Abstract
The Amyloid Precursor Protein (APP) is the source of amyloid peptides that accumulate in Alzheimer’s Disease. However, members of the APP family are strongly expressed in the developing nervous systems of invertebrates and vertebrates, where they regulate neuronal guidance, synaptic remodeling, and injury responses. In contrast to mammals, insects express only one APP ortholog (APPL), simplifying investigations into its normal functions. Recent studies have shown that APPL regulates neuronal migration in the developing insect nervous system, analogous to the roles ascribed to APP family proteins in the mammalian cortex. The comparative simplicity of insect systems offers new opportunities for deciphering the signaling mechanisms by which this enigmatic class of proteins contributes to the formation and function of the nervous system.
Graphical Abstract
Introduction: neuronal migration and the formation of the insect nervous system
The directed migration of neurons and glia along specific pathways is a universal feature of developing nervous systems [1,2], during which cells navigate through a dynamic environment of potential guidance cues. The phenomenon of neuronal migration was first recognized in vertebrate development, where it is critical to the formation of both the central nervous system (CNS) and peripheral nervous systems (PNS) [3,4], and more modern methods have revealed extraordinary complexity in the modes of migration that give rise to the mammalian cortex [5••, 6•]. The initiation, extent, and termination of migration must be precisely regulated, and a variety of evolutionarily conserved guidance cues have been identified that influence particular aspects of migratory behavior [7, 8•]. The significance of the migratory process has been underscored by the numerous developmental defects and neurological diseases arising from errors in migration [9, 10••], although the precise mechanisms underlying many of these defects have proven more difficult to ascertain.
Neuronal migration also plays important roles in invertebrate nervous systems, including mollusks [11], crustaceans [12], and nematodes [13,14], where the molecular pathways regulating the migratory process can be investigated within intact organisms. Until recently, however, the contribution of migration to the formation of insect nervous systems was under-appreciated. Although the differentiation of the embryonic CNS in insects typically involves relatively small displacements of newly generated neurons from their neurogenic niches [15, 16•], more dramatic patterns of neuronal and glial migration have now been documented in both the embryonic PNS [17] and the developing adult CNS [18,19, 20••]. A particularly striking example of migration was recently identified in the developing adult visual system of Drosophila, during which streams of newborn neurons travel into the optic lobes of the brain to establish discrete layers of interneurons with position-specific characteristics [21•, 22••, 23••]. Intriguingly, this process involves Notch-dependent cell fate selection and Slit/Robo-dependent cell positioning (both of which also regulate neurogenesis in the mammalian cortex), providing an elegant illustration of how evolutionarily conserved mechanisms controlling migration play analogous roles in both insect and vertebrate nervous systems [2].
The insect Enteric Nervous System: a dramatic example of neuronal migration
The most dramatic examples of neuronal migration in insects have been described in the developing enteric nervous system (ENS). Analogous to the vertebrate ENS, the insect ENS represents a distinct division of the PNS that provides innervation to the gut and regulates digestion and metabolism [24], as well as modulating a variety of endocrine functions [25,26]. As in other organisms, the insect ENS consists of interconnected peripheral ganglia and nerve plexuses that innervate the gut musculature. In contrast to vertebrates, however, the insect ENS lies superficially on the gut, making it more amenable to direct experimental manipulations. In general, the ENS of all insect species consists of similar components: small subsets of neurons from the brain and abdominal ganglia provide some innervation to the anterior and posterior regions of the gut. In addition, distinct populations of enteric neurons originate from neurogenic regions in the foregut and populate enteric ganglia on the foregut (sometimes called the stomatogastric nervous system) and branching nerve plexuses with more dispersed groups of neurons on the midgut. Notably, both the foregut and midgut populations of enteric neurons achieve their mature distributions via extensive phases of migration [27], analogous to the formation of the mammalian ENS by migrating neural crest cells [28]. The following is a brief summary of the ENS in the tobacco hornworm (Manduca sexta) to illustrate these events (Figure 1A); however the neuroanatomy of the ENS varies dramatically in different species [29,30], reflecting the radically different digestive requirements and lifestyles needed by particular animals.
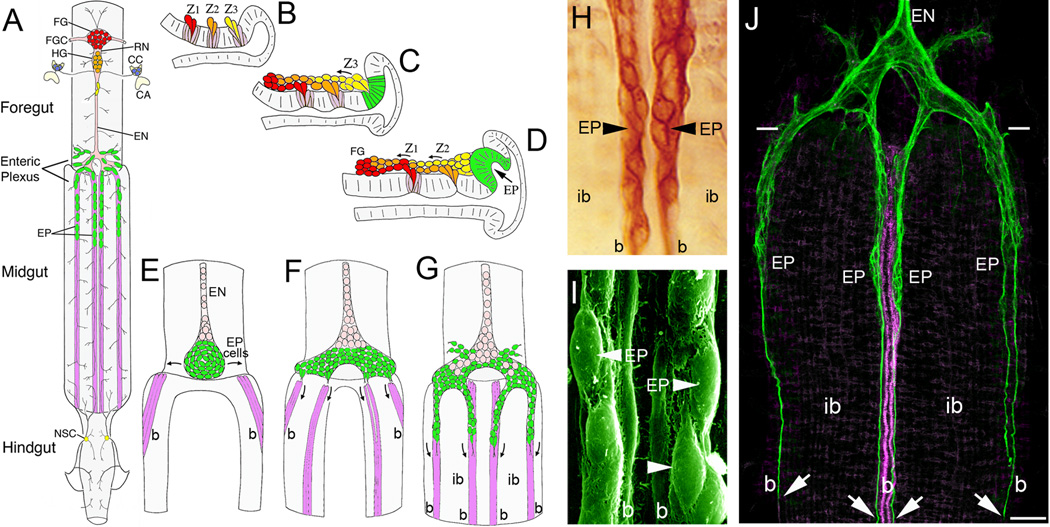
Figure 1. Embryonic development of the insect Enteric Nervous System (ENS) involves extensive patterns of neuronal and glial migration.
(A), Schematic drawing of the ENS and associated neurosecretory organs in the larval stage of the tobacco hornworm Manduca sexta (modified from [27]). The primary ganglion on the foregut is the frontal ganglion (FG; red), connected to the overlying brain lobes by paired frontal ganglion connectives (FGC). Several nerve branches extend anteriorly onto the pharynx, while the recurrent nerve (RN) extends posteriorly to the hypocerebral ganglion (HG; orange), situated below the brain. In Manduca, the hypocerebral ganglion initially forms during embryogenesis but then becomes closely opposed to the frontal ganglion and is no longer readily distinguished in later stages. The HG is also connected to the paired corpora cardiaca (CC; blue), the primary neurosecretory organs of the brain, which are adjacent to the corpora allata (CA; the source of Juvenile Hormone). From the HG, the esophageal nerve (EN) extends posteriorly along the length of the foregut, giving rise to short nerve branches that innervate the foregut musculature. Near the foregut-midgut boundary, the esophageal nerve connects with the enteric plexus that spans the foregut-midgut boundary, which includes nerve branches extending along radial muscles on the foregut and major nerves that extend along eight well-defined muscle bands that lie superficially on the midgut (purple). The enteric plexus contains a population of ~300 distributed neurons (EP cells; green), which includes intermingled groups of neurons expressing a variety of morphological and transmitter phenotypes. The EP cells occupy positions along the anterior 20% of the midgut, and extend long axons posteriorly along the muscle bands with sparse lateral branches that provide a diffuse innervation of the interband midgut musculature. The hindgut is innervated by branches of the proctodeal and rectal nerves that originate in the terminal abdominal ganglion of the ventral nerve cord. Branches of the proctodeal nerve also extend onto the posterior midgut and contain several peripheral neurosecretory cells (yellow). (B–C), Neurogenesis of the developing ENS in Manduca (after [31]). Panels show lateral views of the foregut midline; anterior is to the left, dorsal is to the top. When raised at 25°C, Manduca embryogenesis is complete in 100 hr (1 hr = 1 hour post-fertilization, or hpf). (B), By ~24 hpf, three neurogenic zones (Z1, Z2, & Z3) have formed in the dorsal foregut epithelium, which give rise to a series of mitotically active precursor cells via sequential delamination. Precursors giving rise to neurons typically divide only once (or occasionally twice) after delaminating, similar to midline precursors in the embryonic CNS. (C), By 28 hpf, streams of zone-derived cells have begun to migrate anteriorly along the foregut, while the remaining zone 3 cells delaminate as a group. The epithelium surrounding the original position of zone 3 subsequently differentiates into a distinct placode that will form the EP cells (green). (D), By 33 hpf, migrating zone cells have begun to form the frontal ganglion (FG), while the remaining zone 2 cells delaminate as a group. The EP cell placode has also begun to invaginate from the EP cell packet (described below). Zone 1 continues to generate cells until almost 40 hpf (not shown); late-emerging zone cells derived from all three zones tend to become glial precursors that remain mitotically active throughout much of embryogenesis and establish the glial sheath surrounding the foregut nerves and ganglia. (E–F), Formation of the midgut enteric plexus; panels show dorsal views of the developing ENS at the foregut-midgut boundary (after [88]). (E), By 40 hpf, the EP cells (green) have invaginated en mass from their neurogenic placode located within the posterior dorsal lip of the foregut (D, green). The neurons then commence a bilateral spreading phase of migration (arrows) that almost completely encircles the foregut, adjacent to the foregut-midgut boundary. Concurrently, subsets of longitudinal muscles on the midgut (magenta) begin to coalesce into eight well-defined bands as dorsal closure of the midgut proceeds. Anteriorly, the EP cell packet is in continuity with the developing esophageal nerve (EN), which contains populations of proliferating glial precursors (pink; derived from zone 3) that will subsequently ensheath the enteric plexus. (F), By 55 hpf, the EP cells have almost completely surrounded the foregut, and subsets of the neurons have aligned with each of the midgut muscle bands (only the dorsal four are shown). (G), By 58 hpf, subsets of EP cells have begun to migrate in a chain-like manner along the midgut muscle bands; smaller subsets also migrate onto radial muscles of the foregut (muscles not shown). Proliferating glial cells (pink) subsequently migrate along the pathways established by the neurons, thereby ensheathing the branches of the enteric plexus. (H), Magnified view of EP cell groups migrating on the mid-dorsal band pathways (at 58 hpf) of an embryo immunostained with an antibody recognizing all isoforms of the cell adhesion receptor Fasciclin II (Fas II). The migratory neurons and underlying muscle bands (b) express transmembrane Fas II (TM-Fas II), while the trailing glial cells express GPI-linked Fas II. The migratory EP cells and their processes remain primarily confined to their band pathways while avoiding the adjacent interband musculature (ib). (I), Scanning electron micrograph showing the migratory EP cells on the mid-dorsal band pathways (b) of an embryo at 65 hpf. (J), Lower magnified view of the developing ENS at 62 hpf, in an embryo that was immunostained with anti-TM-Fas II (green). TM-Fas II immunoreactivity in the mid-dorsal muscle bands is shown in magenta to better distinguish the EP cell processes (after [79]). At this stage, the EP cells have migrated ~200 µm and have begun to extend fasciculated axons (arrows) more posteriorly along the muscle bands (b). Throughout this developmental period, the EP cells avoid the adjacent interband regions (ib), extending terminal branches onto the lateral musculature only after migration and axogenesis is complete (~80 hpf). Scale = 20 µm in (H); 5 µm in (I); 60 µm in (J).
In Manduca, the first phase of ENS neurogenesis commences within three neurogenic zones within the mid-dorsal foregut epithelium (Figure 1B–D), which generates a series of neuronal and glial precursors via sequential delamination. Neurons derived from these zones then migrate anteriorly to form two foregut ganglia (frontal and hypocerebral ganglia) that are ensheathed by trailing glial populations [31]. During the second neurogenic phase, a distinct population of ~300 neurons (EP cells) invaginates from a neurogenic placode in the posterior foregut lip to form a discrete packet of post-mitotic neurons (Figure 1D–E) [32]. After spreading bilaterally around the foregut, subsets of these neurons then migrate rapidly onto the midgut via eight muscle bands to form a branching nerve plexus (the Enteric Plexus), (Figure 1F–G). Because of their superficial location on the gut surface, the EP cells and their processes can be readily visualized by a variety of methods throughout their differentiation (Figure 1H–J). However, unlike many neurons in the insect CNS, the migratory EP cells are not uniquely identifiable; rather, the pathways followed by individual neurons are stochastic, and only after migrating do they express particular phenotypes that are regulated in part by their final positions [33,34]. This developmental sequence of directed migration and delayed differentiation is also seen in the developing vertebrate ENS, in which enteric neurons migrate substantial distances to form the nerve plexuses of the gut while delaying their terminal differentiation until migration is largely complete [35,36].
In Manduca, the EP cells only traverse about 20% of the midgut before transitioning to axonal outgrowth and subsequent innervation of the lateral musculature, while in grasshoppers, neurons migrate along the entire length of the midgut [30]. Curiously, this aspect of ENS development has been lost in flies, whereby a substantial portion of the midgut remains uninnervated [37]. In this regard, the insect ENS offers an elegant example of how evolutionary changes in common developmental programs can mold the form and function of the nervous system, providing a rich opportunity to explore the relationship between evolution and development of the nervous system. Meticulous studies have delineated the genetic regulation of the foregut neurogenic zones in Drosophila [38•], providing new tools for investigating how gene mutations that perturb migration in the insect ENS may also contribute to congenital disorders affecting human development. In addition, a number of groups (including our laboratory) have exploited the experimental advantages of the insect ENS to define the roles of particular neuronal guidance factors and signal transduction pathways that regulate different aspects of the migratory process, including the insect ortholog of the Amyloid Precursor Protein (APPL; as summarized below).
Amyloid Precursor Protein: a complicated protein with complex functions
The Amyloid Precursor Protein (APP) is a transmembrane glycoprotein (Figure 2A) that is strongly associated with Alzheimer’s Disease (AD) but that also may serve important functions in neuronal development [39•, 40••]. Although multiple isoforms of APP are generated by alternative splicing [41], the predominant form in neurons is APP695, which undergoes dynamic patterns of expression, trafficking, and cleavage by membrane-associated proteases (called secretases) [42, 43•]. In addition, APP can be processed either via the “non-amyloidogenic” (Figure 2B) or the ‘”amyloidogenic” pathway (Figure 2C); the latter generates β-amyloid peptide fragments (Aβ40–42) that are thought to trigger neuronal dysfunction in AD [44, 45•]. Other APP cleavage fragments have been ascribed a bewildering array of biological activities, although their authentic functions remain controversial [41, 46•]. By comparison, growing evidence suggests that APP695 itself can function as a transmembrane receptor that regulates multiple aspects of neuronal motility, including migration and outgrowth, synaptogenesis, and response to injury [39•, 47••, 48•], albeit via mechanisms that are still poorly understood. Under some conditions, APP has been found to promote neuronal motility, while in other assays, APP signaling restricts growth [49, 50•]. Moreover, APP can potentially interact with dozens of binding partners and cytoplasmic proteins [51, 47••] and is subject to complex patterns of intracellular trafficking that may modulate its bioavailability [52•, 53••]. An added complication is that mammalian neurons express two closely related orthologs of APP (APLP1 and APLP2; Figure 2D) with partially overlapping biological activities [54, 55•]. Although deemed “intellectually unsatisfying” but some authors [56], these paradoxical effects are reminiscent of other guidance receptors that can both promote and restrict motile responses, depending on the developmental context [57•, 58•].
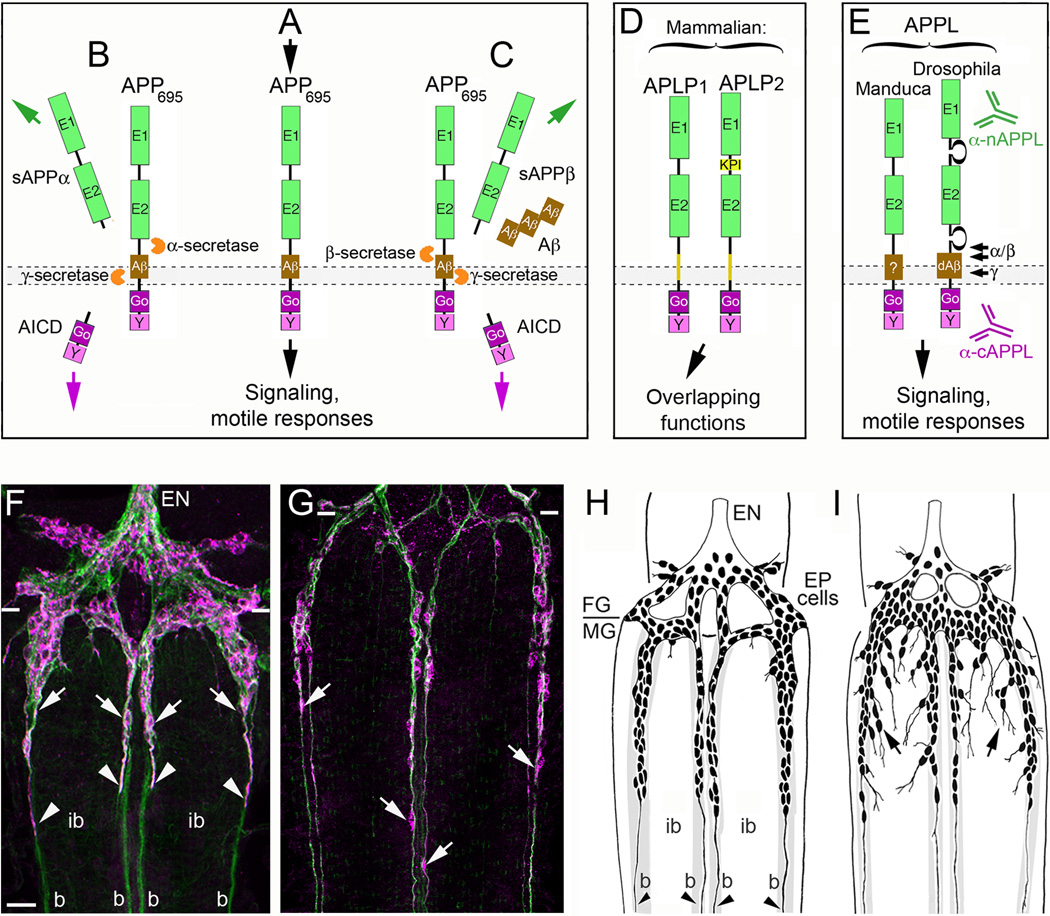
Figure 2. The insect ortholog of Amyloid Precursor Protein regulates neuronal migration in the developing ENS.
(A–E), the structure and processing of APP family proteins is similar in insects and mammals. (A), human APP695 (containing 695 amino acids) has the topology of a type-1 transmembrane glycoprotein, consisting of two extracellular protein interaction domains (E1 and E2); a transmembrane domain that contains the Aβ cleavage fragment; and a short cytoplasmic tail that contains highly conserved binding domains for the heterotrimeric G protein Goα (Go) and a tyrosine-based sorting motif (Y). A wide variety of potential binding proteins and ligands have been identified that can interact with the E1–E2 extracellular domains, while numerous intracellular adapter and signaling proteins besides Goα (are capable of interacting with the cytoplasmic domains. Studies in a variety of systems have shown that APP695 is capable of functioning as a transmembrane receptor, whereby activation with candidate ligands can induce signaling responses that modulate neuronal motility. (B), In the non-amyloidogenic pathway of APP processing, APP is first cleaved by α-secretases at a juxtamembrane site within the Aβ domain, which releases a soluble/secreted ectodomain fragment (sAPPα) and a short transmembrane C-terminal fragment (CTF; not shown). CTF fragments are then rapidly cleaved by the γ-secretase complex (containing presenilins) to produce a cytoplasmic APP intracellular domain (AICD) and a small “p3” peptide of no apparent significance (not shown). (C), In the amyloidogenic pathway, APP is first cleaved by β-secretase (BACE) to generate a slightly shorter sAPPβ ectodomain fragment and a slightly longer CTF fragment containing the Aβ peptide (not shown). This intermediate fragment is then rapidly cleaved by the γ-secretase complex to generate an identical AICD fragment and β-amyloid peptide fragments (Aβ40–42) of varying lengths that accumulate in the brain with aging. Secreted sAPP ectodomain fragments have been ascribed a variety of functions (both beneficial and harmful to neurons), including activation of APP signaling (via interactions with the transmembrane holoprotein); AICD fragments have been shown to induce changes in gene transcription (analogous to the Notch intracellular domain; NICD), although the biological significance of these activities remains under debate. (D), In addition to APP, vertebrates also express to closely related orthologs: APP-Like Protein 1 & 2 (APLP1 and APLP2). Both family members contain extracellular and intracellular protein interaction domains that are closely similar to these domains in APP and have been shown to have partially overlapping functions within the nervous system. (E), Insects only express a single APP family protein, APPL (APP-Like). They also contain similar extracellular and intracellular domains that share considerable sequence conservation with human APP695, including 100% conservation within the Go domain (required for direct interactions with Goα; [79]). Drosophila APPL has also been shown to contain an Aβ-like fragment (dAβ) that is generated by sequential cleavage of APPL by endogenous β- and γ-secretases [70]). Antibodies specific for the n-terminal (α-nAPPL) and c-terminal (α-cAPPL) regions of APPL have been generated that can distinguish the distribution of the holoprotein from its cleavage fragments. (F–G), The embryonic ENS of Manduca at different developmental stages, labeled with anti-TM-Fas II (green) and anti-cAPPL (magenta). (F), At 58 hpf, TM-Fas II is expressed by both the EP cells and their muscle band pathways on the midgut (b). The migratory EP cells also strongly express APPL (arrows) as they travel onto the bands while largely avoiding the adjacent interband regions (ib). Previous studies have shown that transmembrane APPL traffics into their leading processes (arrowheads), where it interacts with Goα [79]). (G), By 65 hpf, the EP cells have transitioned from migration to axon outgrowth, but they continue to robustly express APPL in their cell bodies (arrows) and advancing growth cones (out of the field of view). Paired white hatchmarks indicate the foregut-midgut boundary; scale bar = 30 µm. (H–I), examples of the ENS in embryos that were opened to expose the developing ENS prior to the onset of EP cell migration (~50 hpf) and allowed to develop for an additional 18 hr (through the periods of migration and outgrowth). At the completion of the culture period, embryos were fixed and immunostained with anti-Fas II to reveal the extent of migration and outgrowth, and analyzed by camera lucida methods. (H), Embryo that was treated with control antisense morpholino constructs with no known gene targets in insects; EP cell migration and axon outgrowth (arrowheads) was largely confined to the normal band pathways. (I), Embryo that was treated with antisense morpholino constructs specific for Manduca APPL mRNA; although EP cells that maintained strong contact with their bands migrated and extended axons normally along these pathways, a substantial number of neurons migrated and extended processes inappropriately onto the interband regions (black arrows). A similar pattern of ectopic migration was caused by inhibiting the heterotrimeric G protein Goα or by blocking Goα-dependent Ca2+ influx [79,81].
With respect to neuronal migration, compelling studies have implicated APP695 in regulating motile neurons within the developing mammalian cortex, during which undifferentiated neurons must travel along radial glial progenitors to reach their appropriate cortical layers [5••, 6•]. Once again, however, different experimental methods have yielded contradictory results. Genetic deletion of all three APP family proteins (APP, APLP1 and APLP2) induced a striking pattern of excessive, inappropriate neuronal migration, resulting in heterotopias near the outer layer of the cortex [59]. These results suggest that signaling by APP and its orthologs normally restricts the extent of neuronal migration. In contrast, knocking down APP expression in neuronal precursors resulted in the premature arrest of migration, suggesting that APP normally promotes migration in response to permissive cues [60]. Recent evidence demonstrating that APP family proteins also regulate the mitotic behavior of cortical progenitors may provide an explanation for these disparate results [61]. Nevertheless, deciphering how APP family proteins regulate neuronal migration within the mammalian nervous system remains an ongoing challenge.
Insights from an insect model: APPL and the control of neuronal migration in the ENS
APP is a member of an evolutionary ancient family of transmembrane receptors with orthologs in all higher organisms [62,63]. In contrast to mammals, insects only express one ortholog (APP-Like, or APPL); Figure 2E), which contains the same protein interaction motifs identified in APP695 [64–66], and that is processed by homologous classes of secretases to generate similar fragments [67,68]. Transgenic studies in Drosophila have also shown that human APP695 can rescue defects caused by the loss of APPL [69], while overexpression of Drosophila Aβ-like fragments induces neurodegenerative responses resembling AD [70]. However, insect APPL is only expressed by neurons [64,66], simplifying an analysis of its normal functions.
Similar to mice lacking APP, flies deleted for APPL are viable [69], but they exhibit a variety of neurodevelopmental and behavioral defects [69,71], accompanied by substantially reduced lifespans [72]. In Drosophila, APPL has been found to regulate synaptic growth at the neuromuscular junction [65], axonal targeting by developing photoreceptors, and dendritic sprouting within the metamorphosing brain [65,73,74]. Also like APP695, APPL expression is substantially upregulated in response to injury [75], providing further evidence that APP family members participate in multiple aspects of neuronal motility and growth. In many instances, APPL appears to function as a transmembrane receptor, although both its cleaved ectodomain and AICD fragments have also been implicated in some of these functions [72,73,75]. In adult flies, APPL is required for associative memory [76••] and circadian clock activity [77••], supporting other evidence that perturbing the normal functions of APP may contribute significantly to the behavioral deficits that occur in AD [40••, 47••].
Does APPL play a role in regulating neuronal migration, similar to the roles ascribed to APP695? To investigate this question, we adapted a well-characterized assay of neuronal migration in the developing ENS of Manduca, using an embryonic culture assay that permits direct manipulations and imaging of the migratory EP cells [27]. Initially, we showed that the EP cells first express APPL shortly after emerging onto the foregut, and concentrate the full-length protein in their leading processes throughout their subsequent phases of migration and outgrowth (Figure 2F–G) [66]. Based on provocative evidence that APP695 interacts with the heterotrimeric G protein Goα [78], we also showed that APPL co-localizes with Goα in the EP cells, and we used co-immunoprecipitation and bi-molecular fluorescence assays to demonstrate that the two proteins directly interact [66,79]. We also used an embryonic culture assay to show that inhibiting APPL expression (with antisense constructs) or Goα activity (with pharmacological reagents) induced the same distinctive pattern of ectopic migration and outgrowth onto the interband regions, compared to cultured controls (Figure 2H–I). In contrast, hyperactivating APPL-Goα signaling had the opposite effect, causing a dramatic inhibition and stalling of migration and outgrowth [79,80]. These results are analogous to the ectopic migration seen in mice lacking all three APP family proteins [59], and they substantiated our earlier studies showing that Goα signaling restricts migration via the local activation of a calcium (Ca2+) current in the EP cells [81].
Conclusions and future directions
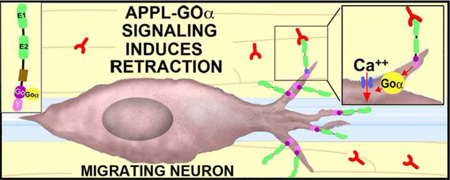
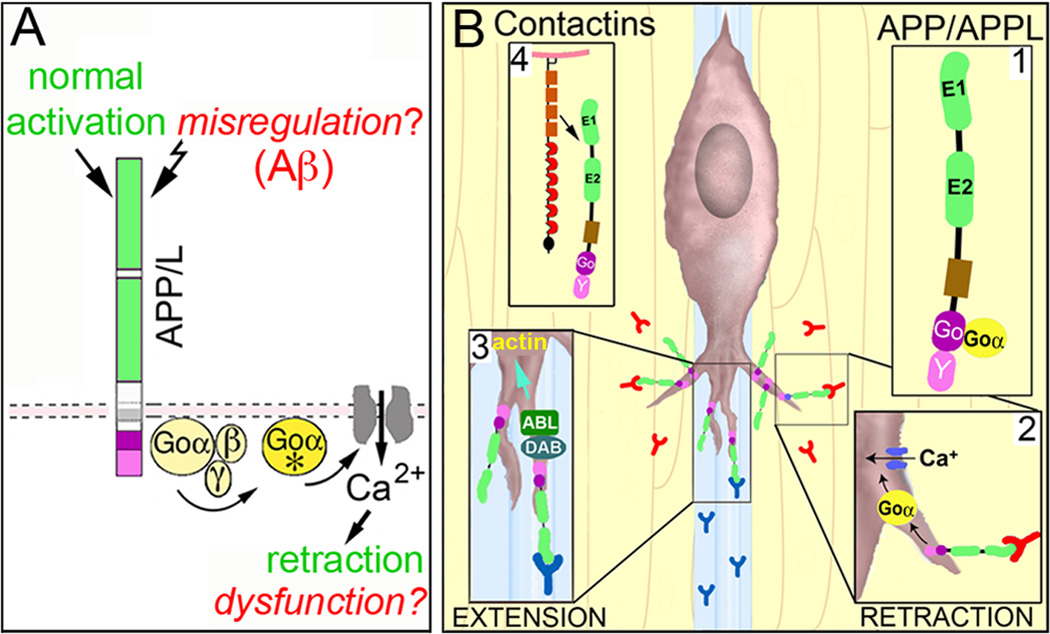
Our results also provide new support for the model that APP family proteins function as unconventional Goα-coupled receptors that regulate neuronal migration. Within the developing insect ENS, we propose that activation of APPL signaling (by ligands associated with the interband regions) stimulates Goα-dependent Ca2+ influx that induces local retraction responses (Figure 3A), thereby helping to maintain the migratory neurons on their pathways. However, several outstanding issues remain to be resolved. (1). What are the ligands that regulate APPL signaling in the developing ENS? Work in mammalian systems has shown that multiple members of the Contactin family of cell adhesion receptors can directly interact with APP [82, 83•]. By comparison, insects express only a single Contactin ortholog that is expressed by glial and epithelial cells [84], and we are currently testing whether Manduca Contactin serves as an APPL ligand within the developing ENS. (2). Does APPL regulate neuronal migration in other regions of the nervous system? Because many examples of migration in Drosophila involve relatively small distances, the modulatory effects of APPL might have been previously overlooked. However, given the robust migratory patterns that were recently discovered in the developing fly visual system [21•, 22••, 23••], a renewed investigation of how APPL signaling affects optic lobe formation might provide new insight into the mechanisms controlling migration in the insect CNS. (3). How does APPL signaling promote neuronal motility in some contexts while inhibiting growth in others? Like other APP family proteins, APPL may interact with a diversity of binding partners and signaling proteins besides Contactins and Goα [71,75,85], supporting the view that APPL can be recruited into distinct signaling complexes in a context-dependent manner (Figure 3B). With the advent of improved protocols for visualizing dynamic protein interactions within neurons [86•, 87•], it may now be possible to exploit the comparative simplicity of insect models to address this challenging issue within the developing nervous system.
Figure 3.
Proposed model for how APP family proteins regulate the motile behavior of developing neurons in response to context-dependent guidance cues. (A), Stimulation of human APP (or insect APPL) by endogenous ligands activates the heterotrimeric G protein Goα (Goα*), which in turn induces Goα-dependent effectors (including Ca2+ influx) that alter cytoskeletal dynamics required for filopodial retraction. During normal development, this signaling pathway helps restrict inappropriate neuronal migration and outgrowth, and might also regulate synaptic pruning. In neurodegenerative conditions like Alzheimer’s Disease, multiple factors (including Aβ) might induce the misregulation of APP signaling, provoking Goα hyperactivation and Ca2+ overload that results in neuronal dysfunction and death. (B), Within the developing ENS of Manduca, APPL acts as a Goα-coupled receptor (B1) for ligands encountered by the migratory EP cells when they extend filopodia off their normal band pathways. Stimulation of APPL induces the local activation of Goα within filopodia (B2), resulting in Goα-dependent Ca2+ influx (via a voltage-independent Ca2+ current). In turn, Ca2-dependent modulation of the actin cytoskeleton results in filopodial retraction, helping to keep the neurons on their correct band pathways. A variety of potential ligands associated with the ensheathing glial cells and interband musculature might trigger APPL-Goα signaling, including insect Contactin (B4). However, in other neurons (and in other regions), ligands associated with permissive regions might activate different APP/L-linked signaling pathways that promote growth. For example APP interactions with the adapter protein Disabled (DAB) can induce the activation of Abl kinase [71], which might enhance actin remodeling to promote outgrowth (B3). In this manner, APP family proteins can function as “molecular hubs”, capable of regulating different types of motile responses in a context-dependent manner.
HIGHLIGHTS.
Neuronal migration is essential to the formation of the insect nervous system
The Amyloid Precursor Protein family regulates multiple types of neuronal motility
The insect ortholog of APP (APPL) is expressed in all developing neurons
APPL regulates neuronal migration in the insect enteric nervous system
APP and APPL may control neuronal motility via similar molecular pathways
Acknowledgments
Authors’ research reported in this publication was supported by grants from the National Institute for Neurological Disease and Stroke of the National Institutes of Health (RO1 AG025525 and R21 NS078363) to PFC, who also received support from an OHSU Presidential Bridge Funding Award. JMR received support from a grant from the Oregon Partners for Alzheimer’s Research and from training grant support from the National Institute on Aging, National Institutes of Health (T32 AG023477). We thank Drs. Doris Kretzschmar for critical input on the manuscript. We are grateful to Dr. Stefanie Kaech and Ms. Aurelie Snyder for their assistance with confocal microscopy and image analysis that was performed in the Advanced Light Microscopy Core, Jungers Center at OHSU, which is supported in part by National Institutes of Health grant # P30 NS061800.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Feng Y, Walsh CA. Protein-protein interactions, cytoskeletal regulation and neuronal migration. Nat Rev Neurosci. 2001;2:408–416. doi: 10.1038/35077559. [DOI] [PubMed] [Google Scholar]
- 2.Hatten ME. New directions in neuronal migration. Science. 2002;297:1660–1663. doi: 10.1126/science.1074572. [DOI] [PubMed] [Google Scholar]
- 3.Cajal Ramón y . Studies in Vertebrate Neurogenesis. Springfield, Ill: Thomas, Charles C; 1929. [Google Scholar]
- 4.Le Douarin NM, Kalcheim C. The Neural Crest. Cambridge, England: Cambridge U. Press; 1999. [Google Scholar]
- 5. Gertz CC, Kriegstein AR. Neuronal Migration Dynamics in the Developing Ferret Cortex. J Neurosci. 2015;35:14307–14315. doi: 10.1523/JNEUROSCI.2198-15.2015. This study used advanced neuronal labeling and imaging methods to investigate how migratory patterns are different in gyrencephalic (folded) brains versus lissencephalic (smooth) brains, providing new insight into the types of migratory errors that might contribute to congenital defects in human brain development.
- 6. Tabata H, Nagata K. Decoding the molecular mechanisms of neuronal migration using in utero electroporation. Med Mol Morphol. 2016;49:63–75. doi: 10.1007/s00795-015-0127-y. This review provides a good summary of recent technical and experimental advances in understanding the complex patterns of neuronal migration that help shape the mammalian cortex.
- 7.Evsyukova I, Plestant C, Anton ES. Integrative mechanisms of oriented neuronal migration in the developing brain. Annu Rev Cell Dev Biol. 2013;29:299–353. doi: 10.1146/annurev-cellbio-101512-122400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thiede-Stan NK, Schwab ME. Attractive and repulsive factors act through multi-subunit receptor complexes to regulate nerve fiber growth. J Cell Sci. 2015;128:2403–2414. doi: 10.1242/jcs.165555. This report contains an excellent update on the different types of neuronal guidance receptors and associated signaling proteins that regulate neuronal migration and outgrowth.
- 9.Ross ME, Walsh CA. Human brain malformations and their lessons for neuronal migration. Annu Rev Neurosci. 2001;24:1041–1070. doi: 10.1146/annurev.neuro.24.1.1041. [DOI] [PubMed] [Google Scholar]
- 10. Stouffer MA, Golden JA, Francis F. Neuronal migration disorders: Focus on the cytoskeleton and epilepsy. Neurobiol Dis. 2016;92:18–45. doi: 10.1016/j.nbd.2015.08.003. This review summarizes recent discoveries from human genetic studies and animal models demonstrating that many neurodevelopmental and neurological disorders are caused by errors in neuronal migration, with a particular focus on epilepsy.
- 11.Jacob MH. Neurogenesis in Aplysia californica resembles nervous system formation in vertebrates. Journal of Neuroscience. 1984;4:1225–1239. doi: 10.1523/JNEUROSCI.04-05-01225.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaves da Silva PG, Benton JL, Sandeman DC, Beltz BS. Adult neurogenesis in the crayfish brain: the hematopoietic anterior proliferation center has direct access to the brain and stem cell niche. Stem Cells Dev. 2013;22:1027–1041. doi: 10.1089/scd.2012.0583. [DOI] [PubMed] [Google Scholar]
- 13.Sundararajan L, Norris ML, Lundquist EA. SDN-1/Syndecan Acts in Parallel to the Transmembrane Molecule MIG-13 to Promote Anterior Neuroblast Migration. G3 (Bethesda) 2015;5:1567–1574. doi: 10.1534/g3.115.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian D, Diao M, Jiang Y, Sun L, Zhang Y, Chen Z, Huang S, Ou G. Anillin Regulates Neuronal Migration and Neurite Growth by Linking RhoG to the Actin Cytoskeleton. Curr Biol. 2015;25:1135–1145. doi: 10.1016/j.cub.2015.02.072. [DOI] [PubMed] [Google Scholar]
- 15.Doe CQ, Kuwada JY, Goodman CS. From epithelium to neuroblasts to neurons: the role of cell interactions and cell lineage during insect neurogenesis. Phil. Trans. R. Soc. Lond. B. 1985;312:67–81. doi: 10.1098/rstb.1985.0178. [DOI] [PubMed] [Google Scholar]
- 16. Kang KH, Reichert H. Control of neural stem cell self-renewal and differentiation in Drosophila. Cell Tissue Res. 2015;359:33–45. doi: 10.1007/s00441-014-1914-9. This report provides an update on the different programs of neurogenesis that contribute to the formation of the fly nervous system, and also lustrates the relatively small displacement of many progeny from proliferating neuroblasts.
- 17.Lhamo T, Ismat A. The extracellular protease stl functions to inhibit migration of v'ch1 sensory neuron during Drosophila embryogenesis. Mech Dev. 2015;137:1–10. doi: 10.1016/j.mod.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Tucker ES, Tolbert LP. Reciprocal interactions between olfactory receptor axons and olfactory nerve glia cultured from the developing moth Manduca sexta. Dev Biol. 2003;260:9–30. doi: 10.1016/s0012-1606(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa E, Kitada Y, Kaido M, Takayama R, Awasaki T, Tabata T, Sato M. Concentric zones, cell migration and neuronal circuits in the Drosophila visual center. Development. 2011;138:983–993. doi: 10.1242/dev.058370. [DOI] [PubMed] [Google Scholar]
- 20. Omoto JJ, Yogi P, Hartenstein V. Origin and development of neuropil glia of the Drosophila larval and adult brain: Two distinct glial populations derived from separate progenitors. Dev Biol. 2015;404:2–20. doi: 10.1016/j.ydbio.2015.03.004. This study contains an elegant analysis of the origins, migration, and differentiation of specific glial populations in the metamorphosing fly brain.
- 21. Apitz H, Salecker I. A region-specific neurogenesis mode requires migratory progenitors in the Drosophila visual system. Nat Neurosci. 2015;18:46–55. doi: 10.1038/nn.3896. This study (and the following studies by Chen et al and Suzuki et al. describe the dramatic patterns of neurogenesis and neuronal migration that give rise to different layers of interneurons in the developing adult optic lobes of Drosophila.
- 22. Chen Z, Del Valle Rodriguez A, Li X, Erclik T, Fernandes VM, Desplan C. A Unique Class of Neural Progenitors in the Drosophila Optic Lobe Generates Both Migrating Neurons and Glia. Cell Rep. 2016 doi: 10.1016/j.celrep.2016.03.061. This study demonstrates that Notch signaling plays an important role in specifying neuronal versus glial fates during the tangential migration of cells into the developing adult optic lobes of Drosophila.
- 23. Suzuki T, Hasegawa E, Nakai Y, Kaido M, Takayama R, Sato M. Formation of Neuronal Circuits by Interactions between Neuronal Populations Derived from Different Origins in the Drosophila Visual Center. Cell Rep. 2016;15:499–509. doi: 10.1016/j.celrep.2016.03.056. This study demonstrates that Slit-Robo signaling helps regulate the positioning of neurons that migrate into the developing adult optic lobes of Drosophila.
- 24.Penzlin H. Stomatogastric nervous system. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry, and Pharmacology. Vol. 5. Pergamon Press; 1985. pp. 371–406. [Google Scholar]
- 25.Ayali A. The role of the arthropod stomatogastric nervous system in moulting behaviour and ecdysis. J Exp Biol. 2009;212:453–459. doi: 10.1242/jeb.023879. [DOI] [PubMed] [Google Scholar]
- 26.Cognigni P, Bailey AP, Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Copenhaver PF. How to innervate a simple gut: familiar themes and unique aspects in the formation of the insect enteric nervous system. Dev Dyn. 2007;236:1841–1864. doi: 10.1002/dvdy.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012;366:64–73. doi: 10.1016/j.ydbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Zitnan D, Endo Y, Sehnal F. Stomatogastric nervous system of Galleria mellonella L. (Lepidoptera: Pyralidae): changes during metamorphosis with special reference to FMRFamide neurons. Int. J. Insect Morphol. & Embryol. 1989;18:227–237. [Google Scholar]
- 30.Ganfornina MD, Sanchez D, Bastiani MJ. Embryonic development of the enteric nervous system of the grasshopper Schistocerca americana. J Comp Neurol. 1996;372:581–596. doi: 10.1002/(SICI)1096-9861(19960902)372:4<581::AID-CNE7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Copenhaver PF, Taghert PH. Origins of the insect enteric nervous system: differentiation of the enteric ganglia from a neurogenic epithelium. Development. 1991;113:1115–1132. doi: 10.1242/dev.113.4.1115. [DOI] [PubMed] [Google Scholar]
- 32.Copenhaver PF, Taghert PH. Neurogenesis in the insect enteric nervous system: generation of pre-migratory neurons from an epithelial placode. Development. 1990;109:17–28. doi: 10.1242/dev.109.1.17. [DOI] [PubMed] [Google Scholar]
- 33.Copenhaver PF, Taghert PH. Development of the enteric nervous system in the moth I. Diversity of cell types and the embryonic expression of FMRFamide-related neuropeptides. Developmental Biology. 1989a;131:70–84. doi: 10.1016/s0012-1606(89)80039-9. [DOI] [PubMed] [Google Scholar]
- 34.Copenhaver PF, Horgan AM, Combes S. An identified set of visceral muscle bands is essential for the guidance of migratory neurons in the enteric nervous system of Manduca sexta. Dev. Biol. 1996;179:412–426. doi: 10.1006/dbio.1996.0271. [DOI] [PubMed] [Google Scholar]
- 35.Gershon MD. Genes and lineages in the formation of the enteric nervous system. Curr Opin Neurobiol. 1997;7:101–109. doi: 10.1016/s0959-4388(97)80127-4. [DOI] [PubMed] [Google Scholar]
- 36.Burns AJ. Migration of neural crest-derived enteric nervous system precursor cells to and within the gastrointestinal tract. Int J Dev Biol. 2005;49:143–150. doi: 10.1387/ijdb.041935ab. [DOI] [PubMed] [Google Scholar]
- 37.Spieß R, Schoofs A, Heinzel HG. Anatomy of the stomatogastric nervous system associated with the foregut in Drosophila melanogaster and Calliphora vicina third instar larvae. J Morphol. 2008;269:272–282. doi: 10.1002/jmor.10581. [DOI] [PubMed] [Google Scholar]
- 38. Hernandez K, Myers LG, Bowser M, Kidd T. Genetic Tools for the Analysis of Drosophila Stomatogastric Nervous System Development. PLoS One. 2015;10:e0128290. doi: 10.1371/journal.pone.0128290. This study describes a new suite of genetic reporter constructs and driver lines that can be used to investigate neurogenesis and migration within the foregut components of the fly enteric nervous system (also called the stomatogastric nervous system).
- 39. Nicolas M, Hassan BA. Amyloid precursor protein and neural development. Development. 2014;141:2543–2548. doi: 10.1242/dev.108712. This review provides a well-balanced update on the different roles that APP and its orthologs may play during the formation of the nervous system in different organisms, including its potential functions in regulating neuronal migration.
- 40. van der Kant R, Goldstein LS. Cellular functions of the amyloid precursor protein from development to dementia. Dev Cell. 2015;32:502–515. doi: 10.1016/j.devcel.2015.01.022. This review complements the report by Nicolas and Hassan, summarizing recent evidence that APP might function as a nexus for different signaling pathways that regulate neuronal migration, outgrowth, and response to injury. Also discussed is the concept that disrupting the normal functions of APP may contribute to the pathologies associated with Alzheimer’s Disease.
- 41.Nalivaeva NN, Turner AJ. The amyloid precursor protein: a biochemical enigma in brain development, function and disease. FEBS Lett. 2013;587:2046–2054. doi: 10.1016/j.febslet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Tam JH, Pasternak SH. Imaging the Intracellular Trafficking of APP with Photoactivatable GFP. J Vis Exp. 2015 doi: 10.3791/53153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niederst ED, Reyna SM, Goldstein LS. Axonal amyloid precursor protein and its fragments undergo somatodendritic endocytosis and processing. Mol Biol Cell. 2015;26:205–217. doi: 10.1091/mbc.E14-06-1049. This study provides a good update on the complex patterns of APP trafficking and processing that regulate the distribution of the holoprotein and its cleavage fragments in mammalian neurons.
- 44.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 45. Karran E, De Strooper B. The amyloid cascade hypothesis: are we poised for success or failure? J Neurochem. 2016 Jun 3; doi: 10.1111/jnc.13632. This review provides an excellent critique of the amyloid hypothesis of Alzheimer’s Disease, including an evaluation of other factors that may contribute to the disease.
- 46. Nhan HS, Chiang K, Koo EH. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol. 2015;129:1–19. doi: 10.1007/s00401-014-1347-2. This review complements the work by Karran and De Strooper, including a discussion of the beneficial and deleterious activities attributed to APP and its cleavage fragments.
- 47. Deyts C, Thinakaran G, Parent AT. APP Receptor? To Be or Not To Be. Trends Pharmacol Sci. 2016;37:390–411. doi: 10.1016/j.tips.2016.01.005. This review provides an comprehensive overview of evidence that APP family proteins can function as transmembrane receptors, including a discussion of the different adapter proteins and signaling cascades associated with APP in different contexts.
- 48. Plummer S, Van den Heuvel C, Thornton E, Corrigan F, Cappai R. The Neuroprotective Properties of the Amyloid Precursor Protein Following Traumatic Brain Injury. Aging Dis. 2016;7:163–179. doi: 10.14336/AD.2015.0907. This report discusses recent evidence that APP (or its cleavage fragments) may serve beneficial functions that protect neurons after traumatic injury.
- 49.Sosa LJ, Bergman J, Estrada-Bernal A, Glorioso TJ, Kittelson JM, Pfenninger KH. Amyloid precursor protein is an autonomous growth cone adhesion molecule engaged in contact guidance. PLoS One. 2013;8:e64521. doi: 10.1371/journal.pone.0064521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Olsen O, Kallop DY, McLaughlin T, Huntwork-Rodriguez S, Wu Z, Duggan CD, Simon DJ, Lu Y, Easley-Neal C, Takeda K, et al. Genetic analysis reveals that amyloid precursor protein and death receptor 6 function in the same pathway to control axonal pruning independent of beta-secretase. J Neurosci. 2014;34:6438–6447. doi: 10.1523/JNEUROSCI.3522-13.2014. This study showed that APP interacts with other membrane receptors to promote axonal pruning rather than outgrowth.
- 51.Rice HC, Young-Pearse TL, Selkoe DJ. Systematic Evaluation of Candidate Ligands Regulating Ectodomain Shedding of Amyloid Precursor Protein. Biochemistry. 2013 doi: 10.1021/bi400165f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Agostinho P, Pliassova A, Oliveira CR, Cunha RA. Localization and Trafficking of Amyloid-beta Protein Precursor and Secretases: Impact on Alzheimer's Disease. J Alzheimers Dis. 2015;45:329–347. doi: 10.3233/JAD-142730. This report provides a critical evaluation of evidence that the intracellular trafficking of APP and its cleavage fragments serve important functions in regulating their bioavailability and activity.
- 53. Muresan V, Ladescu Muresan Z. Amyloid-beta precursor protein: Multiple fragments, numerous transport routes and mechanisms. Exp Cell Res. 2015;334:45–53. doi: 10.1016/j.yexcr.2014.12.014. This report complements the paper by Agostinho et al. (reference 52), and contains a comprehensive review of the complex dynamics of APP trafficking and processing in neurons.
- 54.Klevanski M, Saar M, Baumkotter F, Weyer SW, Kins S, Muller UC. Differential role of APP and APLPs for neuromuscular synaptic morphology and function. Mol Cell Neurosci. 2014;61:201–210. doi: 10.1016/j.mcn.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 55. Cousins SL, Dai W, Stephenson FA. APLP1 and APLP2, members of the APP family of proteins, behave similarly to APP in that they associate with NMDA receptors and enhance NMDA receptor surface expression. J Neurochem. 2015;133:879–885. doi: 10.1111/jnc.13063. This study showed that multiple members of the APP family have overlapping functions in mammalian neurons.
- 56.Shariati SA, De Strooper B. Redundancy and divergence in the amyloid precursor protein family. FEBS Lett. 2013;587:2036–2045. doi: 10.1016/j.febslet.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 57. Finci LI, Kruger N, Sun X, Zhang J, Chegkazi M, Wu Y, Schenk G, Mertens HD, Svergun DI, Zhang Y, et al. The crystal structure of netrin-1 in complex with DCC reveals the bifunctionality of netrin-1 as a guidance cue. Neuron. 2014;83:839–849. doi: 10.1016/j.neuron.2014.07.010. This study provided an elegant structural analysis of how the same protein can trigger either attraction or repulsion responses by motile neurons.
- 58. Kaplan A, Kent CB, Charron F, Fournier AE. Switching responses: spatial and temporal regulators of axon guidance. Mol Neurobiol. 2014;49:1077–1086. doi: 10.1007/s12035-013-8582-8. This review provides a good discussion of how particular guidance cues can interact with different intracellular signaling pathways to elicit both attractive and repulsive responses in motile neurons.
- 59.Herms J, Anliker B, Heber S, Ring S, Fuhrmann M, Kretzschmar H, Sisodia S, Muller U. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. Embo J. 2004;23:4106–4115. doi: 10.1038/sj.emboj.7600390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young-Pearse TL, Chen AC, Chang R, Marquez C, Selkoe DJ. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural Dev. 2008;3:15. doi: 10.1186/1749-8104-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shariati SA, Lau P, Hassan BA, Muller U, Dotti CG, De Strooper B, Gartner A. APLP2 regulates neuronal stem cell differentiation during cortical development. J Cell Sci. 2013 doi: 10.1242/jcs.122440. [DOI] [PubMed] [Google Scholar]
- 62.Coulson EJ, Paliga K, Beyreuther K, Masters CL. What the evolution of the amyloid protein precursor supergene family tells us about its function. Neurochem Int. 2000;36:175–184. doi: 10.1016/s0197-0186(99)00125-4. [DOI] [PubMed] [Google Scholar]
- 63.Virata MJ, Zeller RW. Ascidians: an invertebrate chordate model to study Alzheimer's disease pathogenesis. Dis Model Mech. 2010;3:377–385. doi: 10.1242/dmm.003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo LQ, Martin-Morris LE, White K. Identification, secretion, and neural expression of APPL, a Drosophila protein similar to human amyloid protein precursor. J Neurosci. 1990;10:3849–3861. doi: 10.1523/JNEUROSCI.10-12-03849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torroja L, Packard M, Gorczyca M, White K, Budnik V. The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J Neurosci. 1999;19:7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swanson TL, Knittel LM, Coate TM, Farley SM, Snyder MA, Copenhaver PF. The insect homologue of the amyloid precursor protein interacts with the heterotrimeric G protein Go alpha in an identified population of migratory neurons. Dev Biol. 2005;288:160–178. doi: 10.1016/j.ydbio.2005.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greeve I, Kretzschmar D, Tschape JA, Beyn A, Brellinger C, Schweizer M, Nitsch RM, Reifegerste R. Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. J Neurosci. 2004;24:3899–3906. doi: 10.1523/JNEUROSCI.0283-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolkan BJ, Triphan T, Kretzschmar D. beta-secretase cleavage of the fly amyloid precursor protein is required for glial survival. J Neurosci. 2012;32:16181–16192. doi: 10.1523/JNEUROSCI.0228-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo L, Tully T, White K. Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron. 1992;9:595–605. doi: 10.1016/0896-6273(92)90024-8. [DOI] [PubMed] [Google Scholar]
- 70.Carmine-Simmen K, Proctor T, Tschape J, Poeck B, Triphan T, Strauss R, Kretzschmar D. Neurotoxic effects induced by the Drosophila amyloid-beta peptide suggest a conserved toxic function. Neurobiol Dis. 2009;33:274–281. doi: 10.1016/j.nbd.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soldano A, Okray Z, Janovska P, Tmejova K, Reynaud E, Claeys A, Yan J, Atak ZK, De Strooper B, Dura JM, et al. The Drosophila homologue of the amyloid precursor protein is a conserved modulator of Wnt PCP signaling. PLoS Biol. 2013;11:e1001562. doi: 10.1371/journal.pbio.1001562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wentzell JS, Bolkan BJ, Carmine-Simmen K, Swanson TL, Musashe DT, Kretzschmar D. Amyloid precursor proteins are protective in Drosophila models of progressive neurodegeneration. Neurobiol Dis. 2012;46:78–87. doi: 10.1016/j.nbd.2011.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh J, Mlodzik M. Hibris, a Drosophila nephrin homolog, is required for presenilin-mediated Notch and APP-like cleavages. Dev Cell. 2012;23:82–96. doi: 10.1016/j.devcel.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mora N, Almudi I, Alsina B, Corominas M, Serras F. beta amyloid protein precursor-like (Appl) is a Ras1/MAPK-regulated gene required for axonal targeting in Drosophila photoreceptor neurons. J Cell Sci. 2013;126:53–59. doi: 10.1242/jcs.114785. [DOI] [PubMed] [Google Scholar]
- 75.Leyssen M, Ayaz D, Hebert SS, Reeve S, De Strooper B, Hassan BA. Amyloid precursor protein promotes post-developmental neurite arborization in the Drosophila brain. Embo J. 2005;24:2944–2955. doi: 10.1038/sj.emboj.7600757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bourdet I, Preat T, Goguel V. The full-length form of the Drosophila amyloid precursor protein is involved in memory formation. J Neurosci. 2015;35:1043–1051. doi: 10.1523/JNEUROSCI.2093-14.2015. This study showed that APPL plays an important role in memory formation, possibly involving interactions between transmembrane APPL and its secreted ectodomain fragments.
- 77. Blake MR, Holbrook SD, Kotwica-Rolinska J, Chow ES, Kretzschmar D, Giebultowicz JM. Manipulations of amyloid precursor protein cleavage disrupt the circadian clock in aging Drosophila. Neurobiol Dis. 2015;77:117–126. doi: 10.1016/j.nbd.2015.02.012. This study showed that APPL also plays an important role in maintaining the integrity of the central circadian clock in the fly brain, possibly involving transcriptional changes mediated by its AICD cytoplasmic fragments.
- 78.Okamoto T, Takeda S, Giambarella U, Murayama Y, Matsui T, Katada T, Matsuura Y, Nishimoto I. Intrinsic signaling function of APP as a novel target of three V642 mutations linked to familial Alzheimer's disease. Embo J. 1996;15:3769–3777. [PMC free article] [PubMed] [Google Scholar]
- 79.Ramaker JM, Swanson TL, Copenhaver PF. Amyloid precursor proteins interact with the heterotrimeric G protein Go in the control of neuronal migration. J Neurosci. 2013;33:10165–10181. doi: 10.1523/JNEUROSCI.1146-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horgan AM, Lagrange MT, Copenhaver PF. A developmental role for the heterotrimeric G protein Go alpha in a migratory population of embryonic neurons. Dev Biol. 1995;172:640–653. doi: 10.1006/dbio.1995.8042. [DOI] [PubMed] [Google Scholar]
- 81.Horgan AM, Copenhaver PF. G protein-mediated inhibition of neuronal migration requires calcium influx. J. Neurosci. 1998;18:4189–4200. doi: 10.1523/JNEUROSCI.18-11-04189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osterfield M, Egelund R, Young LM, Flanagan JG. Interaction of amyloid precursor protein with contactins and NgCAM in the retinotectal system. Development. 2008;135:1189–1199. doi: 10.1242/dev.007401. [DOI] [PubMed] [Google Scholar]
- 83. Osterhout JA, Stafford BK, Nguyen PL, Yoshihara Y, Huberman AD. Contactin-4 mediates axon-target specificity and functional development of the accessory optic system. Neuron. 2015;86:985–999. doi: 10.1016/j.neuron.2015.04.005. This study showed that APP interacts with members of the Contactin family of GPI-linked membrane proteins, possibly as a binding partner or co-receptor.
- 84.Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- 85.Ashley J, Packard M, Ataman B, Budnik V. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J Neurosci. 2005;25:5943–5955. doi: 10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sharifai N, Samarajeewa H, Kamiyama D, Deng TC, Boulina M, Chiba A. Imaging dynamic molecular signaling by the Cdc42 GTPase within the developing CNS. PLoS One. 2014;9:e88870. doi: 10.1371/journal.pone.0088870. This study demonstrated a novel method using in vivo FRET imaging to visualize interactions between molecular components of a signal transduction pathway in Drosophila neurons.
- 87. Zschatzsch M, Oliva C, Langen M, De Geest N, Ozel MN, Williamson WR, Lemon WC, Soldano A, Munck S, Hiesinger PR, et al. Regulation of branching dynamics by axon-intrinsic asymmetries in Tyrosine Kinase Receptor signaling. Elife. 2014;3:e01699. doi: 10.7554/eLife.01699. This used fluorescently tagged versions of growth factor receptors to visualize how receptor activation and signaling modulated the motile behavior of neurons in vitro and in vivo.
- 88.Copenhaver PF, Taghert PH. Development of the enteric nervous system in the moth II. Stereotyped cell migration precedes the differentiation of embryonic neurons. Developmental Biology. 1989b;131:85–101. doi: 10.1016/s0012-1606(89)80040-5. [DOI] [PubMed] [Google Scholar]