Abstract
The time to neutrophil engraftment for adult patients after myeloablative double unit umbilical cord blood (UCB) transplantation is 23 days when the two units are given i.v. We hypothesized that the intra-BM injection (IBMI) of one of the two UCB units would reduce systemic loss of hematopoietic progenitors and shorten time to neutrophil recovery after myeloablation. Ten patients with a median age of 35 years were transplanted. The unit to be given by IBMI was randomly assigned; the other unit was given i.v. The median infused graft total nucleated cell dose was 3.7 × 107/kg with no difference between i.v. and IBMI units. All patients tolerated the procedure well, and there was no severe adverse event related to IBMI. The median time to neutrophil engraftment and plt recovery >50 000/μl was 21 and 69 days, respectively. In all, 9 of 10 patients engrafted, 5 with the i.v. unit and 4 with the IBMI unit; 7 of 8 evaluable patients developed acute GVHD and 5 of 10 patients died from treatment-related causes. Survival was 47% at 1 year. Despite safety of administration, IBMI of one of two UCB units did not shorten the time to neutrophil engraftment and offers no advantage over conventional double unit transplantation.
Keywords: double umbilical cord blood transplant, myeloablative, intra-BM injection
Introduction
Umbilical cord blood (UCB) is increasingly used as a source of hematopoietic stem cells for adults as well as children. It is well established that the time to neutrophil engraftment after UCB transplantation (UCBT) is substantially delayed as compared with both BM and mobilized peripheral blood transplantation with neutrophil recovery after UCBT, taking a median of 7–10 days longer than alternative unrelated stem cell sources.1–3 In addition to lower hematopoietic progenitor cell doses, differences in maturational and hematopoietic homing factors may be involved in this engraftment delay.4
Animal models have suggested that injection of hematopoietic stem cells directly into the BM space can improve homing and engraftment after transplantation.5–9 This effect may be attributed to a higher proportion of hematopoietic stem cells reaching the BM microenvironment10 as well as to enhanced colonization and migration to other bones.6,8 Therefore, in an attempt to reduce nonspecific losses of UCB hematopoietic stem cells when it is administered i.v., we evaluated the safety and feasibility of UCB intra-BM injection (IBMI). However, to enhance patient safety, we used a double unit platform with only one unit being injected with IBMI and the other i.v. as an ‘in vivo backup.’ Our hypothesis was that IBMI would confer a selective advantage as suggested in animal models resulting in (1) faster neutrophil recovery and (2) an increased likelihood that the IBMI unit would predominate in engraftment. In addition, we hypothesized that the procedure could be carried out safely on the basis of prior experience with IBMI of BM.11
Patients and methods
Patients
Between July 2005 and September 2007, we conducted a phase I–II study to assess the safety and potential efficacy of UCB IBMI following myeloablative conditioning. Ten patients with a median age of 35 years (range, 20–44), who had advanced or high-risk hematological malignancies12 and who required two UCB units to achieve a graft total nucleated cell (TNC) dose ≥3.0 × 107/kg, were eligible. Thirty-one patients were screened but were ineligible because of an inability to tolerate myeloablation (n = 12), unwillingness to participate (n = 11), disease relapse (n = 3), a double unit graft not being required (n = 2), body habitus unsuitable for IBMI (n = 2), and unsuitability of unit volume reduction owing to the high red blood cell content (n = 1). The study was approved by the University of Minnesota Cancer Protocol Review Committee and Institutional Review Board, and registered at clinicaltrials.gov as NCT00295880. All patients provided informed consent according to the principles of the Declaration of Helsinki.
UCB unit selection
Umbilical cord blood units were matched with the patient and with each other at ≥4/6 HLA-A, B antigens and HLA-DRB1 alleles. Each of the two UCB units was required to have a minimum cryopreserved TNC of 1.5 × 107/kg. Methodology of HLA typing has been detailed elsewhere.1
UCB unit processing and randomization
Before the initiation of the preparative regimen, units were shipped to the University of Minnesota Medical Center Cell Therapy Laboratory in a dry shipper cooled by liquid nitrogen (temperature < −150 °C). They were maintained in the vapor phase of liquid nitrogen until the day of transplantation.
Units were thawed and washed according to previously established techniques.13 Units were randomized to either IBMI or i.v. administration. The IBMI unit was resuspended in approximately 40 ml, and two approximately 20 ml aliquots were administered individually into each posterior superior iliac crest. Subsequently, the i.v. unit was administered within 30 min.
IBMI technique
All patients were pre-medicated with acetaminophen and diphenhydramine, and given mild sedation with i.v. lorazepam and morphine at the bedside. Vancomycin as antibiotic prophylaxis was administered prior to the procedure. Under sterile technique with the patient in the prone position, local anesthesia with 1% lidocaine was injected into each posterior superior iliac crest. Marrow aspiration needles of 15-gauge 2-inch measurement were used for IBMI (Angiotech, Gainesville, FL, USA). Only a single puncture to position the needle in the marrow space was permitted in each hemi-pelvis, with needle positioning confirmed by aspirating approximately 1–2 ml of marrow. Using a 20 ml syringe with a Luer lock, the UCB was infused into the marrow space over approximately 10 min (1–2 ml/min). During the infusion, oxygen saturation was monitored continuously with vital signs every 15 min for 1 h. After infusion was completed, direct pressure was applied to each IBMI site for 10 min/side. Patients were formally re-evaluated at 24 h and 7 days after the IBMI for evidence of local complications.
Treatment
The conditioning regimen consisted of CY 60 mg/kg i.v. every 24 h on days −7 and −6, fludarabine 25 mg/m2/day i.v. every 24 h on days −8, −7 and −6, and TBI 165 cGy twice daily on days −4 to −1. All patients received CYA twice daily from day −3 for at least 3 months with target trough levels of 200–400 ng/ml and mycophenolate mofetil at 1 g i.v. or orally twice daily from day −3 to +30. Supportive care has been described earlier.1,14
Statistical considerations
The primary study end point was the day to neutrophil recovery with the primary objective being to show a 5-day reduction in median time to neutrophil recovery compared with our institutional historical control of 23 days (range 15–41) observed in recipients of two UCB units administered i.v.1 Neutrophil recovery was defined as the first of three consecutive days with ANC ≥500/μl. The second aim was to establish the safety and tolerability of the IBMI technique. The other secondary end points used to describe which unit was responsible for sustained donor engraftment using this transplant strategy included the cumulative incidence of plt recovery ≥50 000/μl, the incidences of acute GVHD at day 100 and chronic GVHD at 1 year and TRM at 6 months and the probabilities of overall survival at 1 year.
Event times for neutrophil recovery were measured from the date of transplantation and were censored for death or disease progression before day 21 without neutrophil recovery. Primary graft failure was defined as lack of neutrophil recovery (ANC ≥500/μl) at day 42. Total donor engraftment was defined as the percentage of cells derived from both units. Diagnosis of acute and chronic GVHD was based on standard clinical criteria with histopathologic confirmation where possible.15
Days to neutrophil and plt recovery were reported as medians and ranges. Cumulative incidence was used to estimate the rate of neutrophil recovery and plt recovery treating non-event deaths as a competing risk.16 The statistical end point of overall survival was estimated by the Kaplan–Meier method.17 The WBC and ANC counts over time were reported as averages with 95% confidence intervals derived from the standard error. Calculation of the likelihood of achieving the primary objective, given the results of the first 10 patients, was performed by assuming a normal distribution of days to engraftment. Conditioning on an average rate of engraftment of 25.6 days in the first 10 patients and an standard deviation of 10.7, the probability of an overall average of 18 days or less was calculated from the normal distribution tables assuming a true average rate of engraftment of 18 days.
Results
Patient and graft characteristics
Twelve patients were eligible and enrolled into the study, but two were subsequently disqualified because the IBMI unit could not be adequately volume reduced in one and unit randomization did not occur in the other. Therefore, 10 patients received UCBT by IBMI. Patient and graft characteristics are summarized in Table 1. The median recipient age was 35 years (range 20–44) and median weight was 73.6 kg (range 56–92). The median infused TNC dose of the graft was 3.7 × 107/kg (range 2.9–5.3) (i.v. unit median infused TNC was 2.0 × 107/kg (range 1.2–3.0) and that of the IBMI unit was 1.8 × 107/kg (range 1.4–3.4)). HLA matching of the i.v. and IBMI units to the recipient (although not dictated per protocol design) was the same in all cases; one patient received two 6/6 units, two received two 5/6 units and seven received two 4/6 units.
Table 1.
Patient and graft characteristics
| UPN | Age (years) |
Weight (kg) |
Diagnosis | Disease stage |
IBMI unit
|
i.v. unit
|
IBMI unit HLA-match |
i.v. unit HLA-match |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NCD (×107/kg) |
CD34 ( ×105/kg) |
CD3 ( ×107/kg) |
CFU-GM ( ×104/kg) |
Viability (%) |
NCD ( ×107/kg) |
CD34 ( ×105/kg) |
CD3 ( ×107/kg) |
CFU-GM ( ×104/kg) |
Viability (%) |
|||||||
| 4226 | 20 | 67 | ALL | CR2 | 2.1 | 1.9 | 0.9 | 0.7 | 78 | 1.5 | 2.8 | 0.6 | 5.0 | 71 | 4/6 | 4/6 |
| 4241 | 32 | 92 | NHLa | CR1 | 1.6 | 6.7 | 0.4 | NA | 65 | 2.0 | 3.4 | 0.8 | NA | 72 | 4/6 | 4/6 |
| 4276 | 44 | 89 | NHLb | Relapse | 1.7 | 3.4 | 0.9 | 2.1 | 50 | 1.9 | 2.5 | 0.9 | 4.8 | 80 | 6/6 | 6/6 |
| 4280 | 41 | 69 | AML, M4 | CR1 | 3.4 | 7.7 | 2.0 | 3.5 | 79 | 1.2 | 2.2 | 0.5 | 3.8 | 70 | 4/6 | 4/6 |
| 4399 | 33 | 62 | ALL, Ph+ CR1 | 1.6 | 2.2 | 0.6 | 1.8 | 70 | 2.0 | 2.1 | 0.7 | 2.2 | 74 | 4/6 | 4/6 | |
| 4451 | 41 | 72 | AML, M1 | CR1 | 1.5 | 1.8 | 0.6 | 1.2 | 62 | 2.8 | 2.5 | 0.8 | 0.6 | 59 | 4/6 | 4/6 |
| 4544 | 28 | 86 | ALL | CR1 | 2.5 | 10.6 | 1.4 | 3.5 | 68 | 2.1 | 2.2 | 0.9 | 1.0 | 80 | 4/6 | 4/6 |
| 4548 | 38 | 90 | ALL | CR1 | 1.4 | 1.5 | 0.6 | NA | 68 | 1.5 | 3.0 | 0.5 | NA | 69 | 5/6 | 5/6 |
| 4555 | 23 | 56 | AML, M0 | CR1 | 1.8 | 1.2 | 0.5 | 1.4 | 71 | 2.0 | 5.0 | 0.7 | 2.8 | 86 | 4/6 | 4/6 |
| 4681 | 44 | 75 | NHLc | Relapse | 2.3 | 2.1 | 0.6 | 1.9 | 65 | 3.0 | 2.5 | 1.5 | 1.5 | 66 | 5/6 | 5/6 |
| Median | 35 | 73.6 | NA | NA | 1.8 | 2.2 | 0.6 | 1.85 | 68 | 2.0 | 2.5 | 0.8 | 2.5 | 71.5 | NA | NA |
Abbreviations: CFU-GM = colony-forming units granulocyte-macrophage; IBMI = intra-BM injection; NA = not available or not applicable; NCD = nucleated cell dose; NHL = non-Hodgkin’s lymphoma; M0 = undifferentiated myeloid leukemia; M1 = minimally differentiated myeloid leukemia; M4 = myelomonocytic myeloid leukemia; PD = progressive disease; UPN = unique patient number.
T-cell lymphoblastic NHL
Large cell B-cell NHL
Mantle cell
IBMI safety and tolerability
All patients were awake and tolerated the IBMI procedure well with a median maximal pain score (scale 0–10) during the infusion of 3.5 (range 0–7). The level of discomfort was no greater than, and in some cases less than, that previously experienced with BM aspirates. It is noted that pain improved by slowing down the IBMI and resolved promptly after completing the procedure.
None of the patients had any discernible adverse event from the IBMI procedure. The median oxygen saturation prior to IBMI was 100% (range 98–100%) and 1 h post IBMI was 100% (range 97–100). The median respiratory rate prior to IBMI was 18/min (range, 16–22) and 1 h post IBMI was 17/min (range, 16–24). The median heart rate prior to IBMI was 95/min (range, 52–114) and 1 h post IBMI was 83/min (range, 53–99). The median blood pressure prior to and 1 h post IBMI were 121/76 mm Hg (range, 104–158/56/91) and 131/79 mm Hg (range, 105–160/69–93), respectively. Inspection of the IBMI sites showed healing and no local pain, mass, erythema or increase in skin temperature at 24 h and 7 days after the procedure.
Hematopoietic recovery
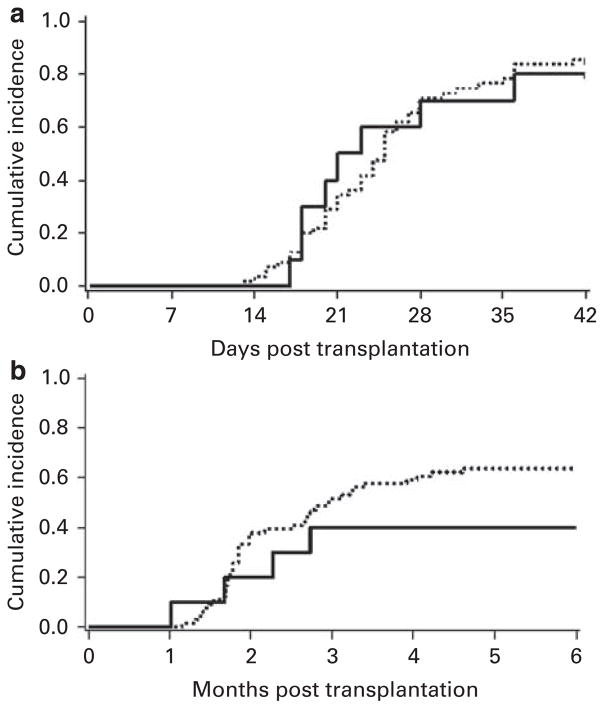
The median time to neutrophil engraftment and plt recovery >50 000/μl was 21 (range 17–49) and 69 (range 30–272) days, respectively (Figures 1a and b). The median WBC count and ANC over time for all patients are shown in Figures 2a and b, respectively. One patient died of graft failure. All of the nine patients who engrafted showed complete chimerism with a single unit that was accounted for by the IBMI unit in four patients and by the i.v. unit in five patients, respectively. Further, the pattern of chimerism was not different from that seen with i.v. double unit UCBT. At days 21–28, the median total donor chimerism was 100% (range, 84–100) with only three patients showing engraftment of both units. Subsequently, donor engraftment was accounted for by a single unit.
Figure 1.
(a) Neutrophil engraftment and (b) plt recovery ≥ 50 000/μl after myeloablative UCB transplantation with IBMI (—) and the historical controls who received two UCB i.v. (- - -).
Figure 2.
(a) Median ANC and (b) WBC count from day 0 until day 60 after IBMI. Solid line (—) denotes median and interrupted lines (- - -) denote the 95% confidence intervals.
Outcomes
Patient outcomes are summarized in Table 2. Seven of eight evaluable patients developed acute GVHD. Five patients developed grade II and two patients developed grade III acute GVHD. Chronic GVHD was observed in two of seven patients at risk. In all, 5 of 10 patients died from treatment-related causes: 2 from infection, 1 from hemorrhage, 1 from graft failure and 1 from acute GVHD. One patient relapsed 127 days after UCBT. However, after tapering the immunosuppression and treatment with rituximab, this patient remains alive and disease free now 22 months after transplantation. Of the patients engrafting, four of nine are alive at a median follow-up of 10 months (range 3.4–31). The probability of survival was 47% (95% confidence interval: 14–80) at 1 year (Figure 3).
Table 2.
Outcomes after umbilical cord blood transplantation with IBMI
| UPN | Time to neutrophil engraftment (days) | Time to plt engraftment ≥50 000/μl (days) | Dominant UCB unit | Grade of acute GVHD | Chronic GVHD | Relapse | Length of follow-up (months) | Alive/dead | Cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 4226 | 28 | 272 | i.v. | 2 | Yes | No | 25 | Alive | NA |
| 4241 | 23 | Not reached | i.v. | 2 | No | NA | 5.0 | Dead | CMV infection |
| 4276 | 21 | 51 | i.v. | 2 | Yes | PD at day 100 | 31 | Alive | NA |
| 4280 | 36 | Not reached | IBMI | 2 | NA | NA | 1.5 | Dead | Hemorrhage |
| 4399 | Graft failure | Graft failure | NA | NA | NA | NA | 1.0 | Dead | Graft failure |
| 4451 | 17 | Not reached | IBMI | 2 | No | NA | 4.8 | Dead | Fungal infection |
| 4544 | 18 | 69 | IBMI | 2 | No | No | 10 | Alive | NA |
| 4548 | 20 | 83 | i.v. | 2 | No | No | 10 | Alive | NA |
| 4555 | 49 | No | IBMI | No | NA | NA | 2.7 | Dead | Acute GVHD |
| 4681 | 18 | 31 | i.v. | No | No | No | 3.4 | Alive | NA |
Abbreviations: IBMI = intra-BM injection; NA = not applicable; NCD = nucleated cell dose; PD = progressive disease; UCB umbilical cord blood; UPN = unique patient number.
Figure 3.
OS after double UCB transplantation with IBMI (—) and the historical controls who received two UCB IV (- - -).
Interim analysis
We expedited an interim analysis that had been planned after 17 patients had been enrolled. Our primary objective was to show a 5-day improvement in time to neutrophil recovery over the historical control group when two UCB units were given i.v. (median 23 days).1 We observed a median time to neutrophil engraftment in the first 10 patients of 21 days (range 17–49). For the interim analysis, we tested two hypotheses to assess the futility of a definitive result given the planned trial size of 29 patients. If we assumed that the patients do benefit from the IBMI infusion, the probability to achieve an average engraftment rate of 14.6 days or less among the remaining 19 patients is 0.02 (2%). As this hypothesis was unlikely, the study was closed for statistical projection of futility.
Discussion
Our study has a number of significant findings. First, we observed that the procedure of IBMI at the bedside was both safe and well tolerated. This is similar to what was reported by Hagglund et al.11 who described nine patients who received a sibling marrow graft by IBMI. However, in their series, one patient was unable to receive the whole graft by IBMI owing to pain. As patients may have a variable tolerance to pain, different supportive care may be needed, but our experience suggests that only light i.v. sedation is sufficient for the majority of individuals. Interestingly, Frassoni et al.18,19 recently reported IBMI after patient sedation with i.v. propofol. Our findings show that such anesthesia is not necessary.
Interestingly, despite the UCB unit being thawed and washed as described by Rubinstein et al.,13 the distinct odor of the residual dimethyl sulfoxide was noticed in the room in all patients within minutes of starting the IBMI infusion, suggesting that, after infusion into the marrow space, the UCB product reaches the systemic circulation quickly. This observation supports the notion that there was a potential risk of fat embolism. However, none of the patients developed clinical signs or symptoms of fat embolism such as chest pain, tachypnea or decreased oxygen saturation. This suggests that our IBMI infusion rate was safe and that if any fat embolized, it was not clinically apparent or meaningful.
The second major observation is the lack of any suggestion of superiority of IBMI over i.v. infusion in the double unit setting. The objective of achieving a 5-day reduction in median time to neutrophil recovery, compared with our institutional historical control of 23 days in recipients of two UCB units administered i.v.,1 was based on what would be a clinically meaningful difference that would lead us to consider a change in our clinical practice. Importantly, a shortening from 23 to 18 days would bring the time to neutrophil recovery after UCB, similar to that after unrelated adult BM or peripheral blood.20
The median time to neutrophil (21 days) and plt recovery (69 days) observed in this study is comparable with what we have reported earlier when both units are infused i.v.1,21 with no predominance in engraftment of the IBMI unit. Frassoni et al.18,19 report a similar time to neutrophil engraftment (median 23 days) but substantially faster time to plt recovery (median 38 days) with IBMI of a single UCB unit. This group has suggested that IBMI provides for faster colonization and subsequent seeding of the marrow space by donor cells accounting for the improved hematopoietic recovery.
There are important differences between our study and the one by Frassoni et al.18,19 that could contribute to varying results: we used a double unit graft and a single conditioning regimen, whereas Frassoni et al.18,19 used a single unit graft and multiple conditioning regimens that included anti-thymocyte globulin; our total infused TNC dose (a factor known to influence engraftment22) was a median of 3.7 × 107 and 1.8 × 107/kg for the IBMI unit as compared with 2.3 × 107/kg, and we used a single 20ml IBMI injection on each side as compared with four 5ml injections on the same side in the study by Frassoni et al.18,19 Nonetheless, our findings do not support any assertion of superiority of the IBMI approach.
Most patients in our IBMI study developed acute GVHD, which may be associated with the utilization of the double unit grafts as reported earlier.1,14,23 In contrast, Frassoni et al.,18,19 reported that only 3 of 29 patients who received IBMI UCBT with a single unit developed acute GVHD. However, the incidence of acute GVHD reported by Frassoni et al.19 was low even if compared with children receiving conventional single unit UCBT and may be accounted for by their use of anti-thymocyte globulin. Survival in this study was consistent with our original report of transplantation with two partially HLA-matched UCB units,1 and was similar to the series by Frassoni et al.18,19 that reported 16 of 29 patients alive after a median follow-up of 7.5 months with no suggestion of an advantage using the IBMI approach.
In summary, despite showing the safety and tolerability of the IBMI technique with no adverse events associated with this route of administration, our data suggest that IBMI is only a more cumbersome and uncomfortable method to infuse a UCB unit in the setting of UCB transplantation with two units. Our study was therefore aborted because of the futility of meeting the primary objective. We speculate that potential reasons for not observing improved engraftment that was expected from reports of IBMI in murine transplant models may include the following: (1) our method of IBMI was suboptimal, leading to disruption of the marrow microenvironment24 (that is, rate was too fast or volume was too great for the iliac bones, thus effectively performing i.v. infusion), and (2) the total UCB TNC dose was relatively high, limiting any meaningful improvement in neutrophil or plt recovery. Given the likely biological differences between transplantation of one vs two UCB units, it is possible that IBMI might still be a valuable strategy in patients receiving a single UCB unit graft19 with simplicity and cost-effective advantages as compared, for example, with ex vivo expansion.
Taken together, these data suggest that the IBMI technique is safe but does not improve time to neutrophil recovery or provide an engraftment advantage for patients receiving two partially HLA-matched UCB units i.v. Further prospective studies would be needed to determine whether there is any real benefit in terms of engraftment and GVHD for patients receiving one UCB unit.
Acknowledgments
We thank Marilee Larkin and Kathy Gulberg for their assistance with patient recruiting, clinical monitoring and the execution of the IBMI procedure. This work was supported in part by grants from the National Cancer Institute PO1-CA65493 (JEW, TED), The Children’s Cancer Research Fund (JEW, TED), and the National Marrow Donor Program Prime Award #13468 (JNB)
References
- 1.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 2.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 3.Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 4.Wagner JE. A new route to the stem-cell niche. Lancet Oncol. 2008;9:812–814. doi: 10.1016/S1470-2045(08)70214-6. [DOI] [PubMed] [Google Scholar]
- 5.Zhong JF, Zhan Y, Anderson WF, Zhao Y. Murine hematopoietic stem cell distribution and proliferation in ablated and nonablated bone marrow transplantation. Blood. 2002;100:3521–3526. doi: 10.1182/blood-2002-04-1256. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Kimura T, Asada R, Harada S, Yokota S, Kawamoto Y, et al. SCID-repopulating cell activity of human cord blood-derived CD34− cells assured by intra-bone marrow injection. Blood. 2003;101:2924–2931. doi: 10.1182/blood-2002-09-2782. [DOI] [PubMed] [Google Scholar]
- 7.Kushida T, Inaba M, Hisha H, Ichioka N, Esumi T, Ogawa R, et al. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001;97:3292–3299. doi: 10.1182/blood.v97.10.3292. [DOI] [PubMed] [Google Scholar]
- 8.Mazurier F, Doedens M, Gan OI, Dick JE. Rapid myelo-erythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells. Nat Med. 2003;9:959–963. doi: 10.1038/nm886. [DOI] [PubMed] [Google Scholar]
- 9.Yahata T, Ando K, Sato T, Miyatake H, Nakamura Y, Muguruma Y, et al. A highly sensitive strategy for SCID-repopulating cell assay by direct injection of primitive human hematopoietic cells into NOD/SCID mice bone marrow. Blood. 2003;101:2905–2913. doi: 10.1182/blood-2002-07-1995. [DOI] [PubMed] [Google Scholar]
- 10.Cui J, Wahl RL, Shen T, Fisher SJ, Recker E, Ginsburg D, et al. Bone marrow cell trafficking following intravenous administration. Br J Haematol. 1999;107:895–902. doi: 10.1046/j.1365-2141.1999.01779.x. [DOI] [PubMed] [Google Scholar]
- 11.Hagglund H, Ringden O, Agren B, Wennberg L, Remberger M, Rundquist L, et al. Intraosseous compared to intravenous infusion of allogeneic bone marrow. Bone Marrow Transplant. 1998;21:331–335. doi: 10.1038/sj.bmt.1701116. [DOI] [PubMed] [Google Scholar]
- 12.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 13.Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 16.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Frassoni F, Gualandi F, Podesta M, Raiola AM, Ibatici A, Piaggio G, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9:831–839. doi: 10.1016/S1470-2045(08)70180-3. [DOI] [PubMed] [Google Scholar]
- 19.Ibatici A, Raiola AM, Podestà M, Gualandi F, Sessarego N, Parodi A, et al. Direct intra-bone marrow injection of unrelated umbilical cord blood cells overcomes the problem of delayed engarftment and improves the feasibility of hematopoietic transplant in adult patients. Blood. 2007;110:105a. [Google Scholar]
- 20.Khoury HJ, Loberiza FR, Jr, Ringden O, Barrett AJ, Bolwell BJ, Cahn JY, et al. Impact of posttransplantation G-CSF on outcomes of allogeneic hematopoietic stem cell transplantation. Blood. 2006;107:1712–1716. doi: 10.1182/blood-2005-07-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunstein CG, Defor T, Wagner JE. Umbilical cord blood transplantation (UCBT) at the university of Minnesota: impact of double unit and fludarabine on engraftment after myeloablative conditioning. Blood. 2006;108:888a. [Google Scholar]
- 22.Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 23.MacMillan M, Weisdorf DJ, Brunstein CG, Cao Q, Defor T, Verneris MR, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2008 doi: 10.1182/blood-2008-07-163238. e-pub ahead of print 7 November 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slayton WB, Li XM, Butler J, Guthrie SM, Jorgensen ML, Wingard JR, et al. The role of the donor in the repair of the marrow vascular niche following hematopoietic stem cell transplant. Stem Cells. 2007;25:2945–2955. doi: 10.1634/stemcells.2007-0158. [DOI] [PubMed] [Google Scholar]