy duration predicted by a machine learning algorithm on the basis of network metrics was highly correlated to true epilepsy duration. 3. Three network metrics (modularity, characteristic path length, and efficiency) were independently associated with epilepsy duration after accounting for all other variables. Taken together, these findings suggest that metrics of network architecture have the potential to capture the pathophysiologic impact of this debilitating disease on the cerebral network.
Graph theoretical analyses offer a promising framework in which to explore complex networks. Several studies have used such analyses to quantify brain network architecture in patients with epilepsy (Liao et al., 2010, Bernhardt et al., 2011, Vlooswijk et al., 2011, van Dellen et al., 2009, van Diessen et al., 2013, Bernhardt et al., 2015). These studies have received considerable attention for demonstrating that focal epilepsies are associated with widespread alterations in the architecture of the cerebral network when compared to healthy control subjects. Our findings are not only compatible with the idea of such group level alterations, they further point to the potential for these metrics to capture physiologically-important differences in network architecture between individuals with epilepsy. Although information regarding the relationship between global network measures derived from resting state fMRI and the duration of epilepsy is limited, our findings are compatible with work by Van Dellen et al., who observed epilepsy duration-related alterations in temporal lobe networks using electrocorticography (van Dellen et al., 2009).
In terms of individual network metrics, we observed that modularity made the greatest contribution to accurate prediction of epilepsy duration. Specifically, longer duration of epilepsy was associated with increasing modularity. These findings are consistent with an increasingly fragmentary network over time and, therefore, point to a relative paucity of effective long range connections between sub-specialized modules in the brain. Interestingly we observed that decreasing path length and increasing global efficiency were also important with respect to capturing the impact of epilepsy on the brain. It may seem counterintuitive that increased long range connections are associated with a more fragmentary network. However, in the setting of epilepsy, Hebbian processes strengthen synapses along pathways related to seizure propagation, rather than those contributing to efficient and functional subnetworks (Hernan et al., 2013). Connectivity in this setting, then, is potentiated without regard to network function, resulting in aberrant and potentially maladaptive pathways (Galicia et al., 2009, Koh et al., 2005). Our findings suggest that, although increased in number, many long range connections in epileptic brains are indeed maladaptive as they do not contribute to effective integration of functionally-specialized subnetworks (modules) in the brain.
To date, data regarding the capacity for network metrics to act as a surrogate for the impact of epilepsy on the brain are limited. However, our findings are generally in line with previous reports, most of which have compared epilepsy patients to healthy volunteers at the group level. In a study of children with cryptogenic frontal lobe epilepsy, Vaessen et al. demonstrated increased modularity compared to normal controls (Vaessen et al., 2013). They also observed, among all epilepsy patients, higher modularity in the group with cognitive impairment. Although they did not evaluate the contribution of modularity, Liao et al. observed functional networks characterized by lower clustering coefficient and shorter path length in a cohort of adult patients with temporal lobe epilepsy (Liao et al., 2010). By contrast, Vlooswijk et al. reported longer path lengths in a cohort of adult patients with cryptogenic localization-related epilepsy (Vlooswijk et al., 2011). These discrepancies regarding the impact of epilepsy on path length may relate to age of the cohort in question. Shorter path lengths were observed in children (our study) and young adults (Liao et al.), whereas longer path lengths were observed in older adults (Vlooswijk et al.). Together these findings point to a potentially important interaction between developmental physiology, which prioritizes growth and reorganization, and the impact of epilepsy on the brain.
This study has several limitations. First, this work is based on a cross-sectional design. A longitudinal study would be of great value toward defining the changes in resting-state functional connectivity that occur over time, particularly as they relate to cognitive decline. Second, we acknowledge that all patients in this study were being evaluated for epilepsy surgery and may not be representative of a more general epilepsy population. Third, the duration of each patient's epilepsy is not a perfect marker of the cumulative impact of their disease and likely does not encapsulate all the nuances of an individual's clinical course relevant to their disease burden. Specifically, it does not capture the frequency of seizures or their severity, nor does it account for the age of onset. It is worth noting, however, that our findings persisted after adjusting for age of epilepsy onset and, further, there is good reason to question the importance of seizure frequency with regard to measuring the impact of epilepsy on cognitive function (Sherman et al., 2012). It is worth noting, however, that duration of disease has been shown to be a valid marker of the cumulative impact of epilepsy on the brain, particularly as it relates to cognitive function (van Iterson et al., 2014). In a related issue, the question of anti-epileptic medications and their contribution to the overall neurologic morbidity of epilepsy could not be addressed by our retrospective design. Future study would be of great interest toward dissociating the impact of seizures on the brain versus that of antiepileptic drugs; differential effects of specific medications could also be addressed in such a study.
In conclusion, we report the following in a cohort of pediatric patients with focal epilepsy: a machine learning algorithm accurately predicted epilepsy duration based on global metrics of network architecture derived from resting state fMRI. These findings suggest that network metrics have the potential to form the basis for statistical models that translate quantitative imaging data into patient-level markers of cognitive deterioration. If these results are confirmed in longitudinal studies, resting state network analyses could be used to personalize clinical decision-making, particularly with regard to the optimal timing for surgical interventions in children with focal epilepsy.
References
- Berg A.T., Berkovic S.F., Brodie M.J. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia. 2010;51(4):676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Chen Z., He Y., Evans A.C., Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb. Cortex. 2011;21(9):2147–2157. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Bonilha L., Gross D.W. Network analysis for a network disorder: the emerging role of graph theory in the study of epilepsy. Epilepsy Behav. 2015;50:162–170. doi: 10.1016/j.yebeh.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Bjornaes H., Stabell K., Henriksen O., Loyning Y. The effects of refractory epilepsy on intellectual functioning in children and adults. A longitudinal study. Seizure. 2001;10(4):250–259. doi: 10.1053/seiz.2000.0503. [DOI] [PubMed] [Google Scholar]
- Breiman L. Random forests. Mach. Learn. 2001;45(1):5–32. [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Czochanska J., Langner-Tyszka B., Losiowski Z., Schmidt-Sidor B. Children who develop epilepsy in the first year of life: a prospective study. Dev. Med. Child Neurol. 1994;36(4):345–350. [PubMed] [Google Scholar]
- DeSalvo M.N., Douw L., Tanaka N., Reinsberger C., Stufflebeam S.M. Altered structural connectome in temporal lobe epilepsy. Radiology. 2014;270(3):842–848. doi: 10.1148/radiol.13131044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elger C.E., Helmstaedter C., Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3(11):663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- Fischl B., Liu A., Dale A.M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., van der Kouwe A.J. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl. 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Freitag H., Tuxhorn I. Cognitive function in preschool children after epilepsy surgery: rationale for early intervention. Epilepsia. 2005;46(4):561–567. doi: 10.1111/j.0013-9580.2005.03504.x. [DOI] [PubMed] [Google Scholar]
- Galicia E., Imai K., Mohamed I.S. Changing ictal-onset EEG patterns in children with cortical dysplasia. Brain Dev. 2009;31(8):569–576. doi: 10.1016/j.braindev.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Guye M., Bettus G., Bartolomei F., Cozzone P.J. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. Magma. 2010;23(5–6):409–421. doi: 10.1007/s10334-010-0205-z. [DOI] [PubMed] [Google Scholar]
- Hagmann P., Cammoun L., Gigandet X. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7) doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann B.P., Seidenberg M., Bell B. The neurodevelopmental impact of childhood onset temporal lobe epilepsy on brain structure and function and the risk of progressive cognitive effects. Prog. Brain Res. 2002;135:429–438. doi: 10.1016/S0079-6123(02)35040-4. [DOI] [PubMed] [Google Scholar]
- Hernan A.E., Holmes G.L., Isaev D., Scott R.C., Isaeva E. Altered short-term plasticity in the prefrontal cortex after early life seizures. Neurobiol. Dis. 2013;50:120–126. doi: 10.1016/j.nbd.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-J., Davis E.P., Sandman C.A. Children's intellectual ability is associated with structural network integrity. NeuroImage. 2016;124:550–556. doi: 10.1016/j.neuroimage.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S., Mathern G.W., Glasser G. Status epilepticus and frequent seizures: incidence and clinical characteristics in pediatric epilepsy surgery patients. Epilepsia. 2005;46(12):1950–1954. doi: 10.1111/j.1528-1167.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- Latora V., Marchiori M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001;87(19):198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu Y., Li J. Brain anatomical network and intelligence. PLoS Comput. Biol. 2009;5(5) doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Zhang Z., Pan Z. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One. 2010;5(1) doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M.E. The structure and function of complex networks. SIAM Rev. 2003;45(2):167–256. [Google Scholar]
- Newman M.E., Girvan M. Finding and evaluating community structure in networks. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2004;69(2 Pt 2):026113. doi: 10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- Paldino M.J., Hedges K., Zhang W. Independent contribution of individual white matter pathways to language function in pediatric epilepsy patients. NeuroImage. 2014;6:327–332. doi: 10.1016/j.nicl.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Weight-conserving characterization of complex functional brain networks. NeuroImage. 2011;56(4):2068–2079. doi: 10.1016/j.neuroimage.2011.03.069. [DOI] [PubMed] [Google Scholar]
- Sherman E.M., Brooks B.L., TB F.-M.C., WS M.A. Detecting epilepsy-related cognitive problems in clinically referred children with epilepsy: is the WISC-IV a useful tool? Epilepsia. 2012;53(6):1060–1066. doi: 10.1111/j.1528-1167.2012.03493.x. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spencer S., Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7(6):525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- Thomas C.G., Harshman R.A., Menon R.S. Noise reduction in BOLD-based fMRI using component analysis. NeuroImage. 2002;17(3):1521–1537. doi: 10.1006/nimg.2002.1200. [DOI] [PubMed] [Google Scholar]
- Vaessen M.J., Braakman H.M., Heerink J.S. Abnormal modular organization of functional networks in cognitively impaired children with frontal lobe epilepsy. Cereb. Cortex. 2013;23(8):1997–2006. doi: 10.1093/cercor/bhs186. [DOI] [PubMed] [Google Scholar]
- van Dellen E., Douw L., Baayen J.C. Long-term effects of temporal lobe epilepsy on local neural networks: a graph theoretical analysis of corticography recordings. PLoS One. 2009;4(11) doi: 10.1371/journal.pone.0008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.P., Stam C.J., Kahn R.S., Hulshoff Pol H.E. Efficiency of functional brain networks and intellectual performance. J. Neurosci. 2009;29(23):7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Diessen E., Diederen S.J., Braun K.P., Jansen F.E., Stam C.J. Functional and structural brain networks in epilepsy: what have we learned? Epilepsia. 2013;54(11):1855–1865. doi: 10.1111/epi.12350. [DOI] [PubMed] [Google Scholar]
- van Iterson L., Zijlstra B.J., Augustijn P.B., van der Leij A., de Jong P.F. Duration of epilepsy and cognitive development in children: a longitudinal study. Neuropsychology. 2014;28(2):212–221. doi: 10.1037/neu0000034. [DOI] [PubMed] [Google Scholar]
- Vlooswijk M.C., Vaessen M.J., Jansen J.F. Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology. 2011;77(10):938–944. doi: 10.1212/WNL.0b013e31822cfc2f. [DOI] [PubMed] [Google Scholar]
- Xia M., He Y. Magnetic resonance imaging and graph theoretical analysis of complex brain networks in neuropsychiatric disorders. Brain Connect. 2011;1(5):349–365. doi: 10.1089/brain.2011.0062. [DOI] [PubMed] [Google Scholar]