Abstract
Objectives
Maddrey discriminant function (MDF) score is a measure of disease prognosis in alcoholic hepatitis (AH) used to identify patients at highest risk of mortality and determine the need for initiation of pharmacologic treatment. The purpose of this study was to evaluate the effects of pharmacologic therapy for hospitalized AH patients as stratified by MDF score.
Methods
A retrospective review of patients with an AH diagnosis admitted to a Methodist LeBonheur Healthcare adult hospital between 06/2009 and 06/2014 was conducted. Patients ≥18 years of age with an ICD-9 code for AH were evaluated.
Results
Of the 493 patients screened, 234 met the inclusion criteria, comprised of 62 patients with an MDF ≥ 32 (treatment, n = 42 vs. no treatment, n = 20) and 172 patients with an MDF < 32 (treatment, n = 15 vs. no treatment, n = 157). For the patients with an MDF ≥ 32, there was no statistically significant difference between the treatment group vs. non-treatment group regarding 28-day mortality (31% vs. 11%, respectively; P = 0.18) and 6-month mortality (45% treatment vs. 38% non-treatment; P = 0.75). For the patients with an MDF <32, there was no statistically significant difference between the treatment group vs. non-treatment group regarding 28-day mortality (0% vs. 7%, respectively; P > 0.99) and 6-month mortality (11% treatment vs. 13% non-treatment; P > 0.99). There was no difference in incidence of acute kidney injury, hepatorenal syndrome, development of infection or hepatic encephalopathy between the treatment vs. non-treatment groups.
Conclusions
Pharmacologic treatment showed no survival benefit, regardless of disease severity. Given the mortality risk seen in mild–moderate AH patients not receiving treatment and concern for a possible treatment ceiling effect in severe AH patients, more data are needed to adequately assess the utility of MDF in selecting appropriate candidates for AH treatment.
Abbreviations: AASLD, American Association for the Study of Liver Diseases; AH, alcoholic hepatitis; AKI, acute kidney injury; ALD, alcoholic liver disease; ARR, absolute risk reduction; eGFR, estimated glomerular filtration rate; GI, gastrointestinal; HRS, hepatorenal syndrome; INR, international normalized ratio; MDF, Maddrey discriminant function; MELD, Model for End-Stage Liver Disease; PT, prothrombin time; SCr, serum creatinine; SD, standard deviation
Keywords: Maddrey discriminant function, pentoxifylline, prednisolone
Alcoholic liver disease (ALD) is a form of liver injury resulting directly from alcohol consumption with injury manifesting as reversible fatty liver to alcoholic hepatitis (AH) or cirrhosis.1 ALD is associated with increased healthcare costs resulting from multiple hospital readmissions, and has been identified as the second most leading indication for liver transplantation.1 ALD encompasses AH that occurs as a result of prolonged alcohol consumption and ranges in severity from asymptomatic to liver failure.2 AH is associated with a high mortality burden, up to 15% at 30 days, which is dependent on disease severity at presentation.1 Per American Association for the Study of Liver Diseases (AASLD) guidelines, disease severity should be initially established upon presentation in all AH patients to aid in therapeutic management.3 One measure of disease severity and prognosis specific to AH patients is the Maddrey discriminant function (MDF) score, which utilizes the patient's prothrombin time (PT) and total bilirubin to predict short-term mortality. An MDF score greater than or equal to 32 indicates severe disease with a 1-month mortality rate up to 30–50%.3 Patients with severe disease and concurrent hepatic encephalopathy appear to be at the highest risk of death.3 The MDF score can be utilized in clinical practice for early identification of patients at highest risk of mortality and initiation of pharmacological therapy to prevent further liver damage and development of complications.3, 4
Corticosteroids have been most commonly studied in AH patients and are regarded as the treatment of choice. However, the results from small placebo-controlled trials have varied from reduction in short-term mortality to no effect.3 The most recent meta-analysis data provide evidence that corticosteroid treatment is primarily beneficial in severe AH patients.5, 6 While a 28-day duration of prednisolone 40 mg daily is regarded as the gold standard of therapy, it may not be ideal in patients with an active gastrointestinal (GI) bleed or infection due to risk of further exacerbation of these conditions. One alternative to corticosteroid therapy that has been studied is an oral phosphodiesterase inhibitor, pentoxifylline. This therapy has been shown to reduce 28-day (absolute risk reduction (ARR): 21.6%) as well as 3-month mortality (ARR: 20.6%), with its beneficial effects mostly attributed to the reduction of hepatorenal syndrome (HRS).7, 8 Given the potential benefits of pentoxifylline, it was postulated that combination therapy with corticosteroids may lead to improvement in outcomes surpassing what is recognized with steroids alone. However, two small studies have concluded that combination therapy does not improve 4-week or 6-month survival.9, 10
Although limited and conflicting evidence is available in this patient population, current AASLD practice guidelines recommend pharmacologic therapy in AH patients with an MDF score ≥32 with prednisolone or utilizing pentoxifylline as an alternative agent to improve survival. In contrast, pharmacologic therapy is not recommended for less severe patients (MDF <32), specifically those without hepatic encephalopathy, as these patients are unlikely to benefit from treatment.3 Therefore, the purpose of this study was to evaluate the effects of pharmacologic therapy in hospitalized AH patients, examining outcomes in both mild-to-moderate and severe AH patients as determined via MDF score.
Methods
A retrospective review of patients admitted to adult hospitals within the Methodist LeBonheur Healthcare System between June 2009 and June 2014 with a diagnosis of AH was conducted. Patients were identified through the corporate patient financial services database using ICD-9-CM code 571.1 for AH. Patients were included if they were at least 18 years of age and had a diagnosis of AH. The diagnosis was further confirmed by chart review for physician documentation of AH. Exclusion criteria consisted of other potential liver injury etiologies, reported current use of steroid or pentoxifylline prior to hospitalization (verified through home medication reconciliation), lack of complete data to calculate an MDF score (lack of total bilirubin or PT labs), history of liver transplantation, received N-acetylcysteine for AH, or experienced death within 24 h of hospital admission.
Patient demographics, as well as pertinent baseline laboratory findings were collected upon admission. Laboratory data included serum albumin, serum creatinine (SCr), estimated glomerular filtration rate (eGFR) via MDRD equation, international normalized ratio (INR), PT, and total bilirubin. Utilizing these data, an MDF score and a Model for End-Stage Liver Disease (MELD) score were calculated to assess disease severity. MDF score was calculated using the following equation: 4.6 (patient's prothrombin time – control prothrombin time) + total bilirubin, with a control prothrombin time of 14.5 based on the upper limit of normal for the lab assay utilized. Patients with a score ≥32 were defined as severe AH, while those with a score <32 were classified as mild-to-moderate disease. Clinical data including date of initiation and duration of inpatient AH treatment, preexisting or in-hospital development of AH complications, and mortality were also collected. AH treatment was defined as pharmacological treatment with prednisolone, prednisone, and/or pentoxifylline for more than 24 h as documented in the medication administration record. AH complications evaluated in this study included HRS or acute kidney injury (AKI), infection, GI bleed, and hepatic encephalopathy, which were all based on physician documentation of occurrence in the patient's medical record during index hospitalization 24 h after treatment was initiated. Mortality data were determined by subsequent readmissions and/or death within our hospital system or by follow-up visits in our outpatient hepatology clinic.
Patients were initially divided via MDF score into those with an MDF score <32 and those with an MDF score ≥32 to assess outcomes based on disease severity. These groups were further divided into treatment or non-treatment groups. The primary outcome of this study was to describe the 28-day and 6-month mortalities in patients who received AH treatment vs. no treatment based on disease severity, as stratified by their MDF score. Secondary outcomes included assessment of overall mortality and the incidence of AH complication development in the treatment vs. non-treatment groups, along with mortality outcomes in patients who received monotherapy with corticosteroid vs. combination therapy (corticosteroid plus pentoxifylline).
Categorical patient characteristics were compared using a chi-square test or Fisher's exact test. Continuous data were analyzed using a Student t test and are expressed as the mean ± standard deviation (SD). Incidence of 28-day and 6-month mortalities, as well as AH complication development were all identified and compared between the treatment and non-treatment groups utilizing a chi-square test. Data were analyzed using SPSS Statistics for Windows, version 21 (IBM Corporation, Armonk, NY). P values less than 0.05 was considered statistically significant. The study was approved by the University of Tennessee Health Science Center Institutional Review Board and in accord with the Helsinki Declaration of 1975. The study did not receive any financial grants or support from outside sources.
Results
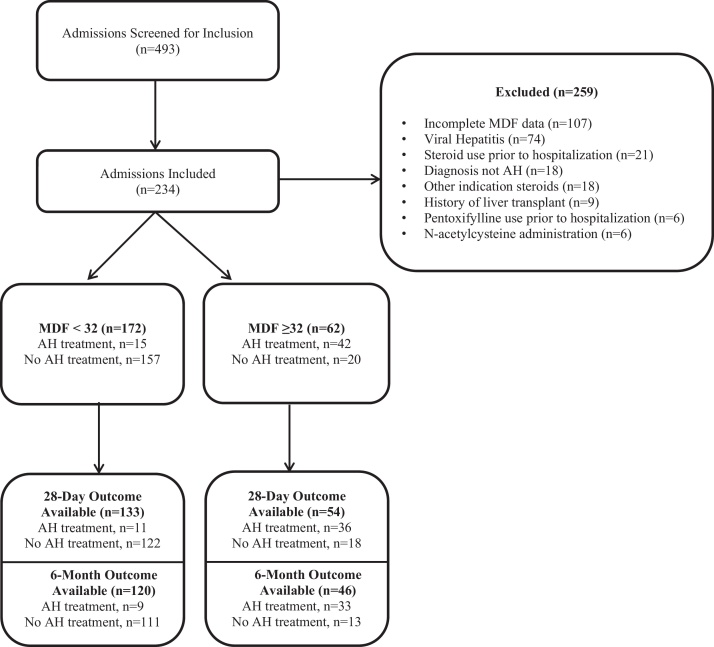
Overall, 493 admissions were screened with 234 meeting inclusion criteria. Of these 234 admissions, 62 patients had an MDF ≥ 32 and 172 patients had an MDF < 32. Of those with an MDF ≥ 32, 42 patients received treatment, while only 15 patients with an MDF < 32 received treatment (Figure 1). Baseline demographics are shown in Table 1. PT, INR, and total bilirubin were all significantly higher in patients with an MDF ≥ 32, yielding higher MDF and MELD scores compared to patients with an MDF < 32 (P < 0.05). Overall, the majority of the treatment group was composed of patients with an MDF ≥ 32 (73.7%), whereas the no treatment group was mainly comprised of patients with an MDF < 32 (88.7%). Average treatment duration with steroid therapy was 6.5 ± 5.9 inpatient days compared to 9.6 ± 9.4 inpatient days with pentoxifylline. Excluding patients who experienced inpatient mortality, 88% of patients originally initiated on a steroid and 100% of patients originally initiated on pentoxifylline and continued therapy upon discharge for an anticipated 28-day course of therapy.
Figure 1.
Patient selection.
Table 1.
Baseline Characteristics.a
| Characteristicsa | MDF < 32 (n = 172) | MDF ≥ 32 (n = 62) | P-value |
|---|---|---|---|
| Age, years | 51 ± 10 | 48 ± 9 | <0.05 |
| Female, n (%) | 52 (22.8) | 53 (29.9) | <0.05 |
| African American, n (%) | 68 (39.5) | 17 (27.4) | 0.09 |
| Albumin (g/dL) | 3.2 ± 0.8 | 2.2 ± 0.5 | <0.05 |
| SCr (mg/dL) | 1.1 ± 0.77 | 1.7 ± 2.0 | <0.05 |
| PT | 15.5 ± 2.1 | 24.5 ± 11.0 | <0.05 |
| INR | 1.2 ± 0.2 | 2.3 ± 1.7 | <0.05 |
| Total bilirubin (mg/dL) | 4.1 ± 4.4 | 16.2 ± 9.3 | <0.05 |
| MELD | 13.9 ± 5.0 | 27.7 ± 7.2 | <0.05 |
| MDF | 10.3 ± 9.5 | 57.2 ± 28.5 | <0.05 |
| Treatment, n (%) | 15 (8.7) | 42 (67.7) | <0.05 |
All data presented as mean ± standard deviation unless otherwise specified.
Patients with a 28-day or 6-month outcome available are listed in Figure 1. Of the patients with an MDF ≥ 32 and a mortality outcome available, there was no significant difference in 28-day mortality between the treatment group vs. no treatment groups (31% vs. 11%, respectively; P = 0.18) (Table 2). There was also no significant difference in 6-month mortality (45% treatment vs. 38% no treatment; P = 0.75). Similarly, patients with an MDF < 32 had no significant difference in 28-day mortality between the treatment group vs. no treatment group (0% vs. 7%, respectively; P > 0.99) and no significant difference in 6-month mortality (11% treatment vs. 13% no treatment; P > 0.99).
Table 2.
Mortality Outcomes.a
| Treatment, n (%) | No treatment, n (%) | P-value | |
|---|---|---|---|
| 28-Day mortality | |||
| MDF ≥32, (n = 54) | 11 (31) | 2 (11) | 0.18 |
| MDF <32 (n = 133) | 0 (0) | 9 (7) | 1.00 |
| 6-Month mortality | |||
| MDF ≥32 (n = 46) | 15 (45) | 5 (38) | 0.75 |
| MDF <32 (n = 120) | 1 (11) | 14 (13) | 1.00 |
n = total number of patients with a 28-day & 6-month mortality endpoint respectively.
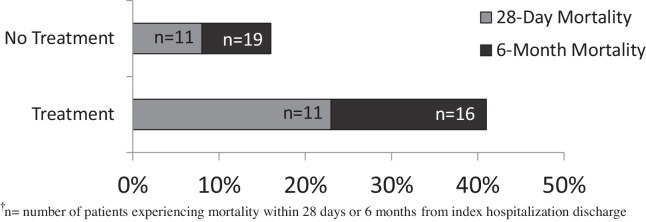
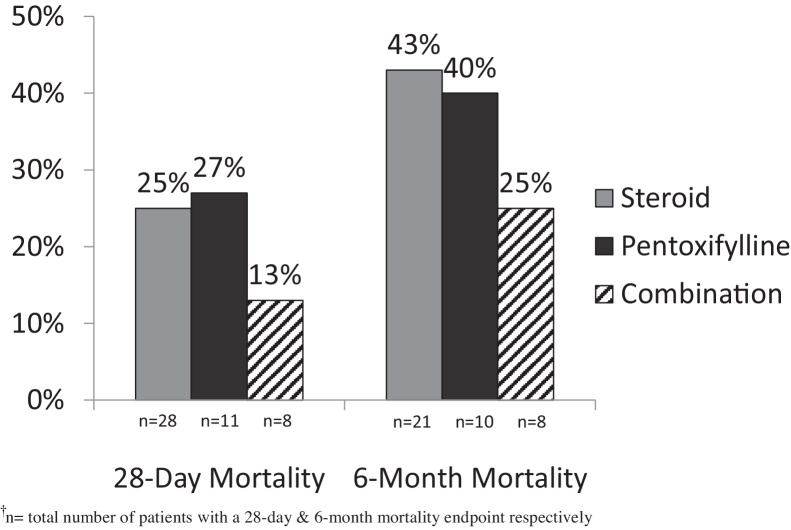
When assessing overall mortality (Figure 2), there was a significantly higher rate of death at 28 days in the treatment group vs. no treatment group (23% vs. 8%, respectively; P < 0.05). The cause of death at 28 days was contributed by worsening liver failure in 15 patients, while sepsis or an infection was cited as the cause in 7 patients, 3 of who received steroid treatment. Examining 6-month mortality, rates were also significantly higher in the treatment group vs. no treatment group (41% vs. 16%, respectively; P < 0.01). Further evaluating outcomes by treatment agent (Figure 3), combination therapy decreased rates of 28-day mortality (25% corticosteroid monotherapy vs. 13% combination therapy; P = 0.65) and 6-month mortality (43% corticosteroid monotherapy vs. 25% combination therapy; P = 0.67); however, these findings were not statistically significant.
Figure 2.
Overall 28-day and 6-month mortality.†
Figure 3.
28-Day and 6-month mortality by treatment agent.†
In terms of new-onset complications (Table 3), a higher incidence of AKI was observed in the treatment group vs. no treatment group (16% vs. 6%, respectively; P < 0.05). Higher incidences of HRS development were seen as well (14% treatment vs. 2% no treatment; P < 0.01). Rates of infection were also higher in patients who received treatment (19% treatment vs. 6% no treatment; P < 0.01). Of the 7 patients who experienced mortality at 28 days due to sepsis or an infection, 3 patients received previous treatment with steroids. However, when patients were stratified by MDF score, no differences in AKI, HRS development, or infection were observed between treatment and no treatment groups. The only GI bleed reported in the study occurred within the no treatment group.
Table 3.
AH Complication Incidence.
| Event | Treatment (n = 57) | No Treatment (n = 177) | P-value |
|---|---|---|---|
| Acute kidney injury, n (%) | 9 (15.8) | 11 (6.2) | P = 0.03 |
| Hepatorenal syndrome, n (%) | 8 (14.0) | 4 (2.3) | P = 0.002 |
| Hepatic encephalopathy, n (%) | 4 (7.0) | 16 (9.0) | P = 0.79 |
| Infection, n (%) | 11 (19.3) | 10 (5.6) | P = 0.005 |
Discussion
Our study showed that regardless of MDF score, patients receiving pharmacologic therapy did not yield a short-term or long-term survival benefit. While meta-analysis of several older, small trials of steroid therapy have shown short-term mortality benefit, its utilization remains controversial due to concern for infection development and the emergence of new literature challenging previous findings.5, 6, 11 Along the same accord, a recent meta-analysis of pentoxifylline demonstrated a decreased incidence of fatal HRS but no short-term survival benefit compared to placebo.12 Lack of survival benefit was most recently described by Thursz and colleagues in the STOPAH trial, which evaluated the effects of prednisolone and pentoxifylline therapy in 1103 severe AH patients with higher average MDF scores in the treatment group compared to our study (MDF = 63 in STOPAH vs. MDF = 42 in our study). The STOPAH investigators found that while prednisolone was associated with a non-significant reduction in mortality, neither steroid (14% treatment vs. 18% non-treatment, P = 0.06) nor pentoxifylline (16% treatment vs. 16% non-treatment, P = 0.69) were effective in reducing 28-day mortality.13 Long-term survival was unaffected by treatment as no differences in mortality between treatment groups were noted at 90-day or 1-year follow-up.13 While not without its limitations, such as lack of liver biopsy diagnosis confirmation and appreciably lower mortality rates than previous trials, STOPAH is the largest, most comprehensive piece of AH literature currently available. Our findings align with the results of the STOPAH trial that prednisolone and/or pentoxifylline pharmacotherapy is not associated with a significant survival benefit in severe patients, a finding that may simply speak to the rapidly progressive nature of the disease itself.
When comparing the treatment vs. no treatment groups in our study, patients who received treatment had a higher mortality rate than those who did not receive treatment. This may have been driven by the higher disease severity of the treatment group, as it was mainly comprised of patients with an MDF ≥ 32. One hypothesis that has been proposed to explain higher mortality rates in patients with more severe disease is a ceiling effect of drug therapy in preventing the inflammatory cascade and ultimate liver damage.2, 5 Mendenhall and colleagues first described this phenomenon after observing that steroid treatment increased mortality in patients with an MDF > 54.5 This cutoff should be further studied to determine if a true MDF range of treatment benefit exists before reaching a treatment ceiling. Treating all patients with an MDF ≥ 32 could inappropriately place the most severe patients at increased risk of complications such as infection or GI bleed, and further increase their mortality risk. While our study found an increased incidence of infection in the treatment group, we saw no difference in GI bleed incidence, which occurred at a low frequency.
Due to concerns regarding the high mortality rates observed in the severe AH population and the application of PT, a non-standardized laboratory value, in the MDF equation, alternative stratification methods have been explored.14 One proposed alternative method for stratification of AH patients is the MELD score. MELD has the advantage of including serum creatinine in its calculation, an elevation of which in the setting of AKI or HRS has been associated with poor outcomes in AH patients.14 Goyal and colleagues compared these two stratification methods in a small population and found that MELD score is as effective as MDF in predicting 30-day mortality and found that MELD greater than 14 at admission and greater than 12 at day 7 were strongly correlated with short-term mortality.15 Dunn and colleagues found that MELD was also comparable to MDF in predicting 30-day and 90-day mortalities, with MELD being the only independent predictor of 90-day mortality.14 However, the authors concluded that AH patients with a MELD greater than 21 should be considered for pharmacologic therapy, as this score maintained a greater sensitivity and specificity at predicting 90-day mortality.14 Current AASLD guidelines indicate a poor prognosis in AH patients with a MELD ≥ 18 and propose a treatment algorithm utilizing this score as an alternative to MDF ≥ 32 when establishing disease severity, but make no specific treatment recommendations utilizing this score.3, 16 Although promising data are starting to emerge utilizing MELD as an alternative method to stratify AH patients for treatment, the majority of the small studies find it comparable to MDF.16, 17, 18 When stratifying patients in our study by MELD score, we found no difference in mortality outcomes when utilizing a cutoff of 18. Further studies are needed to assess the true utility of the MELD score in AH patients and its ability to yield a greater survival benefit when compared to MDF.
Following current guideline recommendations, most trials, including both the STOPAH and COPE trial, exclude mild-to-moderate patients as these patients typically display lower mortality and complication rates. However, the AASLD guidelines do not explicitly recommend avoiding treatment in this patient population, only stating that they will likely not require or benefit from medical intervention.3 Based on clinical presentation at hospital admission or clinical developments during index hospitalization, a practitioner may choose to exercise clinical judgment and treat certain mild-moderate patients. Therefore, we sought to determine if patients with an MDF <32 experienced benefit from pharmacologic treatment. Ultimately, we found no benefit from pharmacologic therapy, confirming guideline recommendations for this subset of patients. However, it is concerning that 7% of patients with an MDF <32 experienced 28-day mortality without treatment. It is important to note that only 1 of these patients had concurrent hepatic encephalopathy, as this AH complication has been associated with poor prognosis regardless of MDF score.3 These results further highlight a limitation of the current MDF score cutoff failing to capture certain mild-moderate patients who may have otherwise benefited from early identification and treatment. These findings need to be confirmed in larger, randomized controlled trials assessing outcomes in this less severe population.
There are several limitations to this study that should be considered when interpreting the results. First, this was a retrospective study that relied on ICD-9 code for diagnosis of AH. However, we did further confirm the diagnosis based on physician documentation of AH. Despite our methods, clinical judgment still could have classified some patients as AH inaccurately, as biopsy results were not frequently available to confirm the diagnosis in the acute setting. However, it is important to note that AASLD guidelines do not require a biopsy to confirm AH diagnosis, particularly not in those who are acutely ill.3, 11 While our sample size may be considered an adequate patient population, only a small number of our patients received treatment for AH, especially those with an MDF < 32. Due to short hospitalization stays and inpatient treatment durations, we were unable to assess Lille score on day 7 in those patients receiving steroid therapy to assess early response. Furthermore, mortality data could only be abstracted based on hospital readmissions and clinic visits within our health system, which limited our sample size for mortality outcomes. Therefore, patients without a 28-day or 6-month mortality outcome available were not included in our mortality assessment, which could potentially underestimate the true mortality data. Lastly, we were unable to control for recidivism or verify outpatient compliance for discharged patients, which may have influenced mortality results in those patients who were noncompliant and/or failed to abstain from alcohol.
Conclusion
Regardless of disease severity, prednisolone and/or pentoxifylline pharmacologic treatment failed to yield a short-term or long-term mortality benefit in hospitalized AH patients in this retrospective analysis. Higher mortality and complication rates in patients who received treatment could be attributed to the severity of the disease in this group or the presence of a treatment ceiling effect. Therefore, caution should be exercised when viewing prednisolone or pentoxifylline therapy as a bridge to future procedures or liver transplant due to the rapid progression of disease. Given the current state of inconclusive and to some extent contradictory study results, future research should focus not only on identifying novel pharmacologic treatment modalities, but assessing the utility of MDF outside the current 32 cutoff to determine if a specific MDF range exists that yields a true survival advantage in AH patients. Alternative assessment tools, such as MELD score, should also be explored in further detail to determine if utilizing a different severity scoring system would afford any advantage.
Conflicts of Interest
The authors have none to declare.
References
- 1.Basra S., Bhupinderjit S.A. Definition epidemiology and magnitude of alcoholic hepatitis. World J Hepatol. 2011;3(5):108–113. doi: 10.4254/wjh.v3.i5.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fairbanks K.D. Cleveland Clinic; 2015, November. Alcoholic Liver Disease. Available from: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hepatology/alcoholic-liver-disease/ Accessed 05.11.15. [Google Scholar]
- 3.O'Shea R.S., Dasarathy S., McCullough A.J. Alcoholic liver disease. Hepatology. 2010;51(January (1)):307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 4.Mendenhall C., Roselle G.A., Gartside P., Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcohol Clin Exp Res. 1995;19(3):635–641. doi: 10.1111/j.1530-0277.1995.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 5.Rambaldi A., Saconato H.H., Christensen E., Thorlund K., Wettersley J., Gluud C. Systematic review: glucocorticoids for alcoholic hepatitis-a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. Aliment Pharmacol Ther. 2008;27(June (12)):1167–1178. doi: 10.1111/j.1365-2036.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- 6.Mathurin P., Mendenhall C.L., Carithers R.L. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36(4):480–487. doi: 10.1016/s0168-8278(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 7.Akriviadis E., Botla R., Briggs W., Han S., Reynolds T., Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119(6):1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 8.De B.K., Gangopadhyay S., Dutta D., Baksi S.D., Pani A., Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15(13):1613–1619. doi: 10.3748/wjg.15.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathurin P., Louvet A., Duhamel A. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. JAMA. 2013;310(10):1033–1041. doi: 10.1001/jama.2013.276300. [DOI] [PubMed] [Google Scholar]
- 10.Sidhu S.S., Goyal O., Singla P. Corticosteroid plus pentoxifylline is not better than corticosteroid alone for improving survival in severe alcoholic hepatitis (COPE trial) Dig Dis Sci. 2012;57(6):1664–1671. doi: 10.1007/s10620-012-2097-4. [DOI] [PubMed] [Google Scholar]
- 11.Sohali U., Satapathy S.K. Diagnosis and management of alcoholic hepatitis. Clin Liver Dis. 2012;16(4):717–736. doi: 10.1016/j.cld.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Parker R., Armstrong M.J., Corbett C., Rowe I.A., Houlihan D.D. Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Ailment Pharmacol Ther. 2013;37:845–854. doi: 10.1111/apt.12279. [DOI] [PubMed] [Google Scholar]
- 13.Thursz M.R., Richardson P., Allison M. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2014;372(17):1619–1628. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 14.Dunn W., Jamil L.H., Brown L.S. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41(2):353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 15.Goyal S.K., Dixit V.K., Jain A.K. Assessment of the model for end-stage liver disease (MELD) score in predicting prognosis of patients with alcoholic hepatitis. J Clin Exp Hepatol. 2014;4(1):19–24. doi: 10.1016/j.jceh.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soultati A.S., Dourakis S.P., Alexopoulou A., Deutsch M., Vasilieva L., Archimandritis A.J. Predicting utility of a model for end stage liver disease in alcoholic liver disease. World J Gastroenterol. 2006;12(25):4020–4025. doi: 10.3748/wjg.v12.i25.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadian M., Kakkar R., Dhar M., Kaushik R.M. Model for end-stage liver disease score versus Maddrey discriminant function score in assessing short-term outcome in alcoholic hepatitis. J Gastroenterol Hepatol. 2014;29(3):581–588. doi: 10.1111/jgh.12400. [DOI] [PubMed] [Google Scholar]
- 18.Goyal S.K., Dixit V.K., Jain A.K., Mohapatra P.K., Ghosh J.K. Assessment of the model for end-stage liver disease (MELD) score in predicting prognosis of patients with alcoholic hepatitis. J Clin Exp Hepatol. 2014;4(1):19–24. doi: 10.1016/j.jceh.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]