Abstract
Background
Performance during cognitive control functional magnetic resonance imaging (fMRI) tasks are associated with frontal lobe hypoactivation in patients with bipolar disorder, even while euthymic. Here, we study the structural underpinnings for this functional abnormality simultaneously with brain activation data.
Methods
In a sample of ninety adults (45 with inter-episode Bipolar I disorder and 45 healthy controls), we explored whether abnormal functional activation patterns in bipolar euthymic subjects during a Go-NoGo fMRI task are associated with regional deficits in cortical gray matter thickness in the same regions. Cross-sectional differences in fMRI activation were used to form a-priori hypotheses for region-of-interest cortical gray matter thickness analyses. fMRI BOLD to structural magnetic resonance imaging (sMRI) thickness correlations were conducted across the sample and within patients and controls separately.
Results
During response inhibition (NoGo minus Go), bipolar subjects showed significant hypoactivation and reduced thickness in the inferior frontal cortex (IFC), superior frontal gyrus and cingulate compared to controls. Cingulate hypoactivation corresponded with reduced regional thickness. A significant activation by disease state interaction was observed with thickness in left prefrontal areas.
Conclusions
Reduced cingulate fMRI activation is associated with reduced cortical thickness. In the left frontal lobe, a thinner cortex was associated with increased fMRI activation in patients, but showed a reverse trend in controls. These findings suggest that reduced activation in the IFC and cingulate during a response inhibition task may have an underlying structural etiology, which may explain task-related functional hypoactivation that persists even when patients are euthymic.
Keywords: Bipolar I disorder, bipolar euthymia, fMRI, Go-NoGo task, cortical thickness, structure function correlation
Introduction
Bipolar disorder affects ~3% of the US adult population each year (1), and is characterized by dramatic shifts of mood between euthymia, mania and depression. Converging evidence suggests that dysfunction in anteriorly oriented fronto-limbic network(s), including specific prefrontal regions (e.g. inferior frontal cortex (IFC), anterior cingulate) that project to specific subcortical areas (e.g. amygdala, striatum) (2, 3), may contribute to mood dysregulation associated with bipolar disorder (2, 4–9). Functional imaging studies of bipolar subjects have consistently revealed a functional deficit in IFC that is seen both during mania (10–15) and euthymia (4, 16–20). The IFC is comprised of the pars triangularis, pars opercularis and pars orbitalis in the inferior frontal gyrus (Brodmann’s areas (BA) 44, 45 and 47), and the persistence of a decrease in IFC function in euthymic bipolar subjects suggests its independence from mood state.
The underlying etiology of IFC hypofunction is not known. As changes in the hemodynamic response measured with fMRI are linked with neural activity, subtle disorganization in underlying gray matter structure may contribute to functional deficits. Structural anatomic studies have reported extensive anatomical connectivity between the IFC and brain regions associated with mood regulation and emotional responses (21–23), including the anterior cingulate cortex (ACC) and the amygdala. A structural abnormality in the IFC could lead to fMRI-related hypofunction and consequently to disconnectivity with other “affective” brain regions.
Structural studies have shown that overall brain volumes appear within the normal range in persons with bipolar disorder (8, 24, 25). However, regional differences have been observed in prefrontal cortical, subcortical and medial temporal structures (4, 26, 27). Most studies of prefrontal cortex (PFC) have examined relatively large frontal lobe regions (28–32). Of the fewer studies segmenting more functionally distinct frontal regions, some (33–43) but not all (44–46) have found differences between patient and control groups.
No imaging study, to our knowledge, has simultaneously collected and evaluated structural MRI (sMRI) and fMRI data to assess the relationship between fMRI and sMRI gray matter abnormalities in bipolar disorder. The current study was thus designed to 1) assess disease-specific alterations in neural function in the IFC using an fMRI task that activates this region; 2) explore structural contributions to IFC hypofunction using sophisticated techniques to measure regional variations in brain morphology; and 3) relate gray matter alterations to neural function. Specifically, we tested whether regionally specific differences in gray matter thickness measured with sMRI may be associated with the functional deficits seen in this region in our (19) and others’ prior fMRI studies of bipolar disorder (16).
Methods and Materials
Participants
Participants consisted of 90 adult subjects. Forty-five subjects with DSM-IV diagnosed bipolar I disorder, currently euthymic, (24 male) ranging in age from 20 to 61 years (M=39.9, SD=12.1) were recruited through the UCLA Mood Disorders Clinic and advertisements. An additional 45 healthy controls (23 male) ranging in age from 20 to 63 years (M=37.7, SD=10.5) were recruited by local advertising.
In bipolar subjects, the Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version (SCID) (47) was used to confirm bipolar I disorder diagnosis. Bipolar subjects with a past history of alcohol or drug use disorder could participate if they were sober for >3 months, as confirmed by self-report, and had no mood episode within 30 days of the scan per SCID assessment. At the time of scanning, all bipolar subjects were euthymic, operationally defined as a score < 7 on both the Young Mania Rating Scale (YMRS) (48) and the Hamilton Rating Scale for Depression (HRSD) (49). The SCID was used to confirm that control participants were free of any current or past Axis I psychiatric illness. Exclusion criteria for all subjects included left-handedness, head injury with loss of consciousness > 5 min, ferrous metal implants, neurologic illness, and pregnancy. All participants provided written informed consent in accordance with the Institutional Review Board at the University of California, Los Angeles (UCLA).
fMRI Paradigm
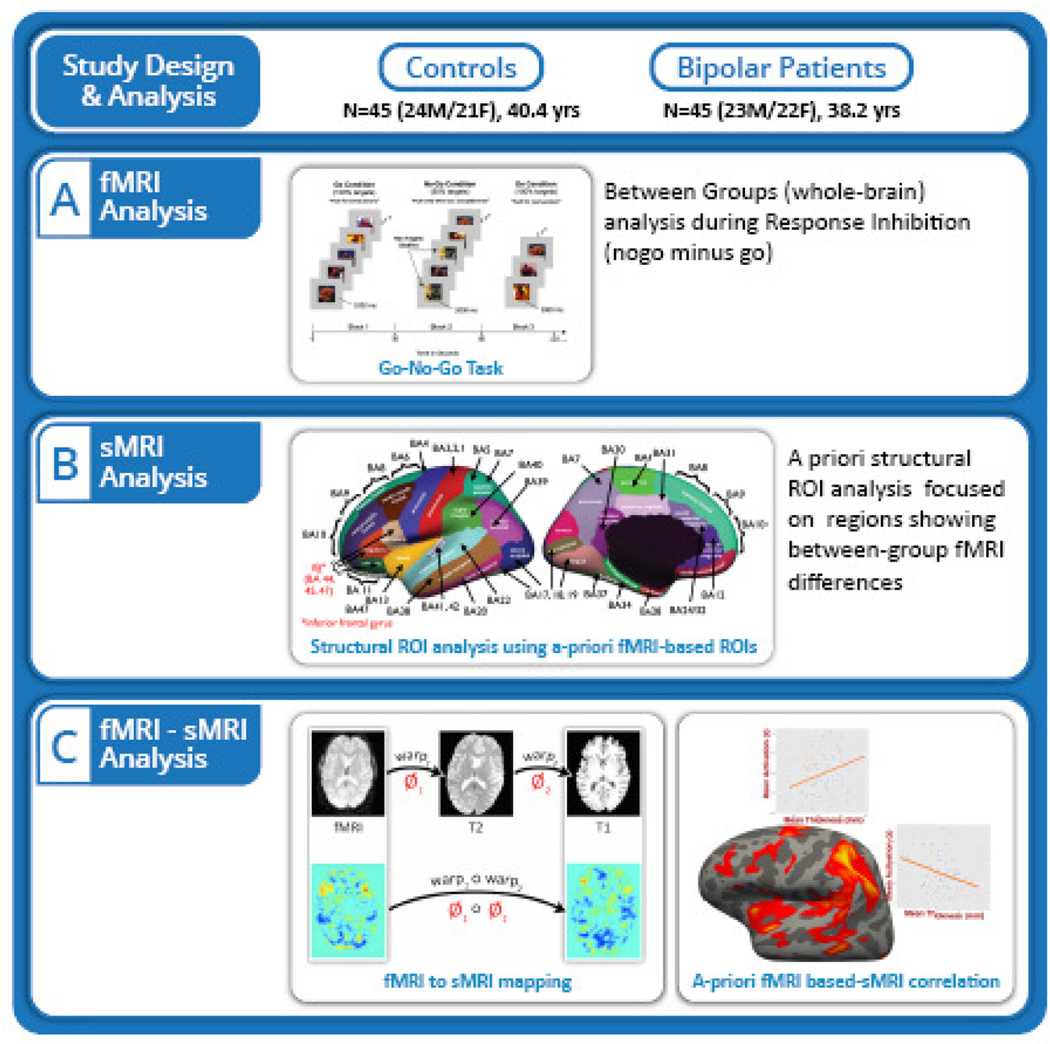
A well-validated response inhibition task (Go-NoGo) (50) was used to probe brain regions involved in cognitive control, including IFC, other OFC regions (BA 10, 11, 47) and the cingulate (BA 24/32). Task details have been published previously (50) and are illustrated in Figure 1A and presented in the Supplement.
Figure 1.
Schematic of the research study design including the block design and timing, and the functional and structural image processing and analysis techniques.
fMRI Behavioral Analysis
Means and standard deviations were computed for accuracy and response times for the Go and NoGo conditions. The distribution for accuracy was non-normal because most subjects made few or no errors. Consequently, accuracy was dichotomized (high and low performance) and differences were assessed non-parametrically. Differences in accuracy and response time were tested independently using 2-tailed Fisher’s exact test and Mann-Whitney U tests, respectively.
fMRI Acquisition
Imaging data were collected on a Siemens Trio 3T scanner at the UCLA Ahmanson-Lovelace Brain Mapping Center. Here, an echo planar image (EPI) gradient-echo pulse sequence (TR/TE=2500/25ms; flip angle=78°; FOV=192mm; 64×64 matrix; 3 × 3 × 3 mm in-plane resolution; slice thickness=3 mm; 0.75 mm gap; 30 total interleaved slices) with integrated parallel acquisition technique (IPAT) was acquired covering the entire brain. Scan time was 4 min and 48 sec, or 112 volumes. EPI T2-weighted images for intra- and inter-subject registration were acquired with the following parameters: TR/TE=5000/34ms; flip angle=90°; FOV=192mm; 128×128 matrix; in-plane voxel size 1.5 × 1.5 × 3.0 mm, slice thickness=3 mm, and 30 total slices.
sMRI Acquisition
To evaluate brain structure, high-resolution T1-weighted MPRAGE (magnetization-prepared rapid-acquisition gradient echo) scans were acquired (TR/TE=1900/2.26ms; flip angle=9°; FOV 250mm by 250 mm; 256 × 256 matrix; voxel size: 1×1×1mm; and total sequence time 6 min and 50 sec) in the same imaging session.
Neuroimaging Data Analysis
Figure 1 summarizes analysis procedures. Briefly, fMRI data were first analyzed separately to determine group differences in brain activation during the NoGo minus Go contrast (Figure 1A). A priori structural ROIs were then chosen based on significant between-group differences, and analyzed for cortical thickness differences (Figure 1B). Structure-function relationships were subsequently determined by assessing whether regions that showed abnormalities in cortical thickness between diagnostic groups overlapped with those showing abnormalities in task dependent fMRI activation (Figure 1C).
fMRI Preprocessing and Analyses
Functional data were processed using FEAT, Version 6.0, part of FSL (www.fmrib.ox.ac.uk/fsl). FSL’s Brain Extraction Tool (53) was used to skull strip the structural images. Motion correction was performed using Motion Correction using FMRIB’s Linear Image Registration Tool (54). Spatial smoothing used a Gaussian kernel of 5 mm Full Width between Half Maximum (FWHM). Grand-mean intensity normalization (by a single multiplicative factor) and high-pass temporal filtering (using a Gaussian-weighted least-squares straight line fitting, with sigma = 45.0s) were conducted on the 4D datasets. Using FMRIB’s Improved Linear Model, time-series statistical analyses were performed with local autocorrelation correction (55). FMRIB’s Linear Image Registration Tool (54, 56) was used to register functional images using a two-step transformation 1) to co-planar high-resolution structural images using a 7-parameter affine registration, and 2) to MNI standard space using a 12-parameter affine registration. The NoGo minus Go contrast was the focus of fMRI analysis, as this represents activation related to response inhibition and has been previously shown to reveal differences in bipolar versus normal subjects (19, 57). For first-level analyses, time-series statistical analyses were carried out at a single-run intra-subject level using a GLM that modeled each block using a synthetic hemodynamic response function and its first derivative. Six motion parameter estimates were modeled as covariates of no interest. Subject-specific activation maps were carried to higher-level group analyses using FLAME stage 1 and stage 2 (58–60) to assess within- and between-group patterns in activation. Regions of activation with a height threshold of Z>2.0 and cluster probability of P < 0.05 corrected for multiple comparisons using Gaussian random field theory were considered significant (61).
sMRI Preprocessing and Cortical Thickness Analysis
Preprocessing of high resolution T1-weighted images included removal of non-brain tissue, automated registration, segmentation of sub-cortical white and deep gray matter tissue types, surface extraction and surface registration to the FreeSurfer Desikan atlas (51, 52). Cortical thickness was estimated and smoothed (20 mm FWHM) in each subject.
To assess whether areas showing a main effect of group in fMRI analyses (Table 2) showed a similar main effect of group in cortical thickness, a priori ROI-based analyses of mean cortical thickness were performed. A main effect of group was conducted using a general linear model in SPSS controlling for age.
Correlation of fMRI activation with Cortical Thickness
To investigate associations between fMRI activations with cortical thickness, functional and structural maps were aligned within subject and then aligned to the Freesurfer Desikan atlas (51, 52) to bring them into a common space. Alignment of the functional and structural images within each subject was achieved using three steps. First, the low resolution fMRI image was registered to the T2 weighted structural MR image using a 6 parameter transformation; the resulting image was rigidly aligned to the high-resolution T1-weighted MR image using FLIRT (56). The resulting transformation was used to project and resample the smoothed (Gaussian kernel, FWHM=1mm), task related functional activation maps on the T1 image for individual subjects. Finally, activation maps were projected onto subjects’ individual cortical surfaces by performing a 3D convolution with a spherical kernel of radius 1mm and then resampling them to the nearest point on each vertex using the ShapeTools libraries (62). Functional activations (z-score for NoGo minus Go) were averaged over anatomical ROIs from the Desikan atlas for each subject. To avoid ROI selection bias (63), anatomical ROIs were exclusively chosen over ‘significant’ functional clusters. To test the relationships between cortical thickness and activation, the subject-specific mean thickness and average z-score for each cortical ROI were then fed into separate partial correlation analyses in SPSS that controlled for age. We also tested for interaction effects of disease state on functional activations as a predictor for the cortical ROI thickness controlling for age.
Results
Demographic and Clinical Data
Bipolar and control groups did not differ significantly in age, gender or ethnicity. Eleven (24%) of bipolar subjects were medication-free. The remaining 34 bipolar subjects were receiving medications (see Table 1).
Table 1.
Demographic and behavioral performance data, presented as mean ± standard deviation or n (%).
| Bipolar (n = 45) |
Controls (n = 45) |
p Value | |
|---|---|---|---|
| Age (Years) | 39.9 ± 12.1 | 37.7 ± 10.5 | 0.346 |
| Gender (Male/Female) | 24/21 | 23/22 | 0.833 |
| Ethnicity | 0.255 | ||
| Caucasian | 25 | 25 | |
| African American | 12 | 12 | |
| Asian | 4 | 8 | |
| American Indian/Alaskan Native | 2 | 0 | |
| Pacific Islander/Native Hawaiian | 2 | 0 | |
| YMRS Score | 1.7 ± 2.0 | 0.4 ± 0.9 | <0.001 |
| HRSD-21 Score | 3.8 ± 2.4 | 1.2 ± 1.5 | <0.001 |
| Age of onset (years)1 | 20.7 ± 9.5 | - | |
| Duration of bipolar Illness (years)1 | 19.2 ± 13.3 | - | |
| Duration of euthymic mood (weeks)1 | 106.1 ± 282.3 | - | |
| Current Medications2 | |||
| None | 11 (24%) | 45 | |
| Lithium | 1 | - | |
| Valproic Acid (Depakote, Divalproex Sodium) | 6 | - | |
| Lamotrigine (Lamictal) | 11 | - | |
| Antipsychotic | 31 | - | |
| SSRI Antidepressant (e.g. Zoloft, Celexa) | 9 | - | |
| Other Antidepressant (e.g. Wellbutrin) | 13 | - | |
| Other Anticonvulsant | 4 | - | |
| Benzodiazepine | 2 | - | |
| Current Comorbidity | |||
| Posttraumatic stress disorder (PTSD) | 1 | - | |
| Anorexia nervosa | 1 | - | |
| Panic disorder without
Agoraphobia, Specific Phobia, and PTSD |
1 | - | |
| fMRI Task Accuracy (%) | |||
| Go condition | 93.6 ± 6.9 | 95.8 ± 6.7 | .09 |
| NoGo condition | 97.3 ± 3.4 | 98.7 ± 1.8 | .09 |
| fMRI Task Reaction time (sec) | |||
| Go condition | 0.52 ± 0.14 | 0.47 ± 0.11 |
U =
808, p = .135 |
| NoGo condition | 0.56 ± 0.12 | 0.51 ± 0.08 |
U =
803, p = .125 |
YMRS, Young Mania Rating Scale; HRSD-21, Hamilton Rating Scale for Depression (21 item).
Course of illness information (i.e., bipolar illness duration, duration of euthymic mood) was obtained by self-report at the time of SCID interview and confirmed by referring to psychiatric care records when available. Duration of euthymic mood data is missing for one bipolar participant.
The number of reported bipolar subjects taking medication is greater than the total N of 45 due to 28 bipolar subjects taking more than 1 medication.
fMRI Performance and Activation
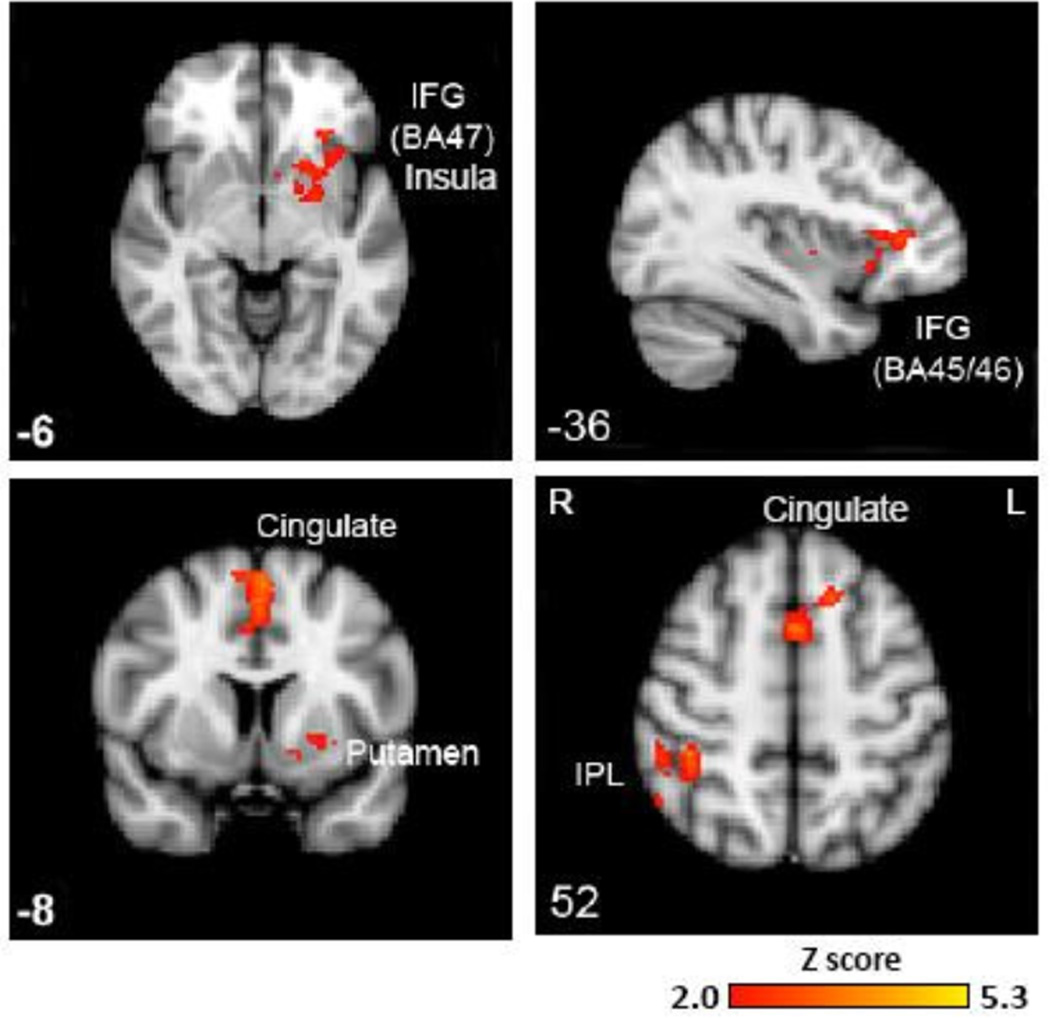
As shown in Table 1, there were no significant between-group differences in response times or accuracy for either the Go or NoGo conditions. Within-group results showed significant activation of cognitive control regions as previously demonstrated in controls (19, 57) (Supplementary Figure S1). During response inhibition (NoGo minus Go), significant between-group activation differences (controls>bipolar) were found in three distinct clusters with voxel sizes of 433, 491, and 484, corresponding to left prefrontal areas (Tal xyz = −1, 9, 52, Z=3.72), right inferior parietal lobule (Tal xyz = 41, −34, 48, Z=3.62), and left globus pallidus (Tal xyz = −16, 0, −3, Z=3.56), respectively. Table 2 presents the peak local maxima within these clusters. These clusters included the IFC (corresponding to BA 47 and BA 45/56), medial frontal gyrus (BA 6), insula, left and right superior frontal gyri (BA 6), and cingulate gyrus (BA 32), and right parietal lobule (BA 40/7) (Z>2.0, p < 0.05, corrected) (Figure 2 and Table 2). There were no areas of significantly greater activation in bipolar subjects for this contrast.
Table 2.
Between-group results show significantly reduced functional activation in bipolar versus control subjects during response inhibition.
| BA | x | y | z | Z-statistic | |
|---|---|---|---|---|---|
| Frontal lobe | |||||
| L Inferior Frontal Gyrus* | 47 | −29 | 26 | −3 | 2.73 |
| L Inferior Frontal Gyrus | 45 | −33 | 31 | 5 | 3.29 |
| L Inferior Frontal Gyrus | 45/46 | −35 | 33 | 9 | 3.08 |
| L Superior Frontal Gyrus* | 6 | −1 | 9 | 52 | 3.72 |
| R Superior Frontal Gyrus** | 6 | 4 | 12 | 44 | 2.82 |
| L Medial Frontal Gyrus | 6 | −1 | 12 | 44 | 3.33 |
| L Insula | −29 | 17 | 9 | 2.33 | |
| Limbic lobe | |||||
| L Cingulate Gyrus | 32 | −1 | 11 | 39 | 2.32 |
| R Cingulate Gyrus | 32 | 4 | 9 | 39 | 2.65 |
| Subcortical Regions | |||||
| L Globus Pallidus | −16 | 0 | −3 | 3.56 | |
| L Putamen | −23 | −2 | 0 | 3.12 | |
| Parietal lobe | |||||
| R Inferior Parietal Lobule** | 40 | 41 | −34 | 48 | 3.62 |
| R Superior Parietal Lobule | 7 | 43 | −57 | 51 | 3.58 |
BA = Brodmann area; L = left; R = right; (x, y, z) are Talairach coordinates of local maxima significant at Z>2.0 and p<0.05, corrected for multiple comparisons across whole-brain using Gaussian random field theory. For reporting purposes, Montreal Neurological Institute (MNI) coordinates were transformed to Talairach space using the MNI to Talairach Conversion Applet (www.bioimagesuite.org). Anatomical localization and assignment of the corresponding Brodmann area was then performed using a Talairach stereotaxic atlas (98) .
More than one local maxima within 10 mm corresponds to this anatomical label and BA region.
More than one local maxima cluster outside 10 mm corresponds to this anatomical label and BA region.
Figure 2.
Between-group higher-level analyses during response inhibition (NoGo minus Go) showed significantly reduced fMRI activation in the left inferior frontal gyrus corresponding to Brodmann area’s 45/46/47, left insula, bilateral cingulate gyrus, left striatal regions (putamen and globus pallidus) and right inferior parietal lobule at Z> 2.0, p<0.05 corrected for whole-brain multiple comparisons. IFG = inferior frontal gyrus; IPL = inferior parietal lobule; BA = Brodmann’s area. Right = Left.
Cortical Thickness
An ROI structural analysis investigating the brain regions where significant fMRI activation differences were seen between patients and controls (Table 2) revealed significant cortical thickness reductions in bipolar versus control subjects in several regions. Decreased thickness was evident in bipolar subjects in the left IFC (p = 0.017), corresponding to pars triangularis, pars opercularis, and pars orbitalis (Table 3). Relative to controls, decreased thickness in bipolar subjects was also evident in the right superior frontal gyrus (p = 0.009) and right cingulate gyrus (p = 0.031) (Table 3). There were no significant group differences in cortical thickness in additional regions (left insula, left cingulate gryus, bilateral parietal lobule) where between-group functional differences were observed. No areas showed significantly increased thickness in bipolar patients vs. controls. For exploratory purposes, we additionally conducted a whole-brain analysis of cortical thickness and results from all regions are presented in the Supplement (Table S1 and Table S2).
Table 3.
P-values showing significant decreases in cortical ROI thickness in bipolar patients in those brain regions that demonstrated significant BOLD hypoactivations.
| BA | Thickness P-value |
Effect sizes (Cohen’s d) |
|
|---|---|---|---|
| Frontal lobe | |||
| L Par sorbitalis | 47 | NS | -- |
| L Inferior Frontal
Gyrus (merged pars triangularis, pars opercularis, pars orbitalis) |
44, 45, 47 | 0.017 | 0.42 |
| L Superior Frontal Gyrus | 6/8/9/10 | NS | -- |
| R Superior Frontal Gyrus | 6/8/9/10 | 0.009 | 0.58 |
| L Medial Frontal
Gyrus (same as L Superior Frontal Gyrus) |
6 | NS | -- |
| L Insula | NS | -- | |
| Limbic lobe | |||
| L Cingulate
Gyrus (caudal-anterior cingulate) |
32 | NS | -- |
| R Cingulate
Gyrus (caudal-anterior cingulate) |
32 | 0.031 | 0.46 |
| Parietal lobe | |||
| R Inferior Parietal Lobule | 40 | NS | -- |
| R Superior Parietal Lobule | 7 | NS | -- |
Only the ROIs in Table 2 are shown. BA = Brodmann area; L = left; R = right; NS = not significant. The regions that appear in parentheses are labels from the Desikan atlas.
Associations between Functional Activation and Cortical Thickness
To determine significant linkages between brain structure and function, partial correlations between functional activations (Z-score for No-Go minus Go) and cortical thickness, controlling for age, were performed for those areas, presented in Table 2 and Figure 2, showing significant fMRI effects of group.
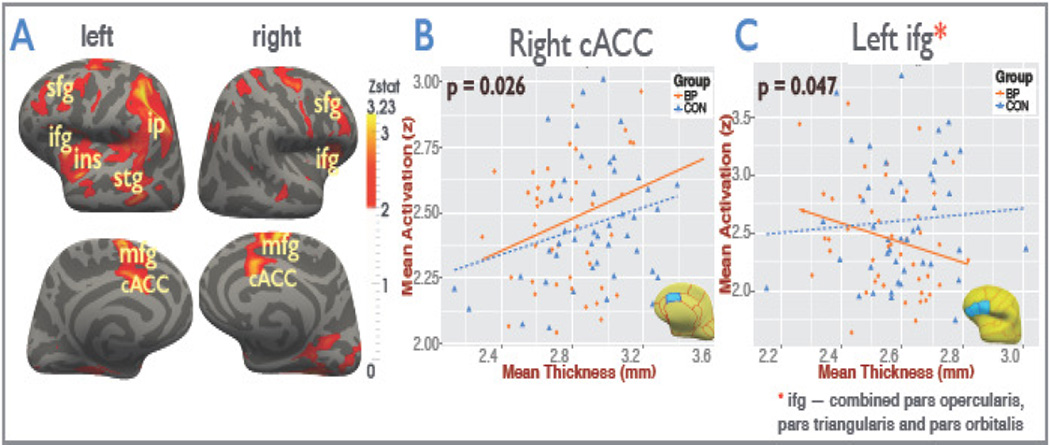
Figure 3A shows the functional hypoactivation (Z-score) in bipolar patients compared to controls visualized on the inflated Freesurfer atlas. As shown in Figure 3B, a significant positive correlation between activation and cortical thickness was found in the right caudal ACC across the sample (i.e. controls and patients combined) (r = 0.26, p = 0.0136) and in the patient group alone (r = 0.33, p = 0.026), but not in controls subjects separately.
Figure 3.
(A) Functional hypoactivation (Z-score) in bipolar patients compared to controls visualized on the inflated Freesurfer atlas. Significant clusters of reduced activation show the inferior frontal gyrus (ifg), superior frontal gyrus (sfg), medial frontal gyrus (mfg), insula (ins), caudal anterior cingulate gyrus (cACC), superior temporal gyrus (stg), and inferior parietal (ip) regions. (B) Partial correlations between mean BOLD activation and a priori mean region-of-interest (ROI) cortical thickness in the right caudal anterior cingulate (cACC), controlling for age, are displayed separately for bipolar (BP) patients and controls (CON). Only patients demonstrated a significant correlation (r = 0.33, p = 0.026) between thickness and functional activation. (C) Significant disease interaction effect (p=0.047) was found for the functional activation and cortical thickness in the left inferior frontal gyrus which consists of the caudal middle frontal, lateral orbitofrontal, medial orbitofrontal, pars orbitalis, pars triangularis, pars opercularis, rostral middle frontal, superior frontal, and the frontal pole ROIs merged together. It was also observed that the left frontal lobe mean activations were significantly negatively correlated (r = −0.34, p = 0.021) with the respective mean cortical thickness in patients when controlled for age and the duration of the euthymic period but not in controls.
We also found a significant interaction effect (p=0.047) of fMRI activations by disease state as predictors for cortical thickness in the left IFC (Figure 3C), corresponding to pars opercularis, pars triangularis and pars orbitalis (also see Table 3 and Figure S2 and Figure S3).
We examined correlations with illness duration (years) and the period of euthymic state (weeks) for both ROI thickness and functional activations (Table S4). Cortical thickness in the left middle temporal (r=0.31, p=0.039), right transverse temporal (r=-0.31, p=0.039), right parahippocampal (r=0.31, p=0.04) and the right posterior cingulate (r=0.32, p=0.029) gyri were significantly correlated with illness duration when controlling for age.
Discussion
This study sought to i) confirm prior findings that IFC hypofunction occurs in patients with bipolar disorder even in the euthymic state and ii) to assess whether structural deficits occur in regions of functional hypoactivation. Functional and structural analyses surveyed local effects, and additional regions were explored using the appropriate corrections for multiple comparisons to better understand how functional deficits in bipolar disorder relate to abnormalities in underlying neural architecture.
Consistent with prior literature (7, 64), we found that euthymic bipolar subjects showed significant hypoactivation in core regions of inhibitory control circuits, including the IFC and ACC. The superior frontal gyrus (SFG; BA 6), which serves motor planning and decision-making, is a region that includes supplementary (SMA) and premotor areas, and has previously been implicated in the inhibition of response (65). We also showed fMRI hypoactivation in this region as consistent with prior work demonstrating reduced activation during a Go-NoGo task in bipolar I euthymic subjects (19) as well as bipolar II depressed subjects (57) versus controls. It has been posited that hypoactivation in the SMA, IFC, and striatum may explain some of the disinhibition (e.g., impulsivity) characteristics observed in bipolar patients even while euthymic (19). Reduced fMRI activation in BA 6 has also been implicated in the cognitive control of emotion in MDD subjects also while euthymic (66), suggesting that this may represent an endophenotypic marker of future depression risk.
Additionally, results showed disease-related reductions in cortical thickness in cortical areas exhibiting functional deficits - specifically, the IFC, ACC and the SFG. These structural deficits suggest a potential etiology for frontally-mediated functional deficits seen in prior fMRI studies of bipolar disorder (7) and could explain why IFC functional deficits, in particular, appear trait- rather than state-related. That is, IFC hypoactivation has been reported both in bipolar mania (67, 68) and euthymia (18, 19, 50). Further, prior work shows patients with focal lesions to BA 6 have an increased number of false alarms during a simple Go-NoGo task (65) suggesting that structural abnormalities in this region impair performance. The present study extends this prior work by demonstrating PFC thickness deficits in euthymic bipolar patients vs. controls despite equivalent task performance.
Linking measures of brain structure and function, partial correlations analyses revealed a disease state interaction effect for the left IFC with patients showing a negative relationship between activation and thickness and controls showing the opposite trend. In contrast, a positive linear relation between structure and function was observed in the right ACC. The ACC may contribute to behavior by modifying responses in reaction to challenging cognitive or physical states that require additional efforts at cognitive control. Thus, cortical thinning in the ACC in patients with bipolar disorder may explain the hypoactivation seen in this region during tasks like the Go-NoGo that require increased cognitive control in order to successfully inhibit habituated motor responses, potentially via suppression of thalamic response (69).
Functional deficits
The current study replicates our prior work in an independent sample of euthymic bipolar subjects where we found IFC hypoactivation during the Go-NoGo task (19). A growing number of functional neuroimaging studies using different behavioral probes have reported abnormal IFC function in bipolar disorder across mood states (10, 11, 13, 17, 18, 57, 70–73). A meta-analysis of fMRI activation and neurocognitive studies investigating response inhibition in bipolar disorder (including 667 controls and 635 patients) report reduced activation in the IFC in bipolar subjects regardless of current mood state and behavioral performance (16). These findings are consistent with our current results showing hypoactivations in BA 47, 44/45 and 46. Notably, IFC deficits are detectable even during euthymia suggesting a trait related disturbance (16). In line with meta-analyses (17, 74), we have previously reported abnormal functional connectivity between the IFC and the amygdala in manic subjects (12), where reduced IFC function was significantly correlated with heightened amygdala activation. Amygdala overactivation may be a primary source of the pathophysiologic change in bipolar subjects. Alternatively, chronic hypoactivity in a cortical region such as the IFC could disrupt a primarily inhibitory prefrontal-amygdala circuit. Inhibitory input from the IFC to the basolateral amygdala may be a mechanism by which the PFC modulates amygdala output and suppresses amygdala-mediated behaviors (75). In bipolar disorder, there may be specificity for a ventral PFC—limbic/amygdala circuit abnormality, as these abnormal regional brain findings have not been reported in schizophrenia (where a dorsolateral PFC—hippocampal circuit appears to be primarily disrupted) (17, 76).
Structural deficits
Early studies of coarsely demarcated PFC have frequently yielded negative findings (for review, see Strakowski et al. (4, 24) and Soares (77)). However, studies using more refined PFC segmentations suggest regional abnormalities (33–41, 74). Foland-Ross et al. (42) reported reduced thickness in the PFC and ACC in euthymic bipolar I subjects vs. controls. Similarly, Lyoo et al. (39) found decreased cortical thickness in multiple prefrontal, sensory and sensory association regions in bipolar I and II subjects vs. controls. Almeida et al. (41) reported reduced gray matter volumes (GMV) within the ventral/medial PFC in bipolar I subjects vs. controls, while Eker et al. (38) found left OFC deficits both in euthymic bipolar I subjects and their healthy siblings, suggesting reductions in OFC volume may be associated with the heritability of bipolar disorder. Drevets et al. (33) and Hirayasu et al. (34) reported GMV reductions in the left sub-genual PFC in patients with bipolar disorder. Nugent et al. reported (78) lower GMV in lateral OFC in bipolar I and II subjects compared to controls. The findings of BA47 gray matter deficits might provide an explanation for the functional abnormalities seen in this area in our data. The ACC is also specifically implicated. Sassi et al. (35) reported a significant reduction in GMV in the left ACC in untreated bipolar I and II patients in line with other groups showing reduced ACC (36, 40, 79) and precentral gyrus (38, 40) volume in subjects with bipolar illness compared to controls.
Neuroimaging studies have demonstrated a role for medial and lateral regions of the OFC in mood regulation (80, 81) and in associative emotional memory functions (22, 82–84). A reduction in PFC GMV may be related to duration of illness (37, 39), increased age (85) and may have prognostic implications. Sax et al. (86) found that decreases in PFC volumes inversely correlate with performance on tests probing deficits of sustained attention in bipolar disorder (86). Consistent with this, another study showed reduced PFC volume in bipolar patients associate with diffuse neuropsychological impairments (87). The ACC, widely described to play a role in cognitive control, forms an anterior component of the default mode network (DMN) (88). Given that medial frontal gyrus (BA 6) is another component of the default mode network, structural abnormalities in this network may contribute to functional studies of the DMN showing abnormalities in bipolar disorder (88).
Structure and function relations
Our study found reductions in cortical thickness in the IFC, ACC and SFG, regions that also exhibited functional deficits. Patients with a relatively thicker left frontal cortex, corresponding to pars opercularis, pars triangularis and pars orbitalis, showed decreased functional activation in this same region during performance of the response inhibition task, whereas control subjects showed the opposite trend. At the same time, we also found a positive linear correlation between cortical thickness and activation in the right ACC in the bipolar group and in the combined sample. Interestingly, structure-function associations in the right ACC during response inhibition have been reported in healthy controls (89). A recent review (90) summarizing both functional and structural neuroimaging studies has suggested that there is a disruption of the neural circuitry in the ventrolateral prefrontal cortex (including the IFC and OFC) along with the medial prefrontal cortex (including the ACC) that mediates both voluntary and automatic emotion processing and regulation processes. Our study provides supporting evidence that structural deficits in a node of the inhibitory network such as the ACC may link with downstream disruption of functional activity in the IFC.
Both increases and decreases in cortical thickness can impact neural processing as dependent on the underlying mechanisms. For example, aberrant neural pruning or decreased intracortical myelination may account for greater thickness values together with altered neurotransmission. The current results might suggest that task performance relies on the structural and functional integrity on a network of structures in controls, but that a structural disturbance within the IFC forming a component of this network contributes to altered task performance in patients selectively. Previous unrelated studies by our group (91, 92) have shown reduced activation with increasing IFC thickness in healthy pediatric cohorts during syntactic and orthographic processing tasks. In contrast, others (93) have shown patterns of negative correlations of thickness and activations in auditory tasks in other clinical populations. A large multimodal meta-analysis (94) of structure-function changes in first episode psychosis has shown patterns of hypoactivation in the medial PFC together with increases in GMV. This meta-review (94) speculates that the reductions in gray matter may cause compensatory changes to function, leading to increased vascularization and thus hyperactivation. Since fMRI BOLD is not a direct measurement of blood flow, it is not straightforward to draw causal conclusions relating gray matter thickness and functional activations. Furthermore, since we focused on a response inhibition task, it is likely that these findings are task-specific. While our findings warrant further replication, they do provide new evidence to suggest a structural basis for altered neural processing in bipolar disorder and stress the importance of inferior-frontal and ventro-limbic circuitry where both increased and decreased thickness appear related to altered functional signatures.
Limitations
The typical lower spatial resolution of functional imaging data, spatial smoothing and partial volume effects (95) remain potential confounders for structure-function mapping studies. Consequently, the lack of structure-function relationships in the IFC in particular may be attributable to methodological limitations including a mismatch between structural and functional anatomy or to microscopic changes not detectable at the macroscopic level. Further, functional deficits may be attributable to structural disturbances in connected areas rather than the region itself.
Conclusion
Our results suggest an underlying structural deficit in bipolar subjects in brain regions where functional deficits are seen. This study has a number of implications. The discovery of specific anatomic abnormalities with clear functional consequences may serve as biomarkers for intervention studies. IFC deficits reported here also appear a promising potential endophenotype given that they appear state-independent. Future studies may address the heritability of this disease signature and its co-segregation in families (96, 97), the coupling of structure-function relationships with respect to illness severity and clinical history and explore structural and functional deficits reported across other functional domains with high clinical relevance.
Supplementary Material
Acknowledgments
The authors thank: Ana R. Aquino, Jeffrey Fischer and the UCLA Deutsch Mood Disorders Fellows for diagnostic interviewing and assessment; Jacquelyn Dang and Isabella Cordova for assistance with data entry; and Tara Pirnia for assistance with image processing.
Funding for this project was provided by the National Institute of Mental Health (R21 MH075944 [LLA], R01084955 [LLA], K24MH102743 [KN]), the National Center for Research Resources (RR12169, RR13642 and RR00865) by way of the UCLA Ahmanson-Lovelace Brain Mapping Center, and Neuroscience Fellowships provided by the MSST and Swift foundations.
Financial Disclosures
Dr. Altshuler has received past funding from Takeda Pharmaceuticals North America, Inc. for an advisory board meeting, Sunovion Pharmaceuticals Inc. for an advisory board meeting, and from the law offices of Hughes-Sokol-Piers-Resnick DYM Ltd for review of medical records and conference call.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions: Dr. Altshuler was the project PI and supervised the collection and analysis of fMRI data by Dr. Vizueta. Drs. Narr and Thompson supervised the analysis of structural MRI and structure-function mapping analyses by Dr. Joshi. Drs. Joshi and Vizueta wrote the Methods and Results section. Ms. Townsend and Drs. Foland-Ross, Vizueta and Altshuler refined the literature review and discussion. Dr. Bookheimer provided guidance on the fMRI data analyses and contributed to the first and final drafts.
All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archives of General Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Annals of the New York Academy of Sciences. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 3.Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 4.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Molecular Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 5.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13(829):833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. The British Journal of Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- 7.Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares JC, Mann JJ. The anatomy of mood disorders--review of structural neuroimaging studies. Biological Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 9.Strakowski SM, DelBello MP, Adler C, Cecil DM, Sax KW. Neuroimaging in bipolar disorder. Bipolar Disord. 2000;2:148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- 10.Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, et al. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biological Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Research. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, et al. A functional magnetic resonance imaging study of bipolar disorder: state-and trait-related dysfunction in ventral prefrontal cortices. Archives of General Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, White T, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. The American Journal of Psychiatry. 1999;156:1986–1988. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- 15.Rubinsztein JS, Fletcher PC, Rogers RD, Ho LW, Aigbirhio FI, Paykel ES, et al. Decision-making in mania: a PET study. Brain. 2001;124:2550–2563. doi: 10.1093/brain/124.12.2550. [DOI] [PubMed] [Google Scholar]
- 16.Hajek T, Alda M, Hajek E, Ivanoff J. Functional neuroanatomy of response inhibition in bipolar disorders--combined voxel based and cognitive performance meta-analysis. Journal of Psychiatric Research. 2013;47:1955–1966. doi: 10.1016/j.jpsychires.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Delvecchio G, Sugranyes G, Frangou S. Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychological Medicine. 2013;43:553–569. doi: 10.1017/S0033291712001432. [DOI] [PubMed] [Google Scholar]
- 18.Foland-Ross LC, Bookheimer SY, Lieberman MD, Sugar CA, Townsend JD, Fischer J, et al. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. NeuroImage. 2012;59:738–744. doi: 10.1016/j.neuroimage.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend JD, Bookheimer SY, Foland-Ross LC, Moody TD, Eisenberger NI, Fischer JS, et al. Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task. Bipolar Disord. 2012;14:442–450. doi: 10.1111/j.1399-5618.2012.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Molecular Psychiatry. 2015 In Press. [Google Scholar]
- 21.Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 22.Price JL. Comparative aspects of amygdala connectivity. Annals of the New York Academy of Sciences. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- 23.Morris JS, Dolan RJ. Dissociable amygdala and orbitofrontal responses during reversal fear conditioning. NeuroImage. 2004;22:372–380. doi: 10.1016/j.neuroimage.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Strakowski SM, Adler CM, DelBello MP. Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder? Bipolar Disord. 2002;4:80–88. doi: 10.1034/j.1399-5618.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- 25.Hoge EA, Friedman L, Schulz SC. Meta-analysis of brain size in bipolar disorder. Schizophrenia Research. 1999;37:177–181. doi: 10.1016/s0920-9964(98)00149-2. [DOI] [PubMed] [Google Scholar]
- 26.Beyer JL, Krishnan KR. Volumetric brain imaging findings in mood disorders. Bipolar Disord. 2002;4:89–104. doi: 10.1034/j.1399-5618.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- 27.Haller S, Xekardaki A, Delaloye C, Canuto A, Lovblad KO, Gold G, et al. Combined analysis of grey matter voxel-based morphometry and white matter tract-based spatial statistics in late-life bipolar disorder. Journal of Psychiatry & Neuroscience. 2011;36:391–401. doi: 10.1503/jpn.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, et al. Decreased regional cortical gray matter volume in schizophrenia. The American Journal of Psychiatry. 1994;151:842–848. doi: 10.1176/ajp.151.6.842. [DOI] [PubMed] [Google Scholar]
- 29.Zipursky RB, Seeman MV, Bury A, Langevin R, Wortzman G, Katz R. Deficits in gray matter volume are present in schizophrenia but not bipolar disorder. Schizophrenia Research. 1997;26:85–92. doi: 10.1016/s0920-9964(97)00042-x. [DOI] [PubMed] [Google Scholar]
- 30.Strakowski SM, Wilson DR, Tohen M, Woods BT, Douglass AW, Stoll AL. Structural brain abnormalities in first-episode mania. Biological Psychiatry. 1993;33:602–609. doi: 10.1016/0006-3223(93)90098-x. [DOI] [PubMed] [Google Scholar]
- 31.Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 32.Lim KO, Rosenbloom MJ, Faustman WO, Sullivan EV, Pfefferbaum A. Cortical gray matter deficit in patients with bipolar disorder. Schizophrenia Research. 1999;40:219–227. doi: 10.1016/s0920-9964(99)00063-8. [DOI] [PubMed] [Google Scholar]
- 33.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 34.Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, et al. Subgenual cingulate cortex volume in first-episode psychosis. The American Journal of Psychiatry. 1999;156:1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, Frank E, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biological Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Lochhead RA, Parsey RV, Oquendo MA, Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biological Psychiatry. 2004;55:1154–1162. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biological Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- 38.Eker C, Simsek F, Yilmazer EE, Kitis O, Cinar C, Eker OD, et al. Brain regions associated with risk and resistance for bipolar I disorder: a voxel-based MRI study of patients with bipolar disorder and their healthy siblings. Bipolar Disord. 2014;16:249–261. doi: 10.1111/bdi.12181. [DOI] [PubMed] [Google Scholar]
- 39.Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 40.Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biological Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Almeida JR, Akkal D, Hassel S, Travis MJ, Banihashemi L, Kerr N, et al. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Research. 2009;171:54–68. doi: 10.1016/j.pscychresns.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foland-Ross LC, Thompson PM, Sugar CA, Madsen SK, Shen JK, Penfold C, et al. Investigation of cortical thickness abnormalities in lithium-free adults with bipolar I disorder using cortical pattern matching. The American Journal of Psychiatry. 2011;168:530–539. doi: 10.1176/appi.ajp.2010.10060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maller JJ, Thaveenthiran P, Thomson RH, McQueen S, Fitzgerald PB. Volumetric, cortical thickness and white matter integrity alterations in bipolar disorder type I and II. Journal of Affective Disorders. 2014;169:118–127. doi: 10.1016/j.jad.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, Strakowski SM. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biological Psychiatry. 2007;61:776–781. doi: 10.1016/j.biopsych.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, Wen W, Malhi GS, Ivanovski B, Sachdev PS. Regional gray matter changes in bipolar disorder: a voxel-based morphometric study. The Australian and New Zealand Journal of Psychiatry. 2007;41:327–336. doi: 10.1080/00048670701213229. [DOI] [PubMed] [Google Scholar]
- 46.Brambilla P, Nicoletti MA, Harenski K, Sassi RB, Mallinger AG, Frank E, et al. Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology. 2002;27:792–799. doi: 10.1016/S0893-133X(02)00352-4. [DOI] [PubMed] [Google Scholar]
- 47.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 48.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 49.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ajilore O, Vizueta N, Walshaw P, Zhan L, Leow A, Altshuler LL. Connectome Signatures of Neurocognitive Abnormalities in Euthymic Bipolar I Disorder. Journal of Psychiatric Research. 2015;68:37–44. doi: 10.1016/j.jpsychires.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 52.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 53.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 55.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 56.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 57.Penfold C, Vizueta N, Townsend JD, Bookheimer SY, Altshuler LL. Frontal lobe hypoactivation in medication-free adults with bipolar II depression during response inhibition. Psychiatry Research. 2015;231:202–209. doi: 10.1016/j.pscychresns.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 59.Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 60.Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 61.Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. New York: Oxford University Press; 2001. pp. 251–270. [Google Scholar]
- 62.Joshi SH, Cabeen RP, Joshi AA, Sun B, Dinov I, Narr KL, et al. Diffeomorphic sulcal shape analysis on the cortex. IEEE Trans Med Imaging. 2012;31:1195–1212. doi: 10.1109/TMI.2012.2186975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 2012;14:326–339. doi: 10.1111/j.1399-5618.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- 65.Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- 66.Smoski MJ, Keng SL, Schiller CE, Minkel J, Dichter GS. Neural mechanisms of cognitive reappraisal in remitted major depressive disorder. Journal of Affective Disorders. 2013;151:171–177. doi: 10.1016/j.jad.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. The American Journal of Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 68.Strakowski SM, Adler CM, Cerullo M, Eliassen JC, Lamy M, Fleck DE, et al. Magnetic resonance imaging brain activation in first-episode bipolar mania during a response inhibition task. Early Interv Psychiatry. 2008;2:225–233. doi: 10.1111/j.1751-7893.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- 70.Vizueta N, Rudie JD, Townsend JD, Torrisi S, Moody TD, Bookheimer SY, et al. Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. The American Journal of Psychiatry. 2012;169:831–840. doi: 10.1176/appi.ajp.2012.11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 72.Brooks JO, 3rd, Vizueta N. Diagnostic and clinical implications of functional neuroimaging in bipolar disorder. Journal of Psychiatric Research. 2014;57:12–25. doi: 10.1016/j.jpsychires.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 73.Kaladjian A, Jeanningros R, Azorin J-M, Nazarian B, Roth M, Mazzola-Pomietto P. Reduced brain activation in euthymic bipolar patients during response inhibition: An event-related fMRI study. Psychiatry Research: Neuroimaging. 2009;173:45–51. doi: 10.1016/j.pscychresns.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Houenou J, Frommberger J, Carde S, Glasbrenner M, Diener C, Leboyer M, et al. Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. Journal of Affective Disorders. 2011;132:344–355. doi: 10.1016/j.jad.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 75.Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. The Journal of Neuroscience. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Archives of General Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 77.Soares JC. Contributions from brain imaging to the elucidation of pathophysiology of bipolar disorder. The International Journal of Neuropsychopharmacology. 2003;6:171–180. doi: 10.1017/S1461145703003390. [DOI] [PubMed] [Google Scholar]
- 78.Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, et al. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. NeuroImage. 2006;30:485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 79.Oertel-Knochel V, Reinke B, Feddern R, Knake A, Knochel C, Prvulovic D, et al. Episodic memory impairments in bipolar disorder are associated with functional and structural brain changes. Bipolar Disord. 2014;16:830–845. doi: 10.1111/bdi.12241. [DOI] [PubMed] [Google Scholar]
- 80.Baker SC, Frith CD, Dolan RJ. The interaction between mood and cognitive function studied with PET. Psychological Medicine. 1997;27:565–578. doi: 10.1017/s0033291797004856. [DOI] [PubMed] [Google Scholar]
- 81.Northoff G, Richter A, Gessner M, Schlagenhauf F, Fell J, Baumgart F, et al. Functional dissociation between medial and lateral prefrontal cortical spatiotemporal activation in negative and positive emotions: a combined fMRI/MEG study. Cereb Cortex. 2000;10:93–107. doi: 10.1093/cercor/10.1.93. [DOI] [PubMed] [Google Scholar]
- 82.Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- 83.Dapretto M, Bookheimer SY. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 84.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 85.Brambilla P, Harenski K, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, et al. Differential effects of age on brain gray matter in bipolar patients and healthy individuals. Neuropsychobiology. 2001;43:242–247. doi: 10.1159/000054897. [DOI] [PubMed] [Google Scholar]
- 86.Sax KW, Strakowski SM, Zimmerman ME, DelBello MP, Keck PE, Jr, Hawkins JM. Frontosubcortical neuroanatomy and the continuous performance test in mania. The American Journal of Psychiatry. 1999;156:139–141. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- 87.Coffman JA, Bornstein RA, Olson SC, Schwarzkopf SB, Nasrallah HA. Cognitive impairment and cerebral structure by MRI in bipolar disorder. Biological Psychiatry. 1990;27:1188–1196. doi: 10.1016/0006-3223(90)90416-y. [DOI] [PubMed] [Google Scholar]
- 88.Torrisi S, Moody TD, Vizueta N, Thomason ME, Monti MM, Townsend JD, et al. Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar Disord. 2013;15:156–166. doi: 10.1111/bdi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hegarty CE, Foland-Ross LC, Narr KL, Townsend JD, Bookheimer SY, Thompson PM, et al. Anterior cingulate activation relates to local cortical thickness. Neuroreport. 2012;23:420–424. doi: 10.1097/WNR.0b013e3283525a95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and roadmap for future research. The American journal of psychiatry. 2014;171:829–843. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu LH, Dapretto M, O’Hare ED, Kan E, McCourt ST, Thompson PM, et al. Relationships between Brain Activation and Brain Structure in Normally Developing Children. Cerebral Cortex. 2009;19:2595–2604. doi: 10.1093/cercor/bhp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nuñez SC, Dapretto M, Katzir T, Starr A, Bramen J, Kan E, et al. fMRI of syntactic processing in typically developing children: Structural correlates in the inferior frontal gyrus. Developmental Cognitive Neuroscience. 2011;1:313–323. doi: 10.1016/j.dcn.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anurova I, Renier LA, De Volder AG, Carlson S, Rauschecker JP. Relationship Between Cortical Thickness and Functional Activation in the Early Blind. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire PK, et al. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neuroscience & Biobehavioral Reviews. 2012;36:2325–2333. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 95.Oakes TR, Fox AS, Johnstone T, Chung MK, Kalin N, Davidson RJ. Integrating VBM into the General Linear Model with voxelwise anatomical covariates. NeuroImage. 2007;34:500–508. doi: 10.1016/j.neuroimage.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bearden CE, Freimer NB. Endophenotypes for psychiatric disorders: ready for primetime? Trends in genetics : TIG. 2006;22:306–313. doi: 10.1016/j.tig.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 97.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. The American journal of psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 98.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.