Abstract
Objective
Inflammation in diseases such as rheumatoid arthritis (RA) stimulates osteoclast-mediated articular bone erosion and inhibits osteoblast-mediated bone formation, leading to a net loss of bone. Pro-inflammatory cytokines and antagonists of the Wnt signaling pathway have been implicated in the inhibition of osteoblast differentiation and activity in RA, contributing to the erosive process and impairing erosion healing. Importantly, osteoblast differentiation and function are also regulated by the osteogenic bone morphogenetic protein (BMP) signaling pathway, which is antagonized by BMP3. We therefore examined the potential role of BMP3 in inflammatory arthritis.
Methods
Two murine models of RA, K/BxN serum transfer arthritis and antigen-induced arthritis, were used to establish the temporal expression of BMP3 and the cellular sources of BMP3 mRNA and protein in inflammatory arthritis. To determine the effects of inflammation on expression of BMP3 in osteoblasts, murine calvarial osteoblasts were treated with pro-inflammatory cytokines and BMP3 expression was assessed.
Results
In both murine models of RA, BMP3 mRNA and protein are highly expressed by osteoblasts lining inflammation-bone interfaces late in the course of arthritis. Synovial tissues are not a significant source of BMP3. BMP3 expression is induced in osteocalcin-expressing osteoblasts in vitro following stimulation by TNF.
Conclusion
These data implicate BMP3 as a novel factor that may act locally to contribute to the erosive process and inhibit the repair of articular bone in RA through inhibition of osteoblast differentiation and function.
Keywords: bone morphogenetic protein (BMP), osteoblast, rheumatoid arthritis, inflammation, TNF
Introduction
Rheumatoid arthritis (RA) is characterized by articular bone erosion. Pro-inflammatory cytokines central to RA pathogenesis, including TNF, IL-1β, and IL-17, promote osteoclastogenesis (1). Importantly, inflammatory cytokines also inhibit the differentiation and function of osteoblasts (2, 3). Mature osteoblasts in turn mediate bone formation and erosion repair in RA as inflammation resolves (4). However, the mechanisms by which inflammation inhibits osteoblast function to limit bone formation and impede healing of articular erosions have not been fully elucidated.
Osteoblast differentiation and function is dependent on the bone morphogenetic protein (BMP) signaling pathway; BMP2, 4, and 7 are critical for the commitment of mesenchymal precursor cells to osteoblasts, and maintaining adult bone mass. BMP-induced signaling is regulated by secreted antagonists, including a unique member of the BMP family, BMP3, which antagonizes the BMP type II receptor ActRIIB, and suppresses BMP-induced osteogenic responses to negatively regulate bone density (5, 6). BMP3 overexpression causes spontaneous rib fractures due to decreased bone mineralization (7). In contrast, BMP3-deficient mice have increased bone volume and density (6) and ActRIIB receptor-deficient mice exhibit defects in bone mineralization similar to BMP3 transgenic mice (7).
Pro-inflammatory cytokines also directly impact the BMP signaling pathway. TNF inhibits BMP-induced bone formation (8) and upregulates BMP3 expression in dental follicle cells in vitro (9). Alterations in BMP signaling, specifically the upregulation of BMP3 expression, may be a novel pathway by which inflammation inhibits osteoblast function. We have determined a potential role for BMP3 in perpetuating articular erosions, and demonstrate that BMP3 is expressed late in the course of inflammatory arthritis by osteoblasts lining erosion sites. We further demonstrate that TNF, but not IL-1β or IL-17, induces BMP3 expression in osteoblasts, identifying a potential mechanism for the inhibition of osteoblast function in RA.
Methods
Animals and mouse models
All animal procedures were performed in accordance with protocols approved by the IACUC at the University of Massachusetts Medical School. KRN T cell transgenic mice (provided by Dr. Benoist, and the Institut de Genetique et de Biologie Moleculaire et Cellulaire, Illkirch, France) were crossed with NOD mice (Jackson) to generate K/BxN mice that spontaneously develop arthritis (10). Arthritogenic serum was obtained and injected into twelve-week old male C57BL/6J mice (Jackson) to induce K/BxN serum transfer arthritis (11, 12). Induction of antigen-induced arthritis was adapted from previously published procedures (13). Twelve-week old male C57BL/6J mice were immunized subcutaneously with an emulsion of mBSA/CFA (Sigma), supplemented with 2.5mg/ml M. tuberculosis H37 RA (Difco). Arthritis was induced by knee injection of 6µg mBSA. Mice were injected intraperitoneally with 20µg lipopolysaccharide (Sigma) to synchronize arthritis induction.
Cell Culture
Primary calvarial osteoblasts were isolated from neonatal C57BL/6J mice (P0–P2) by three sequential digestions with 2mg/ml collagenase P (Roche) and 0.25% trypsin (Gibco). 0.8×104 cells/cm2 were plated and fed with MEM (Gibco) supplemented with 10% FBS (Atlanta), 50µg/ml ascorbic acid (Sigma), and 10mM β-glycerophosphate (Sigma) to induce differentiation at confluency (day 0). On days 14 and 21 of differentiation, cells were stimulated with TNF (0.75ng/ml or 7.5ng/ml, R&D Systems), IL-1β (10ng/ml, Fitzgerald) or IL-17 (50ng/ml, R&D Systems).
RNA isolation and qRT-PCR
Synovium was dissected from the tibio-talar or knee joints and processed for RNA (12). RNA from osteoblasts was prepared in Trizol, genomic DNA was removed (Turbo DNase-free kit, Ambion), and cDNA was synthesized (iScript cDNA synthesis kit, BioRad). qRT-PCR was performed for reference gene hydroxymethylbilane synthase (HMBS) and BMP3 (Qiagen) using iScript Sybr Green Supermix (BioRad). BMP3 mRNA expression was normalized to HMBS. Using the 2−ΔΔCt method (14), data from synovial tissue were expressed as fold increase in arthritic compared to average non-arthritic gene expression. Calvarial osteoblast data were expressed as fold increase in TNF-treated compared to unstimulated cells. Osteoblast differentiation was determined by comparing mRNA expression of osteocalcin (Qiagen) at days 7, 14 and 21 to day 0.
Western blotting
Protein was extracted from calvarial osteoblasts in RIPA buffer. 100µg protein was loaded onto a 10% SDS-PAGE gel, transferred to PVDF membrane and probed with antibodies for BMP3 (Santa Cruz, sc-7404) or β-actin (Cell Signaling, #4967). After incubation with secondary antibody (anti-goat IgG HRP (Santa Cruz, sc-2020) or anti-rabbit IgG HRP (Santa Cruz, sc-2004)), protein was detected by chemiluminescence (Thermo Scientific).
Histopathologic analyses
Immunohistochemistry for BMP3 protein was adapted from previously described methods (11, 12). Slides were stained with two goat polyclonal anti-BMP3 antibodies (Abcam (ab18864) and Santa Cruz (sc-9031)) or biotin-conjugated rabbit anti-goat IgG secondary antibody (DAKO), followed by HRP-conjugated streptavidin (DAKO). Antibody staining was visualized using DAB liquid chromogen (DAKO). For in situ hybridization, BMP3 specific primers (NM_173404.3 from 1330–1733bp) were used to generate a 391bp murine BMP3 cDNA fragment which was cloned into the pGEMT-easy vector (Promega). Digoxigenin labeled riboprobes were generated and in situ hybridization was performed (12).
Statistical analysis
Statistical analysis of qRT-PCR data was determined by student’s unpaired t-test using Prism 6.0 (GraphPad). P value <0.05 was considered statistically significant.
Results
BMP3 is expressed in osteoblasts late in the course of arthritis
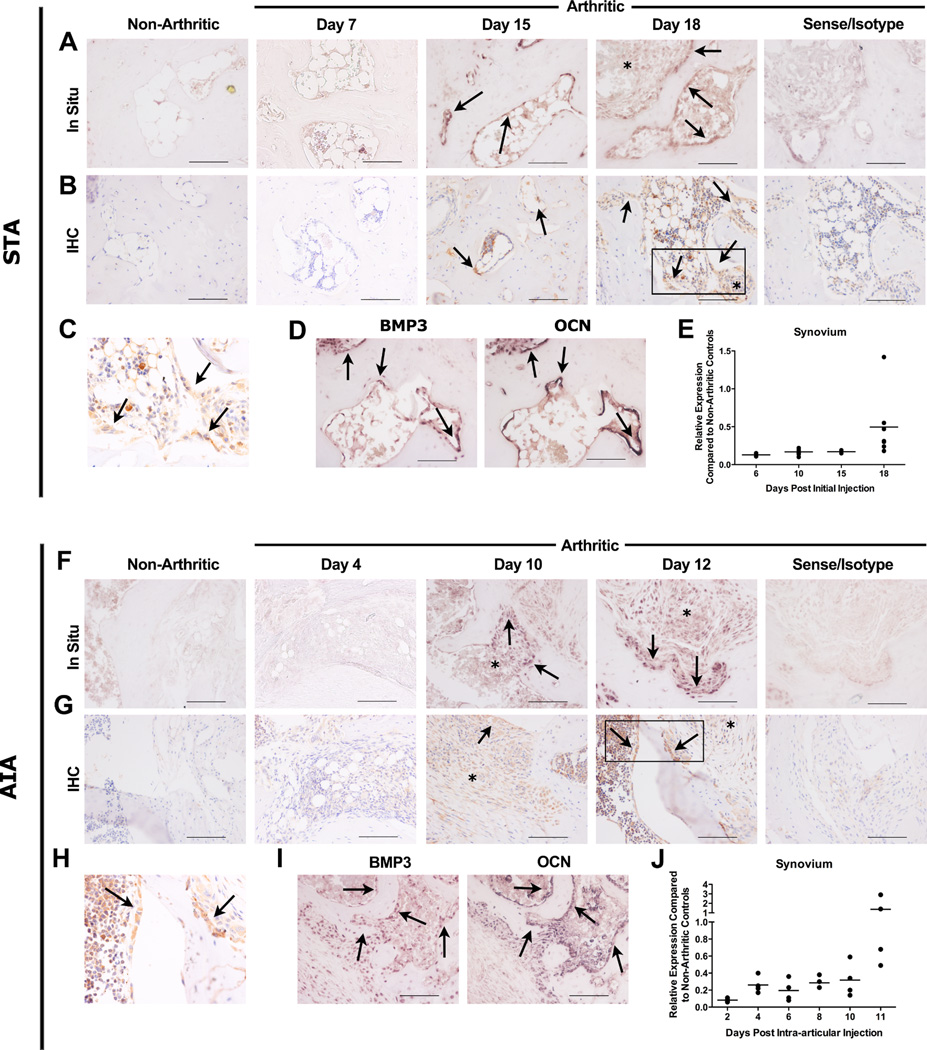
To determine the timing and localization of BMP3 expression in arthritic erosions in vivo, two animal models of RA with different pathologic mechanisms were studied: K/BxN serum transfer arthritis (STA) and antigen-induced arthritis (AIA). BMP3 expression was not observed in non-arthritic mice (Figures 1A–B (STA) and F–G (AIA), far left panels). STA is an immune complex-mediated arthritis dependent on production of both IL-1β and TNF. Robust BMP3 mRNA (Figure 1A) and protein expression (Figures 1B,C) was observed in osteoblasts lining the endosteal surfaces of eroded bone late in the course of arthritis (days 15 and 18). BMP3 expression was identified in serial sections in osteocalcin-expressing (mature) osteoblasts at inflammation-bone interfaces (Figure 1D). Importantly, these BMP3-expressing osteoblasts were seen only at late stages when inflammation was resolving and osteoblasts repopulated eroded bone surfaces. Minimal BMP3 mRNA and protein was detected in synovial tissue (Figures 1A,B)** and qPCR demonstrated that BMP3 mRNA expression in arthritic synovium was less than that seen in non-arthritic controls. (Figure 1E).
Figure 1. BMP3 is expressed by osteoblasts lining erosion sites.
Representative in situ hybridization (In Situ) (A and F) and immunohistochemistry (IHC) staining (B and G) for BMP3 mRNA and protein respectively (identified by brown staining), in an eroded midfoot bone (STA) and eroded knee (AIA). Arrows identify osteoblasts expressing BMP3 mRNA or protein. The sense probe and isotype control antibody showed no staining (far right panels). Asterisks (*) mark areas of infiltrating synovial tissues that do not express significant amounts of BMP3. Scale bar: 100µm. (C and H) Higher magnification of boxed area in B and G, respectively, highlights BMP3 protein expression in osteoblasts. (D and I) In situ hybridization in serial sections for BMP3 and osteocalcin (OCN) mRNA expression. Arrows denote osteoblasts that express both BMP3 (left) and OCN (right) mRNA. Scale bar: 20µm. (E and J) qRT-PCR demonstrating relative BMP3 mRNA expression in arthritic compared to non-arthritic mice. Synovial tissue isolated from STA ankle joints (E, n=2–8 mice/time point) and AIA knee joints (J, n=4 mice/time point). Each symbol represents the mean of duplicate qRT-PCR samples. Horizontal line represents means.
We also evaluated BMP3 expression in AIA, a T cell-mediated model of RA. Cells lining the eroded bone surfaces of knees expressed BMP3 mRNA (Figure 1F) and protein (Figures 1G,H) by day 10. Similar to results in STA, induction of BMP3 expression occurred at a late time point in the course of inflammation. As arthritis resolved, cells expressing BMP3 increased in number along the inflammation-bone interface and BMP3 was again identified in mature osteoblasts (serial sections, Figure 1I). BMP3 expression in AIA was also not identified in synovium (days 10 and 12**, Figures 1G,H and J).
TNF induces BMP3 expression in osteoblasts
We examined whether cytokines expressed in inflammatory arthritis could induce BMP3 expression in osteoblasts. TNF (7.5ng/mL) significantly induced BMP3 mRNA expression in maturing calvarial osteoblasts above levels present in unstimulated cells. A 2-fold and 8-fold increase in BMP3 mRNA was seen by 18 hours in mid-stage (day 14) and mature (day 21) osteoblasts, respectively (Figure 2A). Osteoblast differentiation was confirmed by the increasing expression of osteocalcin (Figure 2B). No significant induction of BMP3 mRNA was observed in cells stimulated with TNF at 0.75ng/mL (data not shown), IL-1β or IL-17 (Figure 2C). Induction of BMP3 protein by TNF (7.5ng/mL) was also confirmed in osteoblasts at 24 and 48 hours (Figure 2D). These data demonstrate that BMP3 is induced in mature osteoblasts lining sites of bone erosion as inflammation begins to resolve.
Figure 2. TNF induces BMP3 expression in calvarial osteoblasts at late stages of differentiation.
(A): BMP3 mRNA expression in calvarial osteoblasts treated with TNF (7.5ng/ml) at days 14 and 21 of differentiation. After 18 hours of stimulation, there is an approximately 2-fold (day 14) and 8-fold (day 21) increase in BMP3 mRNA expression compared to the unstimulated, day control cells; *p<0.05, **p<0.01. qRT-PCR is representative of four independent experiments in which each sample was performed in triplicate. (B): Representative osteocalcin mRNA expression over the course of differentiation confirms that osteoblasts are differentiating. (C): Treatment with IL-1β (10ng/ml) or IL-17 (50ng/ml) at day 21 of differentiation does not induce BMP3 mRNA expression in calvarial osteoblasts. (D) Western blot demonstrating induction of BMP3 protein expression by TNF (7.5ng/ml) in calvarial osteoblasts (day 21) at 24 and 48 hours post treatment. β-actin is used as a loading control.
Discussion
Inflammation in RA not only stimulates osteoclastogenesis, but also inhibits osteoblast maturation, contributing to the net loss of bone and inhibiting erosion repair (4, 12). We have shown that once inflammation resolves, osteoblasts populate eroded surfaces and form bone to repair erosions (4). Thus, one reason for the lack of erosion repair in patients with RA undergoing treatment is the presence of continued articular inflammation.
The BMP pathway is critical for osteoblast differentiation and is likely involved in bone erosion in inflammatory arthritis. BMP7-expressing osteoblasts have been localized to erosion sites in murine models (15) and lymphocyte aggregates adjacent to arthritic sites in mice express BMPs that promote bone formation (15), but a direct role for BMP signaling has not been shown. We demonstrate that TNF induces expression of BMP3 in mature osteoblasts. Late in the course of STA and AIA, inflammation begins to abate as a result of the decreased presence of inflammatory stimuli (4, 10). At these early phases of resolving inflammation, maturing osteoblasts at erosion sites are subjected to pro-inflammatory cytokines still present in the joint microenvironment. Thus, residual expression of TNF may impair osteoblast differentiation through BMP3. As an antagonist of the ActRIIB BMP receptor, BMP3 suppresses the phosphorylation of Smad1/5/8 (5), leading to inhibition of BMP-induced osteoblast differentiation. Thus in the context of inflammatory arthritis, BMP3 expression may be a mechanism by which nascent, maturing osteoblasts act in a paracrine manner to inhibit the differentiation of newly committed osteoblast progenitors. Together, these data implicate BMP3 as a new factor that may contribute to inflammatory bone loss through impairment of osteoblast function and erosion repair.
Acknowledgments
We would like to thank Teresa Bowman for technical assistance, and Kara Lindquist and Jonathan Lowery for preliminary studies.
Funding
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number NIH 1RO1-AR055952 (EMG), the Abbott Bioresearch Center Fellowship in Translational Science (MMM) and a grant from the Arthritis National Research Foundation (SK).
Footnotes
Conflict of interest
EMG declares the following competing interests: Grant support from AbbVie, royalties from UptoDate, consulting for Novo Nordisk, GSK and Lilly. MMM is currently employed by AbbVie, but this work was conducted while in the laboratory of Dr. Gravallese.
References
- 1.Gravallese EM, Goldring SR. Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis Rheum. 2000;43:2143–2151. doi: 10.1002/1529-0131(200010)43:10<2143::AID-ANR1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Nanes MS. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. doi: 10.1016/s0378-1119(03)00841-2. [DOI] [PubMed] [Google Scholar]
- 3.Stashenko P, Dewhirst FE, Rooney ML, Desjardins LA, Heeley JD. Interleukin-1 beta is a potent inhibitor of bone formation in vitro. J Bone Miner Res. 1987;2(6):559–565. doi: 10.1002/jbmr.5650020612. [DOI] [PubMed] [Google Scholar]
- 4.Matzelle MM, Gallant MA, Condon KW, Walsh NC, Manning CA, Stein GS, et al. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum. 2011;64:1540–1550. doi: 10.1002/art.33504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokabu S, Gamer L, Cox K, Lowery J, Tsuji K, Raz R, et al. BMP3 suppresses osteoblast differentiation of bone marrow stromal cells via interaction with Acvr2b. Molecular endocrinology. 2012;26:87–94. doi: 10.1210/me.2011-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, et al. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet. 2001;27:84–88. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 7.Gamer LW, Cox K, Carlo JM, Rosen V. Overexpression of BMP3 in the developing skeleton alters endochondral bone formation resulting in spontaneous rib fractures. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:2374–2381. doi: 10.1002/dvdy.22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakase T, Takaoka K, Masuhara K, Shimizu K, Yoshikawa H, Ochi T. Interleukin-1 beta enhances and tumor necrosis factor-alpha inhibits bone morphogenetic protein-2-induced alkaline phosphatase activity in MC3T3-E1 osteoblastic cells. Bone. 1997;21:17–21. doi: 10.1016/s8756-3282(97)00038-0. [DOI] [PubMed] [Google Scholar]
- 9.Yao S, Prpic V, Pan F, Wise GE. TNF-alpha upregulates expression of BMP-2 and BMP-3 genes in the rat dental follicle--implications for tooth eruption. Connective tissue research. 2010;51:59–66. doi: 10.3109/03008200903019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, et al. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 11.Pettit AR, Ji H, von Stechow D, Muller R, Goldring SR, Choi Y, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–1699. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh NC, Reinwald S, Manning CA, Condon KW, Iwata K, Burr DB, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res. 2009;24:1572–1585. doi: 10.1359/jbmr.090320. [DOI] [PubMed] [Google Scholar]
- 13.van Lent PL, Grevers L, Lubberts E, de Vries TJ, Nabbe KC, Verbeek S, et al. Fcgamma receptors directly mediate cartilage, but not bone, destruction in murine antigen-induced arthritis: uncoupling of cartilage damage from bone erosion and joint inflammation. Arthritis Rheum. 2006;54:3868–3877. doi: 10.1002/art.22253. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Gortz B, Hayer S, Redlich K, Zwerina J, Tohidast-Akrad M, Tuerk B, et al. Arthritis induces lymphocytic bone marrow inflammation and endosteal bone formation. J Bone Miner Res. 2004;19:990–998. doi: 10.1359/JBMR.040205. [DOI] [PubMed] [Google Scholar]