Abstract
Background
Although liver biopsy remains the gold standard for the diagnosis of non-alcoholic fatty liver disease [NAFLD], many non-invasive markers of liver fibrosis have recently been proposed and assessed as surrogates of liver biopsy.
Aims and objective
To evaluate the degree of liver fibrosis by different non-invasive fibrosis scoring systems and to compare each non-invasive fibrosis scoring system with histological fibrosis stage.
Materials and methods
The study population consists of consecutive patients with biopsy proven NAFLD. Complete medical history was taken and physical examination was done in all patients along with appropriate biochemical evaluations. NAFLD fibrosis score, BARD score, BAAT score and APRI score were calculated and each score was compared with histological fibrosis staging.
Results
The study population consisted of 60 patients having mean age 39.73 years (SD 9.62, range 17–63 years) including 51 (85%) males and 9 (15%) females. On histology fibrosis was present in 68.3% (41/60) patients. Out of 60 patients 41 had fibrosis and among them 17, 22, 2 patients had grade 1, 2, 3 fibrosis respectively and no one had grade 4 fibrosis. 61.67% (37/60) had definite NASH. Comparing the fibrosis of histology with the noninvasive scoring systems, the sensitivity and specificity of NAFLD fibrosis score were 5.56% and 100% respectively. BARD score had 45.83% sensitivity and 80.55% specificity. The sensitivities of BAAT score and APRI score were 0% and 29.16% respectively and the specificities were 100% and 97.22% respectively.
Conclusion
The noninvasive scoring systems like NFS, BARD, BAAT, and APRI are not sensitive enough to detect fibrosis but highly specific to include fibrosis if scores are more than cut-off values in our cohort, however they cannot replace liver biopsy. Newer more efficient non-invasive scoring systems have to be devised for the Indian NAFLD population.
Abbreviations: ALT, alanine aminotransferase; APRI, aspartate aminotransferase (AST)-to-platelet ratio index; AST, aspartate aminotransferase; BMI, body mass index; DM, diabetes mellitus; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NAS, NAFLD fibrosis score; NPV, negative predictive value; PPV, positive predictive value; ROC, receiver operating characteristic; SGOT, serum glutamic oxaloacetic transaminases; SGPT, serum glutamic pyruvate transaminases; TPC, total platelet count; TG, triglyceride
Keywords: nonalcoholic fatty liver disease (NAFLD), BARD, BAAT, APRI, fibrosis
Non-alcoholic fatty liver disease (NAFLD) is now considered to be the commonest cause of chronic liver disease in both developed as well as developing countries. NAFLD has become a major public health problem due to the rising prevalence of obesity and T2DM worldwide.1 The prevalence of NAFLD is between 20% to 40% in the general population in the West2, 3 while it varies from 8% to 40% in India.4, 5, 6, 7 Although NAFLD was earlier considered to be a relatively benign condition, up to a third of patients may develop serious consequences, including end-stage liver disease and hepatocellular carcinoma.8, 9, 10, 11 Those at risk are patients with significant hepatic necroinflammation and fibrosis.12, 13, 14
Liver biopsy is currently the gold standard, and has been recommended for confirming the diagnosis and for providing prognostic information in cases of NAFLD.15 The procedure is invasive and prone to complications, with 0.01% risk of death.16, 17 It also has the limitations of sampling error and inter-observer variability.18 As there is high prevalence of NAFLD in our population,4, 19 liver biopsy may not be a logistically feasible option in all. In view of these limitations and incumbent risks, and the high prevalence of the disease and grossly inadequate population of hepatologists who can perform liver biopsy, we need non-invasive tests that can reliably diagnose or exclude significant fibrosis, which would be clinically useful to reduce the need for liver biopsy and to prognosticate patients. Currently several clinical scoring systems based on simple clinical or laboratory indices have been proposed to identify advanced fibrosis in patients with NAFLD and other liver diseases. These include the aspartate aminotransferase (AST)-to-platelet ratio index (APRI),20 the aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio,21 the BARD[BMI, AST/ALT, DM] score,22 the BAAT[BMI, Age, ALT, TG] score,23 the FIB-4[Age, AST, ALT, Platelets] score24 and the NAFLD fibrosis score (NAS).25 However, most of them need to be validated prior to their use in any setting. The present study was undertaken to evaluate the efficacy of some of these noninvasive scoring systems in diagnosing fibrosis in NAFLD patients from coastal eastern India.
Methods
In this observational study, conducted at Cuttack, Odisha from July 2011 to September 2013, 60 consecutive outpatients who had fatty liver on ultrasonography, and biopsy proven NAFLD were included. Ultrasonography was performed using a curvilinear 3–5 mHz probe (Philips) by two experienced sonologists. Men who consumed >20 g and women consuming >10 g of alcohol per day, patients with evidence of concomitant liver diseases, hemolysis, Gilbert's disease, HIV infection or on immunosuppressive therapy were excluded from the study. Serum HBsAg, anti-HCV, HIV, ceruloplasmin by copper oxidase method, anti-nuclear antibody, anti-smooth muscle antibody, anti-LKM antibody, serum protein electrophoresis, urinary copper and KF ring on slit-lamp examination were done wherever indicated to exclude other causes of liver diseases. Written informed consent was obtained from all the subjects. The protocol was approved by the institutional ethics committee.
Anthropometric parameters like height, weight, body mass index (BMI), waist and hip circumferences and waist/hip ratio were recorded in all study subjects. All patients were subjected to hematological workup including complete blood count and biochemical investigations like liver function test, lipid profile and fasting blood glucose. All biochemical assessments were performed in the same laboratory by standard laboratory methods. FBG and lipid profile were assayed by an autoanalyser (BIOLIs 24i Tokyo Boeki, Japan) using standard kit. Liver biopsy was performed after informed consent in 60 patients who agreed for it by using a 16 gauge automated cutting biopsy gun (BARD, USA) through the intercostal approach under ultrasound guidance. Tissue obtained at biopsy were fixed in formalin and subjected to routine paraffin embedding and Hematoxylin and Eosin (H&E) staining, ensuring adequate sections to examine the entire biopsy. Besides H and E staining, sections were also stained with special stains, Reticulin and Masson's trichrome. Histological confirmation of NAFLD was done by using the NAFLD activity score. The histological score was defined as the unweighted sum of the scores for steatosis (0–3), lobular inflammation (0–3), and ballooning (0–2). The patients were categorized into three groups: definite NASH, borderline NASH and ‘not NASH’ on the basis of modified Kleiner et al.’s26 NAS [NAFLD activity score] criteria, wherein they have classified histology of hepatic steatosis into NASH with a NAS of ≥5, and No NASH when NAS was less than 3. We labeled the histologies having intermediate scores of 3 and 4 as borderline NASH in our study. Specimens were evaluated by a two pathologists for necro-inflammation and fibrosis as per the NAS-II system. Significant fibrosis was defined as fibrosis ≥2 and minimal fibrosis as F0 or F1. The potential markers of fibrosis were then correlated with liver biopsy findings of necro-inflammation and fibrosis.
Non-invasive scoring systems like NAFLD fibrosis score [−1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × IFG/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet (×109/L) − 0.66 × albumin (g/dl)],25 BARD score [BMI, AST/ALT,DM],22 BAAT score [BMI, Age, ALT, TG]23and APRI [AST/upper limit of normal × 100/platelet count (×109/L)]20 were calculated and compared. There is significant fibrosis when NFS > 0.676, APRI > 0.88, BARD ≥ 2 and if BAAT score is 0 or 1, then NPV for advanced fibrosis is 100%.
Statistical Methods
Data were checked for normal distribution using Shapiro–Wilk test. Categorical and continuous data were presented as proportion and mean, standard deviation and 95% confidence intervals, respectively. Chi-square and unpaired t tests were used to compare between categorical and continuous data, respectively. P-values below 0.05 were considered significant for all statistical analysis. Statistical analysis was done using SPSS version 16 (SPSS, Inc., Chicago, IL, USA). Sensitivity and specificity for each clinical score were computed and the values obtained were plotted on a ROC (receiver operating characteristic) curve.
Results
The 60 patients with NAFLD had mean age 39.73 years (SD 9.62; range 17–63) and included 51 (85%) males and 9 (15%) females with a gender ratio of 5.67:1. Most patients were in the 4th and 5th decades of life as shown in Table 1. In this cohort, 10 (16.67%) patients had diabetes, 16 (26.67%) patients had hypertension and 41 (68.33%) patients had obesity. Their clinical and biochemical parameters are shown in Table 2.
Table 1.
Age and Gender Distribution.
| Age group (years) | Male | Female | Total | Percentage (%) |
|---|---|---|---|---|
| 11–20 | 1 | 0 | 1 | 1.67 |
| 21–30 | 7 | 1 | 8 | 13.33 |
| 31–40 | 22 | 1 | 23 | 38.33 |
| 41–50 | 16 | 5 | 21 | 35 |
| 51–60 | 5 | 2 | 7 | 11.67 |
| Total | 51 | 9 | 60 | 100 |
Table 2.
Clinical, Biochemical, Histological Data of 60 NAFLD Patients Included in This Study.
| Variable | Value |
|---|---|
| Age (years) | 39.73 ± 9.62 |
| Female/male | 9/51 |
| Diabetes (n) | 10 (16.67%) |
| Hypertension (n) | 16 (26.67%) |
| Obesity (n) | 41 (68.33%) |
| BMI (kg/m2) | 26.48 ± 3.32 |
| SGOT (U/L)a | 41.5 (25–127) |
| SGPT (U/L)a | 60 (23–197) |
| TPC (109/L) | 215 ± 42 |
| TG (mg/dl)a | 170 (70–525) |
| Grade of fibrosis/Stage (0/1/2/3/4) | 19/17/22/2/0 |
Median (range).
BMI = body mass index, SGOT = serum glutamic oxaloacetic transaminases, SGPT = serum glutamic pyruvate transaminases, TPC = total platelet count, TG = triglyceride.
Among the 60 patients with NAFLD, 8 (13.33%) patients had no NASH, 15 (25%) had borderline NASH and 37 (61.67%) patients had definite NASH as shown in Figure 1.
Figure 1.
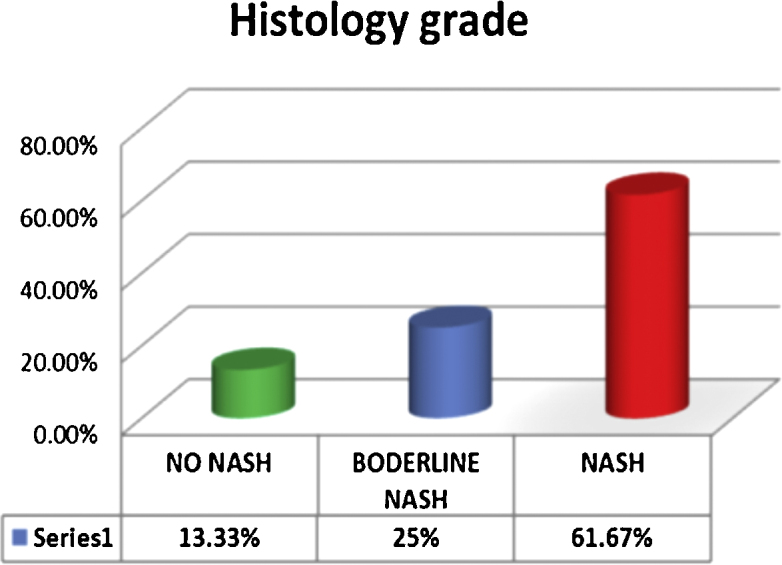
Histological grade of NAFLD.
Liver biopsy (Table 3) revealed macrovesicularsteatosis in all patients; however, in 8 patients microvesicularsteatosis was also seen. Steatosis was predominantly centrilobular. Lobular and portal inflammation was mild in the majority of patients, and it consisted of increased lymphocytes and polymorphonuclear infiltration. Mallory's hyaline was infrequent and seen only in two patients. Fibrosis was seen in 68.3% (41/60), and 32% (19/60) had no fibrosis on histology. Out of 41 patients who had fibrosis. 17, 22, 2 patients had grade 1, 2, 3 fibrosis respectively and no one had grade 4 fibrosis as shown in Table 3.
Table 3.
Histological Profile of 60 NAFLD Patients.
| Parameter | Grade | No (%) |
|---|---|---|
| Steatosis | 0 | 0 |
| 1 | 18 (30) | |
| 2 | 31 (51.67) | |
| 3 | 11 (18.33) | |
| Lobular infiltration | 0 | 14 (23.33) |
| 1 | 23 (38.33) | |
| 2 | 20 (33.33) | |
| 3 | 3 (5) | |
| Ballooning degeneration | 0 | 18 (30) |
| 1 | 28 (46.67) | |
| 2 | 14 (23.33) | |
| Mallory's hyaline | 2 (3.33) | |
| Cirrhosis | 0 |
Table 4 shows the clinical and biochemical data of 60 patients with NAFLD separated in to 2 groups: 41 patients with and 19 patients without fibrosis. Patients with fibrosis had a significantly higher percentage of diabetes (19.51 vs 10.53%; P < 0.05), and higher AST (U/L) level [47 (25–127) vs 35 (26–108); P < 0.05]. The differences between both groups with regards to age, male/female gender ratio, hypertension, and obesity, BMI, ALT, TPC and TG were not statistically significant.
Table 4.
Comparison of Clinical and Biochemical Variables Between NAFLD with (n = 41) and Without Fibrosis (n = 19).
| Variable | No fibrosis (n = 19) | Fibrosis (n = 41) | P value |
|---|---|---|---|
| Age (years) | 39.37 ± 12.2 | 39.9 ± 8.3 | NS |
| Female/male (%) | 15.79/84.21 | 14.63/85.37 | NS |
| Diabetes (%) | 10.53 | 19.51 | <0.05 |
| Hypertension (%) | 42.1 | 43.9 | NS |
| Obesity (%) | 73.68 | 65.85 | NS |
| BMI (kg/m2) | 26.42 ± 3.65 | 26.5 ± 3.2 | NS |
| AST (U/L)a | 35 (26–108) | 47 (25–127) | <0.05 |
| ALT (U/L)a | 52 (23–165) | 68 (26–197) | NS |
| TPC (109/L) | 2.32 ± 0.39 | 2.07 ± 0.42 | NS |
| TG (mg/dl) | 162 (70–274) | 172 (73–525) | NS |
Median (range).
BMI = body mass index, SGOT = serum glutamic oxaloacetic transaminases, SGPT = serum glutamic pyruvate transaminases, TPC = total platelet count, TG = triglyceride.
On histology, only 2 patients had grade 3 fibrosis and no one had grade 4 fibrosis (cirrhosis). Hence, for our study we considered grade 2 and 3 (F ≥ 2) as significant fibrosis, and correlated it with the NAFLD fibrosis score, BARD score, BAAT score and APRI score. In this cohort 24 (40%) patients had significant fibrosis on histology.
Calculation of the NAFLD fibrosis score showed that 51 patients had scores below the lower cut off, one patient had score above the higher cut off, and 8 patients (13.33%) had intermediate values. Among the 51 patients with scores below the lower cut off, while 33 did not have significant fibrosis, 18 had significant fibrosis on liver biopsy. The only patient with score above the higher cut off had significant fibrosis on liver biopsy. Out of 8 patients having intermediate NFS score, 4 had stage 2 fibrosis, 1 had stage 1 and 2 had no fibrosis. Thus, in our cohort of NAFLD patients, the sensitivity and specificity of NAFLD fibrosis score were 5.56 and 100% respectively, and the negative predictive value was 66%. The area under the ROC was 0.47 (95% CI = 0.30–0.64).
Estimation of the BARD score revealed that 42 patients had low scores (0 in 28 patients, 1 point in 14 patients), while 18 patients had high scores (2 points in 10, 3 points in 5 and 4 points in 3 patients). Among the 42 patients with low scores, 30 patients did not have significant fibrosis but 12 had significant fibrosis on liver biopsy. Among the 18 patients with high scores, 11 had significant fibrosis while 7 did not have significant fibrosis on liver biopsy. Thus, in our cohort of NAFLD patients, the sensitivity and specificity of the BARD score were 45.83 and 80.55%, respectively, and the negative and positive predictive values were 61.11 and 69.04% respectively in separating cases with and without significant fibrosis.
Estimation of the BAAT score showed that 30 patients had low scores (0 in 7 patients; 1 point in 23 patients); 30 patients had intermediate scores (2 points in 25; 3 points in 5 patients) while no one had high score of 4. Among the 30 patients with low scores, while 23 did not have significant fibrosis, 7 had significant fibrosis on liver biopsy. Out 30 patients having intermediate BAAT score 15 had stage 2, 7 had no fibrosis, 6 had stage 1 and 2 had stage 3 fibrosis. Thus, in our cohort of NAFLD patients, the sensitivity and specificity of the BAAT scores were 0 and 100% respectively and the negative predictive value was 77% in separating cases with and without significant fibrosis. Calculation of the APRI score showed that 52 patients had scores below the lower cut off and 8 patients (13.33%) had scores above 0.98. Among the 52 patients with score values below the lower cut off, while 35 patients did not have significant fibrosis, 17 had significant fibrosis on liver biopsy. Seven out of eight patients with scores above the higher cut off had significant fibrosis in the liver biopsy. Thus, in our cohort of NAFLD patients, the sensitivity and specificity of APRI score were 29.16 and 97.22% respectively and the negative and positive predictive values were 87.5 and 83.33% respectively in separating patients with and without significant fibrosis. The area under the ROC was 0.36 (95% CI = 0.22–0.51). The comparison between the four non-invasive scores with the histology is shown in Table 5.
Table 5.
Comparison of Non-Invasive Scores with Histological Fibrosis.
| NFS | BARD | BAAT | APRI | |
|---|---|---|---|---|
| Sensitivity | 5.56% | 45.83% | 0 | 29.16% |
| Specificity | 100% | 80.55% | 100% | 97.22% |
| PPV | NA | 61.11% | NA | 87.5% |
| NPV | 66% | 69.04% | 76.66% | 83.33% |
| ROC | 0.47 | NA | NA | 0.36 |
PPV = positive predictive value; NPV = negative predictive value, ROC = receiver operating characteristic.
Discussion
NAFLD has emerged as the commonest liver disease worldwide, and is present in almost one third to one quarter of the general population.4, 7, 19 The universally increasing prevalence of metabolic syndrome, obesity, diabetes, and consequently of NAFLD poses a very important challenge for public health. A recent study from this region showed that most of the patients of NAFLD are asymptomatic and detected incidentally while seeking consultation for other problems.27 In spite of the fact that it is so common, there is little information on the true profile of the average NAFLD patients seen in clinical practice. This is because most studies have been based on selected subsets of patients with metabolic syndrome or with increased liver enzymes for diagnosis of NAFLD.5, 6 This presentation is far away from the real life scenario where most patients of NAFLD are primarily detected incidentally while undergoing imaging studies, especially ultrasonography. In recent years, several noninvasive diagnostic tools, based on liver stiffness measurement (LSM) methods such as FibroScan, acoustic radiation force impulse (ARFI), elastography, and supersonic shear imaging (SSI) have been developed to quantify liver fibrosis through measurement of mechanical or ultrasound shear wave propagation through the hepatic parenchyma. In a recent prospective study, Cassinotto et al.28, confirmed that SSI, FibroScan, and ARFI were valuable diagnostic tools for the staging of liver fibrosis in NAFLD patients. However, these methods have not yet been validated enough to find a place in the guidelines. Most of these tools have been found useful to define the extreme states of little or no fibrosis and advanced fibrosis, but there are difficulties in differentiating between contiguous stages of fibrosis [for example between stage 2 and 3 fibrosis]. In this study, we have taken these four non invasive tests as these tests required only simple laboratory biochemical parameters which can be easily undertaken in a resource constrained setting.
There is conflicting data on the severity of NAFLD among Indians. A study by Madan et al. concluded that NAFLD in North Indian patients is a disease of young over-weight males, most of whom are insulin resistant and they tend to have a mild histological disease at presentation.29 However, the study by Das et al. from rural West Bengal reported that NAFLD was not a mild disease since 4 out of 36 NAFLD patients who underwent liver biopsy in the study had cirrhosis.6 In our study cohort, although 3/5th of the biopsied patients had definite NASH, only 2 had grade 3 fibrosis, and none had cirrhosis. This finding is similar to the study from AIIMS by Madan et al.29in which only 1 out of 51 patients had grade 3 fibrosis and no one had cirrhosis.
In the study by Angulo et al., the NAFLD fibrosis score showed an indeterminate result in only 25% of cases.25 However contrast to that, in our study, the results were quite different. The score was indeterminate in only 13.33% of cases. The sensitivity of the test was rather low (5.56%): among 24 patients with significant fibrosis, the NAFLD fibrosis score was above the higher cut-off in only 1 of them (true positives). On the other hand, the specificity of the test was 100% and the negative predictive value, 66%. Thus we can reliably detect significant fibrosis when the score was higher then cut off value. This finding is similar to the result found by Ruffillo et al.30; in their study, sensitivity was 22.07% and specificity 100%.
In the study by Harrison et al.,22 a low BARD score (0 or 1 point) had a very high negative predictive value (96%) to identify patients without advanced fibrosis, although the positive predictive value of a high score was not so good (43%). When we applied the BARD score in our patients, the results were similar. The test was not very sensitive (45.83%) and had a low PPV (61.11%), but the negative predictive value was 69.04% similar to the findings reported by Ruffillo et al.30 who found that although the BARD score had low sensitivity (51.4%) and low PPV (45.2%), the negative predictive value was 81.3%.
In the study by Ratziu et al.,23 a low BAAT score of 0 or 1 had a NPV of 100% for fibrosis, while a high total score of 4 gave a sensitivity of 14% and a specificity of 100% for the detection of septal fibrosis. When we applied the BAAT scoring system to our cohorts, while sensitivity was found to be 0%, the specificity was 100% and negative predictive value was 77%.
In the study by Kruger et al.,31 AUC for APRI was 0.85 with a cut-off of 0.98, giving a sensitivity of 75% and a specificity of 86% in the advanced fibrosis group. In our study, the sensitivity and specificity of APRI score were 29.16% and 97.22%, respectively, and the negative and positive predictive values were 87.5% and 83.33% respectively in separating cases with and without significant fibrosis. The comparison of different studies with the present study was shown in Table 6.
Table 6.
Comparison of the Findings of This Study with Other Studies.
| Reference of the study | NFS (sen, spe, ppv, npv) (%) | BARD (sen, spe, ppv, npv) (%) | BAAT (sen, spe, ppv, npv) (%) | APRI (sen, spe, ppv, npv) (%) |
|---|---|---|---|---|
| Present Study (India) | 5.56, 100, NA, 66 | 45.83, 80.55, 61.11, 69.04 OR = 3.5 |
0, 100, NA, 76.66 | 29.16, 97.22, 87.5, 83.33 |
| Ruffillo et al.30 (Argentina) | 22.7, 100, 100, 81.3 | 51.4, 77.2, 45.2, 81.3 | NA | NA |
| Angulo et al.25 (Italy) | 77, 71, 52, 88 | NA | NA | NA |
| Harrison et al.22 (USA) | NA | OR = 17, NPV = 96, PPV = 43 | NA | NA |
| Ratziu et al.23 (France) | NA | NA | 14, 100, NA, 100 | NA |
| Kruger et al.31 (South Africa) | 76, 69, 34, 94 | NA | NA | 75, 86, 54, 93 |
sen = sensitivity, spe = specificity, ppv = positive predictive value, npv = negative predictive value.
Limitations
Poor sensitivity could be due to small sample size and even smaller number of patients who had significant fibrosis. Hence, further studies with larger sample size should be carried out. The sensitivity values for all the non-invasive markers used in this study was found to be extremely low compared to the previous studies.22, 23, 25, 30, 31 This could be due to the fact that we used pre-published cut-offs for advanced fibrosis to predict significant fibrosis, and since majority of the patients had F2 fibrosis, they were not picked up by the existing cut-off values resulting in an artificially low sensitivity.
Conclusions
The present study revealed that our subjects with NAFLD were younger, had lower BMI, lower prevalence of diabetes mellitus and lesser necroinflammatory activity score and fibrosis compared to the NAFLD subjects from the West, as reported by Madan et al. from New Delhi earlier.
In our experience, all the four scoring systems (NFS, BARD, BAAT, and APRI) had higher specificity than sensitivity. Therefore, they were useful in identifying patients with significant fibrosis when scores were more than or above the cut off values; in fact, this was also the conclusion from the studies by Harrison et al. and Ruffillo et al. Among the various scoring systems we studied here, APRI is very simple and it can be used to exclude significant liver fibrosis in NAFLD.
From this study we can conclude that all these mathematical models to predict fibrosis could be used to a certain extent to diagnose significant fibrosis in NAFLD patients. However, histopathological evaluation of NAFLD in liver biopsy still remains the gold standard to diagnose fibrosis and inflammation till new reliable mathematical models are formulated.
Conflicts of Interest
The authors have none to declare.
References
- 1.Ratio V., Poniard T. Assessing the outcome of non-alcoholic steatohepatitis? It's time to get serious. Hepatology. 2006;44:802–805. doi: 10.1002/hep.21391. [DOI] [PubMed] [Google Scholar]
- 2.Chitturi S., Farrell G.C., Hashimoto E., Saibara T., Lau G.K., Sollano J.D. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. Asia-Pacific Working Party on NAFLD. J Gastroenterol Hepatol. 2007;22:778. doi: 10.1111/j.1440-1746.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G., Baranova A., Younossi Z.M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Singh S.P., Nayak S., Swain M. Prevalence of non-alcoholic fatty liver disease in coastal eastern India: a preliminary ultrasonographic survey. Trop Gastroenterol. 2004;25:76–79. [PubMed] [Google Scholar]
- 5.Amarapurkar D., Kamani P., Patel N. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6(3):161–163. [PubMed] [Google Scholar]
- 6.Das K., Das K., Chowdhury A. Nonobese population in a developing country has a high prevalence of non-alcoholic fatty liver and significant liver disease. Hepatology. 2010;51(5):1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 7.Mohan V., Farooq S., Deepa M., Ravikumar R., Pitchumoni C.S. Prevalence of non-alcoholic fatty liver disease in south Indians in relations to different grades of glucose intolerance and metabolic syndrome. Diabet Res Clin Pract. 2009;84:84–91. doi: 10.1016/j.diabres.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 8.Adams L.A., Lymp J.F., St. Sauver J. The natural history of non-alcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Bugianesi E., Leone N., Vanni E. Expanding the natural history of non-alcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell S.H., Crespo D. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40:578–584. doi: 10.1016/j.jhep.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Ratziu V., Bonyhay L., Di Martino V. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35(6):1485–1493. doi: 10.1053/jhep.2002.33324. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal A.J., American Gastroenterological Association AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123(5):1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 13.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 14.Ratziu V., Giral P., Charlotte F. Liver fibrosis in overweight patients. Gastroenterology. 2000;118(6):1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 15.Sorbi D., Boynton J., Lindor K.D. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94(4):1018–1022. doi: 10.1111/j.1572-0241.1999.01006.x. [DOI] [PubMed] [Google Scholar]
- 16.McGill D.B., Rakela J., Zinsmeister A.R., Ott B.J. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99(5):1396–1400. doi: 10.1016/0016-5085(90)91167-5. [DOI] [PubMed] [Google Scholar]
- 17.Knawy A.L.B., Shiffman M. Percutaneous liver biopsy in clinical practice. Liver Int. 2007;27:1166–1173. doi: 10.1111/j.1478-3231.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 18.Ratziu V., Charlotte F., Heurtier A. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1889–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 19.Singh S.P., Panda C.R., Swain M. A study of the prevalence of fatty liver disease (NAFLD) in fatal traffic casualties in coastal Orissa. J Gastroenterol Hepatol. 2004;(19 suppl):A838. [Google Scholar]
- 20.Wai C.T., Greenson J.K., Fontana R.J. A simple non-invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 21.Williams A.L., Hoofnagle J.H. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95:734–739. doi: 10.1016/s0016-5085(88)80022-2. [DOI] [PubMed] [Google Scholar]
- 22.Harrison S.A., Oliver D., Arnold H.L. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 23.Ratziu V., Giral P., Charlotte F. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 24.Vallet-Pichard A., Mallet V., Nalpas B. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 25.Angulo P., Hui J., Marchesini G. The NAFLD fibrosis score: a non invasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:847–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 26.Kleiner D.E., Brunt E.M., Van Natta M. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 27.Sigh S.P., Kar S.K., Panigrahi M.K. Profile of patients with incidentally detected non alcoholic fatty liver disease (IDNAFLD) in coastal eastern India. Trop Gastroenterol. 2013;34(3):144–152. doi: 10.7869/tg.118. [DOI] [PubMed] [Google Scholar]
- 28.Cassinotto C., Boursier J., Ledinghen V.D. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817–1827. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 29.Madan K., Batra Y., Gupta S.D. Nonalcoholic fatty liver disease may not be a severe disease at Presentation among Asian Indians. World J Gastroenterol. 2006;12:3400–3405. doi: 10.3748/wjg.v12.i21.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruffillo G., Fassio E., Alvarez E. Comparison of NAFLD fibrosis score and BARD score in predicting fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54(1):160–163. doi: 10.1016/j.jhep.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Kruger F.C., Daniels C.R., Kidd M. A simple bedside marker for advance fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J. 2011;101:477–480. [PubMed] [Google Scholar]


