Abstract
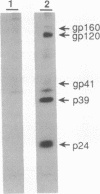
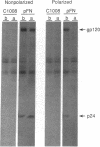
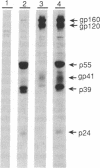
In polarized epithelial cells, the release of enveloped viruses by budding at the cell surface is restricted to a specific cell membrane domain, either the apical or basolateral domain. To investigate the role of the envelope glycoprotein and the capsid proteins of human immunodeficiency virus type 1 (HIV-1) in determining the site of virus assembly, we analyzed virus maturation in a polarized monkey kidney cell line. A line of cells harboring the HIV-1 provirus (VERO-pFN) was found to differentiate into polarized epithelial cell monolayers upon reaching confluency. By electron microscopy, virus maturation was observed predominantly at the basolateral membranes of VERO-pFN cells. Analysis of HIV-1 proteins revealed that virtually all of glycoprotein gp120 and capsid protein p24 were found in the basolateral medium, while no HIV-1 proteins were detected apically. A recombinant vaccinia virus (VV) expressing the HIV-1 gag polyprotein (VVgag) was used to determine the site of release of HIV-1 core particles in polarized epithelial cells in the presence or absence of envelope glycoproteins. When cells were infected with VVgag in the absence of envelope proteins, similar amounts of the p24 capsid protein were released into virus particles at the apical or basolateral surface. In contrast, when cells were doubly infected with VVgag and a recombinant VV expressing the HIV-1 envelope glycoprotein (VVenv), 94% of p24 and all of gp120 were found to be associated with particles released into the basolateral medium. These results indicate that the HIV-1 envelope glycoprotein directly influences the site of release of virus particles containing the gag protein, probably via a specific interaction between the envelope protein and the gag protein.
Full text
PDF

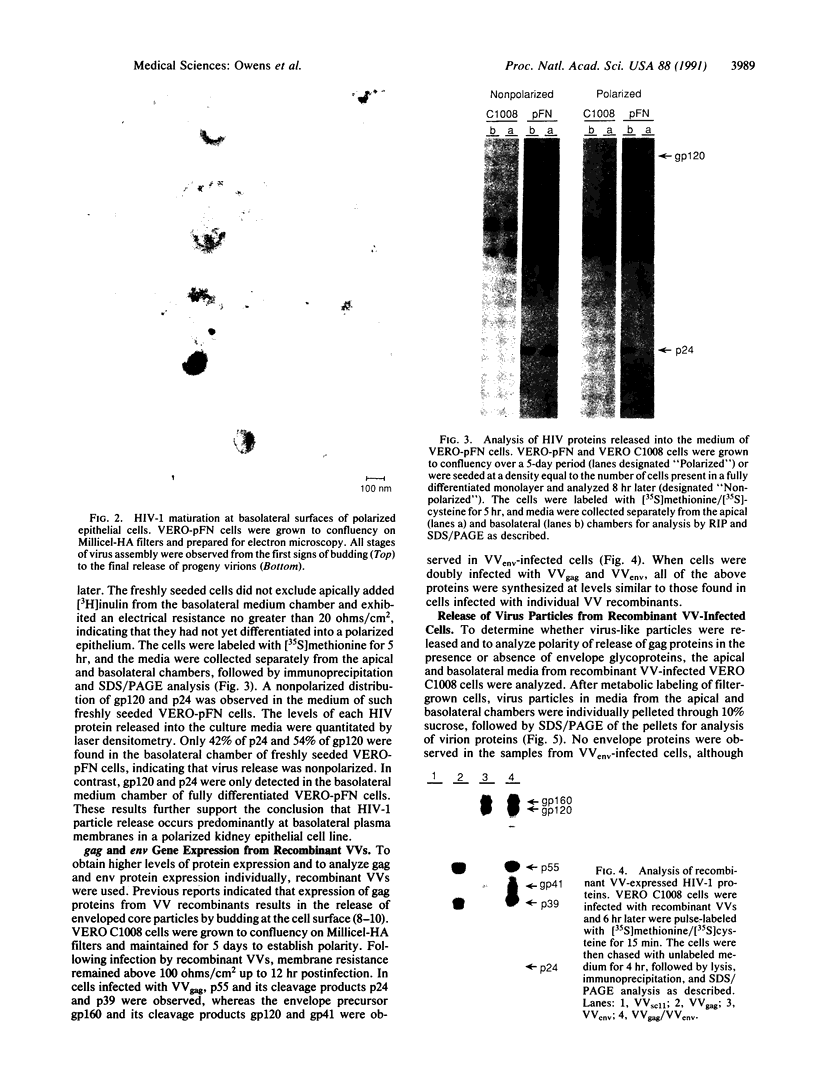


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J. E., Fusco P. J. The M protein of vesicular stomatitis virus associates specifically with the basolateral membranes of polarized epithelial cells independently of the G protein. J Cell Biol. 1988 Nov;107(5):1707–1715. doi: 10.1083/jcb.107.5.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. S., Ariel N., Huang A. S. Membrane anchors of vesicular stomatitis virus: characterization and incorporation into virions. J Virol. 1988 Aug;62(8):2552–2556. doi: 10.1128/jvi.62.8.2552-2556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt A., Bosch J. V., Ziemiecki A., Friis R. R. Rous sarcoma virus p19 and gp35 can be chemically crosslinked to high molecular weight complexes. An insight into virus assembly. J Mol Biol. 1984 Apr 5;174(2):297–317. doi: 10.1016/0022-2836(84)90340-1. [DOI] [PubMed] [Google Scholar]
- Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D., De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989 Oct 6;59(1):103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- Haffar O., Garrigues J., Travis B., Moran P., Zarling J., Hu S. L. Human immunodeficiency virus-like, nonreplicating, gag-env particles assemble in a recombinant vaccinia virus expression system. J Virol. 1990 Jun;64(6):2653–2659. doi: 10.1128/jvi.64.6.2653-2659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. V., Compans R. W., Davis A. R., Bos T. J., Nayak D. P. Surface expression of influenza virus neuraminidase, an amino-terminally anchored viral membrane glycoprotein, in polarized epithelial cells. Mol Cell Biol. 1985 Sep;5(9):2181–2189. doi: 10.1128/mcb.5.9.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. V., Compans R. W., Davis A. R., Bos T. J., Nayak D. P. Surface expression of influenza virus neuraminidase, an amino-terminally anchored viral membrane glycoprotein, in polarized epithelial cells. Mol Cell Biol. 1985 Sep;5(9):2181–2189. doi: 10.1128/mcb.5.9.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Blaug G., Hansen M., Barklis E. Assembly of gag-beta-galactosidase proteins into retrovirus particles. J Virol. 1990 May;64(5):2265–2279. doi: 10.1128/jvi.64.5.2265-2279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karacostas V., Nagashima K., Gonda M. A., Moss B. Human immunodeficiency virus-like particles produced by a vaccinia virus expression vector. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8964–8967. doi: 10.1073/pnas.86.22.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Metsikkö K., Garoff H. Oligomers of the cytoplasmic domain of the p62/E2 membrane protein of Semliki Forest virus bind to the nucleocapsid in vitro. J Virol. 1990 Oct;64(10):4678–4683. doi: 10.1128/jvi.64.10.4678-4683.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsikkö K., Simons K. The budding mechanism of spikeless vesicular stomatitis virus particles. EMBO J. 1986 Aug;5(8):1913–1920. doi: 10.1002/j.1460-2075.1986.tb04444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. J., Alexander N. J., Sutjipto S., Lackner A. A., Gettie A., Hendrickx A. G., Lowenstine L. J., Jennings M., Marx P. A. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989 Oct;63(10):4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A., Wiley C. A., Reynolds-Kohler C., Reese C. E., Margaretten W., Levy J. A. Human immunodeficiency virus detected in bowel epithelium from patients with gastrointestinal symptoms. Lancet. 1988 Feb 6;1(8580):259–262. doi: 10.1016/s0140-6736(88)90348-0. [DOI] [PubMed] [Google Scholar]
- Owens R. J., Compans R. W. Expression of the human immunodeficiency virus envelope glycoprotein is restricted to basolateral surfaces of polarized epithelial cells. J Virol. 1989 Feb;63(2):978–982. doi: 10.1128/jvi.63.2.978-982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez L. G., Davis G. L., Hunter E. Mutants of the Rous sarcoma virus envelope glycoprotein that lack the transmembrane anchor and cytoplasmic domains: analysis of intracellular transport and assembly into virions. J Virol. 1987 Oct;61(10):2981–2988. doi: 10.1128/jvi.61.10.2981-2988.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddington L., Woodgett C., Rose J. K. Replacement of the cytoplasmic domain alters sorting of a viral glycoprotein in polarized cells. Proc Natl Acad Sci U S A. 1987 May;84(9):2756–2760. doi: 10.1073/pnas.84.9.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Amino acid substitutions within the matrix protein of type D retroviruses affect assembly, transport and membrane association of a capsid. EMBO J. 1991 Mar;10(3):535–546. doi: 10.1002/j.1460-2075.1991.tb07980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Structural role of the matrix protein of type D retroviruses in gag polyprotein stability and capsid assembly. J Virol. 1990 Sep;64(9):4383–4389. doi: 10.1128/jvi.64.9.4383-4389.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980 May;20(1):45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman L. M., Garoff H. Alteration of the cytoplasmic domain of the membrane-spanning glycoprotein p62 of Semliki Forest virus does not affect its polar distribution in established lines of Madin-Darby canine kidney cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2607–2618. doi: 10.1083/jcb.103.6.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W., Giusti L., Davis A. R., Nayak D. P., Gething M. J., Sambrook J. Influenza virus hemagglutinin expression is polarized in cells infected with recombinant SV40 viruses carrying cloned hemagglutinin DNA. Cell. 1983 Jun;33(2):435–443. doi: 10.1016/0092-8674(83)90425-7. [DOI] [PubMed] [Google Scholar]
- Roth M. G., Srinivas R. V., Compans R. W. Basolateral maturation of retroviruses in polarized epithelial cells. J Virol. 1983 Mar;45(3):1065–1073. doi: 10.1128/jvi.45.3.1065-1073.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Luftig R. B. Comparative immunofluorescence of murine leukemia virus-derived membrane-associated antigens. Virology. 1983 Jan 30;124(2):259–273. doi: 10.1016/0042-6822(83)90343-4. [DOI] [PubMed] [Google Scholar]
- Satake M., McMillan P. N., Luftig R. B. Effect of vinblastine on distribution of murine leukemia virus-derived membrane-associated antigens. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6266–6270. doi: 10.1073/pnas.78.10.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Shibuta H. Production of human immunodeficiency virus (HIV)-like particles from cells infected with recombinant vaccinia viruses carrying the gag gene of HIV. Virology. 1990 Mar;175(1):139–148. doi: 10.1016/0042-6822(90)90194-v. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Cho M. I., Hammarskjöld M. L., Rekosh D. Human immunodeficiency virus type 1 Pr55gag and Pr160gag-pol expressed from a simian virus 40 late replacement vector are efficiently processed and assembled into viruslike particles. J Virol. 1990 Jun;64(6):2743–2750. doi: 10.1128/jvi.64.6.2743-2750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W. Assembly of animal viruses at cellular membranes. Annu Rev Microbiol. 1988;42:489–516. doi: 10.1146/annurev.mi.42.100188.002421. [DOI] [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W., Earl P., Moss B. Surface expression of viral glycoproteins is polarized in epithelial cells infected with recombinant vaccinia viral vectors. EMBO J. 1986 Feb;5(2):237–245. doi: 10.1002/j.1460-2075.1986.tb04204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Yamakawa M., Tobita K., Seto J. T., Klenk H. D., Rott R. Altered budding site of a pantropic mutant of Sendai virus, F1-R, in polarized epithelial cells. J Virol. 1990 Oct;64(10):4672–4677. doi: 10.1128/jvi.64.10.4672-4677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Garofff H. Subunit composition of the membrane glycoprotein complex of Semliki Forest virus. J Mol Biol. 1978 Jul 5;122(3):259–269. doi: 10.1016/0022-2836(78)90189-4. [DOI] [PubMed] [Google Scholar]