ABSTRACT
As sessile organisms, plants are continuously exposed to various environmental stresses. In contrast to the controlled conditions employed in many researches, more than one or more abiotic and/or biotic stresses simultaneously occur and highly impact growth of plants and crops in the field environments. Therefore, an urgent need to generate crops with enhanced tolerance to stress combinations exists. Researchers, however, focused on the mechanisms underlying acclimation of plants to combined stresses only in recent studies. Plant hormones might be a key regulator of the tailored responses of plants to different stress combinations. Co-ordination between different hormone signaling, or hormone signaling and other pathways such as ROS regulatory mechanisms could be flexible, being altered by timing and types of stresses, and could be different depending on plant species under the stress combinations. In this review, update on recent studies focusing on complex-mode of hormone signaling under stress combinations will be provided.
KEYWORDS: Abscisic acid (ABA), plant hormones, signaling pathways, stress combination, tailored response
Abbreviations
- ABA
abscisic acid
- APX1
ascorbate peroxidase 1
- AUX
auxin
- BR
brassinosteroid
- ET
ethylene
- GSH
glutathione
- JA
jasmonic acid
- JAZ
Jasmonate-zim-domain protein
- MBF1c
multiprotein bridging factor 1c
- NCED
9-cis epoxycarotenoid dioxygenase
- NO
nitric oxide
- ROS
reactive oxygen species
- SA
salicylic acid
As sessile organisms, plants are continuously exposed to various environmental stresses. The major abiotic stresses that impact growth of plants and crops in the field have been extensively studied.1-4 However, the field environment in nature is very different from the controlled conditions employed in many laboratory researches, and often involves the simultaneous exposure of plants to more than one abiotic and/or biotic stresses.5 In addition, current climate prediction models indicate that high temperature will be accompanied by other weather disasters and more detrimental effects on crop production can be expected in future.4,6-9 Therefore, an urgent need to generate crops with enhanced tolerance to stress combinations exists. Researchers, however, focused on the molecular and physiological mechanisms underlying acclimation of plants to stress combinations only in recent studies.5,10
Recent transcriptome and proteome analyses suggested that unique signaling pathways might be tailored in plants in response to different stress combinations.11-13 Unique acclimatory responses of plants to stress combinations cannot be directly deduced from that to each of the different stress applied individually.5,10 Plant hormones could be a key player in the regulation of such tailored responses of plants to stress combinations, because hormone signaling can be flexibly modulated depending on the types of environmental stresses.14-16 In addition, different types of stresses that oppositely affect the hormone synthesis and signaling can simultaneously occur in nature. Thus, precise regulation of hormone synthesis and signaling should be required for the acclimation of plants to various combinations of stresses. In this review, the complex mode of hormone signaling pathways under different combinations of more than one abiotic and/or biotic stresses will be addressed.
Abscisic acid (ABA) has long been known to play integral role in response of plants to abiotic stresses.17 For example, ABA functions as a key regulator of stomatal closure to prevent excess water loss through transpiration under water deficiency and salt stress.18 In addition, ABA also activates signaling pathways involving LATE EMBRYOGENESIS-ABUNDANT class genes and other regulatory genes required for acclimation of plants to abiotic stresses.19 Recent studies suggested that ABA might be involved in tailored response of plants to the drought and heat stress combination as well as drought or heat stress applied individually. For example, accumulation of 9-cis epoxycarotenoid dioxygenase (NCED) protein that is required for ABA synthesis was shown to be gradually up-regulated in poplar in response to drought or heat stress alone.20 Under the drought and heat stress combination, in contrast, NCED protein accumulation initially increased, then declined. These results indicate the different regulatory functions of ABA under these single and combined stresses. Moreover, a recent study demonstrated that Arabidopsis mutants deficient in ABA synthesis (aba1-1) or response (abi1-1) were impaired in their acclimation to the drought and heat stress combination.15 Sensitivity of these mutants to this stress combination might not be due to deficiency in the regulatory mechanisms of stomatal closure. Although stomata of abi1-1 mutant significantly more opened compared to WT plants under drought, its stomatal aperture was reduced to the similar level with WT plants when subjected to the drought and heat stress combination. These results suggest that other signaling pathways, rather than ABA signaling, could play important role in the regulation of stomatal movement under the drought and heat stress combination. Indeed, accumulation of ROS and jasmonic acid (JA) that might be also involved in stomatal closure21 was higher in abi1-1 mutant compared to WT under this stress combination. Furthermore, transcriptional responses to drought, heat and their combination were compared between 2 different wheat cultivars that are tolerant or sensitive to the stress combination.22 In the sensitive cultivar, expression of transcripts encoding phospholipase D and phosphatidyl inositol kinase, key regulators of ABA-dependent stomatal closure,23,24 were up-regulated by all stresses with the highest level under the stress combination. In the tolerant cultivar, in contrast, the highest expression of these transcripts was detected under heat stress, not under the stress combination. Although ABA-dependent stomatal closure does not seem be significant in tailored response of plants to the drought and heat stress combination, ABA was implicated in the regulation of ROS scavenging systems as well as heat response pathways under this stress combination. abi1-1 mutant demonstrated lower accumulation of ascorbate peroxidase 1 (APX1) protein,25 a cytosolic ROS scavenging enzyme, as well as multiprotein bridging factor 1c (MBF1c) protein,26 a master regulator of heat response, compared to WT plants under the drought and heat stress combination.15 These proteins were shown to be required for the acclimation of plants to combinations of water deficit and heat stress.27,28
Recent studies indicated complex mode of co-ordination between different hormone signaling in response of Arabidopsis and other crops to drought, heat and their combination. In citrus, ABA highly accumulated in response to drought applied individually.16 The drought and heat stress combination also induced increase in ABA accumulation, but much lower extent compared to drought alone. In contrast to ABA, higher level of salicylic acid (SA) that might be involved in the signaling pathway antagonizing ABA29 accumulated under the stress combination compared to drought or heat stress applied individually. This pattern of ABA and SA accumulation under these single and combined stresses in citrus was different from that in Arabidopsis which showed the highest or lowest level of ABA or SA accumulation, respectively under the drought and heat stress combination.16 JA whose signaling pathway could be antagonized by SA30 was also highly accumulated in Arabidopsis under the drought and heat combination as well as heat stress alone. In addition, expression of transcript homologous to Arabidopsis Rap2.6L was significantly up-regulated in wheat in response to drought or heat stress applied individually, but not to the drought and heat stress combination.31 Overexpression of Rap2.6L in Arabidopsis was shown to enhance tolerance to abiotic stresses via activating hormone signaling pathways involving ABA, JA, SA and ethylene.32,33 These results suggest that, to some extent, regulatory mechanisms of hormone signaling pathways underlying tailored responses of plants to drought, heat and their combination might be different depending on plant species.
Integration of hormone signaling pathways with ROS regulatory systems was also implicated in acclimation of plants to the drought and heat stress combination. Overexpression of cytokinin oxidase, the cytokinin degrading enzyme, resulted in enhanced tolerance of transgenic tobacco plants to the drought and heat stress combination accompanied by the altered expression patterns of transcripts involved in ROS scavenging.34 In contrast to ABA which is implicated in activation of ROS scavenging mechanism in Arabidopsis,15 down-regulation of cytokinin signaling in tobacco might be associated with regulation of antioxidant mechanisms under the combined stress. To further elucidate co-ordination between hormone signaling pathways and ROS regulatory systems, accumulation of ROS and hormones should be measured in mutants deficient in synthesis or signaling of different hormones.
Response of plants to combinations of drought and other abiotic stresses has been also addressed in recent studies. Although the drought and heat stress combination negatively affects plant growth, the drought and UV-B combination might have beneficial effects on plants compared with each of the individual stress applied individually.35 Tossi et al., (2014) suggested that in Arabidopsis UV-B irradiation increased ABA synthesis as well as NO and ROS production which might lead to stomatal closure.36 In contrast to the tailored response of plants to the drought and heat stress combination, ABA-dependent stomatal closure might be essential for acclimation of plants to the drought and UV-B combination.35 In addition, requirement of ethylene signaling in ABA synthesis was evidenced by the finding that Arabidopsis etr1-1 mutant, deficient in ethylene perception was impaired in ABA synthesis in response to UV-B irradiation.37 Furthermore, drought can induce synthesis of flavonoids that possess antioxidant and UV-B screening functions, as well as ABA that activates proline synthesis and antioxidant systems.38 Both flavonoid synthesis and ABA signaling pathway were shown to be regulated by a transcription factor MYB12 in Arabidopsis.38 It should be important to reveal how ABA signaling pathway and flavonoid synthesis were integrated during the drought and UV-B stress combination in future studies. Response of plants to the osmotic and cold stress combination has been also analyzed using a glutathione (GSH) deficient mutant, pad2.1 in Arabidopsis that showed higher sensitivity to this stress combination. Transcripts responsive to hormones such as auxin, ethylene, brassinosteroid and ABA were differentially expressed in pad2.1 mutant compared to WT plants under the osmotic and cold stress combination.39 Transcripts involved in ethylene synthesis were down-regulated in pad2.1 mutants. The alteration of these transcripts might be due to the increase in ROS by GSH deficiency, or indicating the possibility that GSH might regulate genes and protein expression by thiol mediated modification of various regulatory proteins. In contrast to the ethylene responsive transcripts, transcription factors that are involved in regulation of brassinosteroid and auxin signaling were up-regulated. These findings indicate that hormone signaling pathways might be uniquely tailored depending on the types of stresses that simultaneously occur with drought (Fig. 1).
Figure 1.
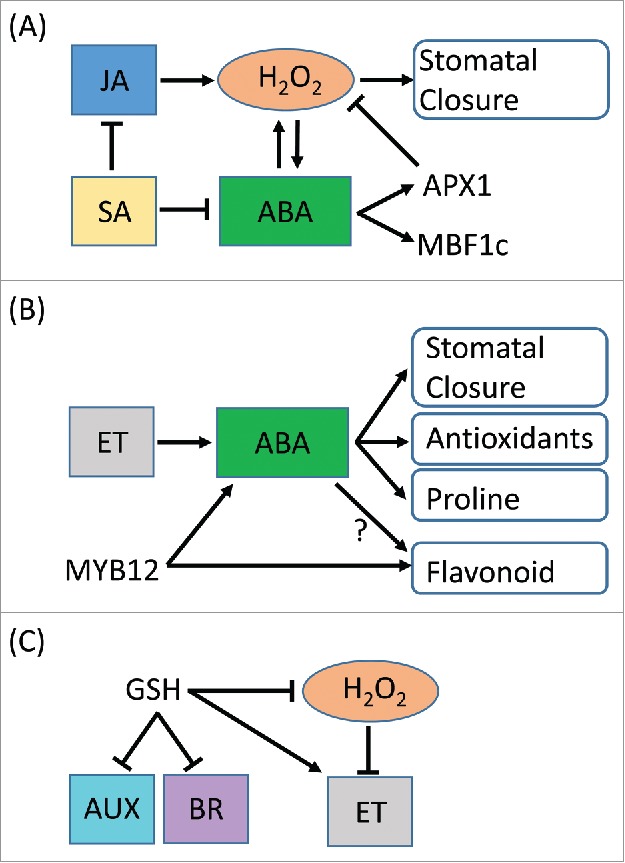
Simplified model of hormone signaling pathways under different abiotic stress combinations in Arabidopsis. The models suggest the tailored response of Arabidopsis to (A) drought and heat combination, (B) drought and UV-B combination, and (C) osmotic and cold combination. The models were generated based on the researches using Arabidopsis subjected to different stress combinations.15,36-39 A model of the signaling pathway under the drought and UV-B combination was also suggested in a recent review35 based the researches using different plant species. ABA; abscisic acid, AUX; auxin, BR; brassinosteroid, ET; ethylene, JA; jasmonic acid, SA; salicylic acid.
In addition to drought, salt stress can be also combined with other abiotic stresses and highly impacts plant growth. Involvement of hormone signaling pathway in acclimation of plants to the salt and heat stress combination has been indicated in a recent study. Transcriptome analysis of Arabidopsis plants subjected to salt, heat and their combination demonstrated that expression of 699 transcripts were specifically enhanced in response to the salt and heat stress combination.14 Interestingly, transcripts associated with ABA signaling pathway were highly represented in these 699 transcripts. In contrast, SA- and gibberellic acid (GA)-associated transcripts that could antagonize ABA signaling pathway29,40 were least represented. Involvement of ABA in the acclimation of plants to the salt and heat stress combination was also supported by the finding that mutants deficient in ABA synthesis (aba1-1) or response (abi1-1) were significantly more sensitive to this stress combination compared to WT plants. ABA was implicated in tailored responses of Arabidopsis both to the drought and salt stress combined with heat stress. However, ABA-dependent pathways that are involved in plant's acclimation to these stress combinations could be different, because little overlap was found between the different sets of transcripts specifically up-regulated in response to the salt and heat stress combination and the drought and heat stress combination.14 Differences and similarities in ABA-dependent tailored responses of Arabidopsis to these stress combinations, however, still need to be investigated in future studies.
In nature, abiotic and biotic stresses can simultaneously occur and defense pathways with a high degree of complexity might be activated in plants. Complex mode of plant responses to the biotic and abiotic stress combinations has also been addressed. A recent study demonstrated that expression of different sets of SA- and JA-associated genes were up-regulated in rice in response to biotic stress combined with drought or salt stress.41 Although both drought and salt stress are able to activate SA- and JA-dependent defense mechanisms, different signaling pathways might be up-regulated by these abiotic stresses. This hypothesis could be supported by the finding that drought and salt stress are able to enhance tolerance of plants to different types of pathogens.42 In some cases, ABA was also shown to be accumulated in response to pathogen infection.42 Higher level of ABA induced by Pst DC 3000 infection suppressed defense pathways against other pathogens.43 However, recent findings demonstrated a positive effect of ABA on biotic stress resistance.44,45 ABA and ROS induced by drought results in stomatal closure that might inhibit penetration of pathogen as second effects, and activation of defense pathways.10 This dual effect makes ABA a controversial molecule that can regulate both positive and negative effects on pathogen responses depending on the environmental conditions.45 Effects of ABA on defense pathways under biotic and abiotic stress combinations could be at least partially modulated via its temporal co-ordination with other hormone signaling pathways. Response of plants to biotic and abiotic stress combinations might be consist of 3 phases.46 In the first phase, ABA induces stomatal closure and maintains water potential. During this phase, synthesis of SA, JA and ethylene can be antagonized. In the second phase, callose accumulation increases via the function of ABA. In the third phase, pathogen-associated molecular patterns stimulate SA, JA and ethylene signaling to activate defense pathways. In addition, hormone signaling pathways might be fine-tuned depending on the order of stress applications. In Arabidopsis, transcripts involved in SA and JA signaling were up-regulated in response to drought and pathogen combination, when drought application was followed by pathogen infection.47 Under this condition, expression of ABA response transcripts was only marginally altered. In contrast, several defense genes including JA related, JASMONATE-ZIM-DOMAIN PROTEIN 10 (JAZ10) were repressed when pathogen infection was followed by drought. These results suggest that hormone-dependent defense mechanisms under the biotic and abiotic stress combinations could be flexible, being altered by timing of stress applications.
Taken together, fine-tuned co-ordination of hormone signaling pathways might be essential in the regulation of tailored responses of plants to different stress combinations (Fig. 1), and these responses to stress combinations cannot be deduced from those under corresponding individual stresses. Although ABA might be involved in responses of plants to a broad range of stress combinations, its integration with other hormones or other signaling pathways such as ROS regulatory systems seems to be different depending on types of stress combinations (Fig. 1). These findings indicate that ABA could function as a key regulators of tailored responses of plants to various stress combinations. In addition, hormone-dependent signaling pathways under the stress combinations could be flexible being altered by intensity, timing and types of stress applications, and could be different depending on plant species. Master regulators that switch on/off the different hormone signaling pathways should be revealed in future studies.
Disclosure of potential conflicts of interest
The author has declared that no competing interests exist.
Acknowledgment
This paper was supported by funding from Sophia University in Japan.
References
- 1.Cavanagh C, Morell M, Mackay I, Powell W. From mutations to MAGIC: resources for gene discovery, validation and delivery in crop plants. Current Opin Plant Biol 2008; 11:215-21; PMID:18295532; http://dx.doi.org/24720847 10.1016/j.pbi.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 2.Munns R, Tester M. Mechanisms of salinity tolerance. Ann Rev Plant Biol 2008; 59:651-81; PMID:18444910; http://dx.doi.org/24720847 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- 3.Chinnusamy V, Zhu JK. Epigenetic regulation of stress responses in plants. Current Opin Plant Biol 2009; 12:133-9; PMID:19179104; http://dx.doi.org/24720847 10.1016/j.pbi.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittler R, Blumwald E. Genetic engineering for modern agriculture: challenges and perspectives. Ann Rev Plant Biol 2010; 61:443-62; PMID:20192746; http://dx.doi.org/24720847 10.1146/annurev-arplant-042809-112116 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R. Abiotic and biotic stress combinations. New Phytol 2014; 203:32-43; PMID:24720847; http://dx.doi.org/ 10.1111/nph.12797 [DOI] [PubMed] [Google Scholar]
- 6.Ahuja I, de Vos RC, Bones AM, Hall RD. Plant molecular stress responses face climate change. Trends Plant Sci 2010; 15:664-74; PMID:20846898; http://dx.doi.org/ 10.1016/j.tplants.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 7.Mittler R, Finka A, Goloubinoff P. How do plants feel the heat? Trends Biocheml Sci 2012; 37:118-25; PMID:22236506; http://dx.doi.org/23359638 10.1016/j.tibs.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 8.Li J, Lin X, Chen A, Peterson T, Ma K, Bertzky M, Ciais P, Kapos V, Peng C, Poulter B. Global priority conservation areas in the face of 21st century climate change. PloS ONE 2013; 8:e54839; PMID:23359638; http://dx.doi.org/ 10.1371/journal.pone.0054839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IPCC Climate change and water Technical Paper of the Intergovernmental Panel on Climate Change. Kundzewicz ZW, Palutikof J, Wu S, eds Cambridge, UK & New York, NY, USA: Cambridge University Press; 2008. [Google Scholar]
- 10.Pandey P, Ramegowda V, Senthil-Kumar M. Shared and unique responses of plants to multiple individual stresses and stress combinations: physiological and molecular mechanisms. Front Plant Sci 2015; 6:723; PMID:26442037; http://dx.doi.org/ 10.3389/fpls.2015.00723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkinson NJ, Lilley CJ, Urwin PE. Identification of genes involved in the response of Arabidopsis to simultaneous biotic and abiotic stresses. Plant Physiol 2013; 162:2028-41; PMID:23800991; http://dx.doi.org/ 10.1104/pp.113.222372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasch CM, Sonnewald U. Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol 2013; 162:1849-66; PMID:23753177; http://dx.doi.org/ 10.1104/pp.113.221044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sewelam N, Oshima Y, Mitsuda N, Ohme-Takagi M. A step towards understanding plant responses to multiple environmental stresses: a genome-wide study. Plant Cell Environm 2014; 37:2024-35; PMID:24417440; http://dx.doi.org/26824246 10.1111/pce.12274 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki N, Bassil E, Hamilton JS, Inupakutika MA, Zandalinas SI, Tripathy D, Luo Y, Dion E, Fukui G, Kumazaki A, et al.. ABA is required for plant acclimation to a combination of salt and heat stress. PloS ONE 2016; 11:e0147625; PMID:26824246; http://dx.doi.org/ 10.1371/journal.pone.0147625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zandalinas SI, Balfagon D, Arbona V, Gomez-Cadenas A, Inupakutika MA, Mittler R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J Exp Bot 2016; 67(18):5381-5390; PMID:27497287; http://dx.doi.org/27121193 10.1093/jxb/erw299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zandalinas SI, Rivero RM, Martinez V, Gomez-Cadenas A, Arbona V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol 2016; 16:105; PMID:27121193; http://dx.doi.org/ 10.1186/s12870-016-0791-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittler R, Blumwald E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015; 27:64-70; PMID:25604442; http://dx.doi.org/ 10.1105/tpc.114.133090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boursiac Y, Leran S, Corratge-Faillie C, Gojon A, Krouk G, Lacombe B. ABA transport and transporters. Trends Plant Sci 2013; 18:325-33; PMID:23453706; http://dx.doi.org/ 10.1016/j.tplants.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 19.Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J Exp Bot 2007; 58:221-7; PMID:17075077; http://dx.doi.org/ 10.1093/jxb/erl164 [DOI] [PubMed] [Google Scholar]
- 20.Li X, Yang Y, Sun X, Lin H, Chen J, Ren J, Hu X, Yang Y. Comparative physiological and proteomic analyses of poplar (Populus yunnanensis) plantlets exposed to high temperature and drought. PloS ONE 2014; 9:e107605; PMID:25225913; http://dx.doi.org/24267539 10.1371/journal.pone.0107605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murata Y, Mori IC, Munemasa S. Diverse stomatal signaling and the signal integration mechanism. Ann Rev Plant Biol 2015; 66:369-92; PMID:25665132; http://dx.doi.org/24267539 10.1146/annurev-arplant-043014-114707 [DOI] [PubMed] [Google Scholar]
- 22.`Aprile A, Havlickova L, Panna R, Mare C, Borrelli GM, Marone D, Perrotta C, Rampino P, De Bellis L, Curn V, et al.. Different stress responsive strategies to drought and heat in two durum wheat cultivars with contrasting water use efficiency. BMC Genomics 2013; 14:821; PMID:24267539; http://dx.doi.org/ 10.1186/1471-2164-14-821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bak G, Lee EJ, Lee Y, Kato M, Segami S, Sze H, Maeshima M, Hwang JU, Lee Y. Rapid structural changes and acidification of guard cell vacuoles during stomatal closure require phosphatidylinositol 3,5-bisphosphate. Plant Cell 2013; 25:2202-16; PMID:23757398; http://dx.doi.org/ 10.1105/tpc.113.110411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X. Phospholipase dalpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 2009; 21:2357-77; PMID:19690149; http://dx.doi.org/ 10.1105/tpc.108.062992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 2005; 17:268-81; PMID:15608336; http://dx.doi.org/ 10.1105/tpc.104.026971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki N, Bajad S, Shuman J, Shulaev V, Mittler R. The transcriptional co-activator MBF1c is a key regulator of thermotolerance in Arabidopsis thaliana. J Biol Chem 2008; 283:9269-75; PMID:18201973; http://dx.doi.org/ 10.1074/jbc.M709187200 [DOI] [PubMed] [Google Scholar]
- 27.Suzuki N, Rizhsky L, Liang H, Shuman J, Shulaev V, Mittler R. Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional coactivator multiprotein bridging factor 1c. Plant Physiol 2005; 139:1313-22; PMID:16244138; http://dx.doi.org/ 10.1104/pp.105.070110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koussevitzky S, Suzuki N, Huntington S, Armijo L, Sha W, Cortes D, Shulaev V, Mittler R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J Biol Chem 2008; 283:34197-203; PMID:18852264; http://dx.doi.org/ 10.1074/jbc.M806337200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moeder W, Ung H, Mosher S, Yoshioka K. SA-ABA antagonism in defense responses. Plant Signal Behav 2010; 5:1231-3; PMID:20861686; http://dx.doi.org/ 10.4161/psb.5.10.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caarls L, Pieterse CM, Van Wees SC. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front Plant Sci 2015; 6:170; PMID:25859250; http://dx.doi.org/ 10.3389/fpls.2015.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Xin M, Qin J, Peng H, Ni Z, Yao Y, Sun Q. Temporal transcriptome profiling reveals expression partitioning of homeologous genes contributing to heat and drought acclimation in wheat (Triticum aestivum L.). BMC Plant Biol 2015; 15:152; PMID:26092253; http://dx.doi.org/ 10.1186/s12870-015-0511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnaswamy S, Verma S, Rahman MH, Kav NN. Functional characterization of four APETALA2-family genes (RAP2.6, RAP2.6L, DREB19 and DREB26) in Arabidopsis. Plant Mol Biol 2011; 75:107-27; PMID:21069430; http://dx.doi.org/ 10.1007/s11103-010-9711-7 [DOI] [PubMed] [Google Scholar]
- 33.Liu P, Sun F, Gao R, Dong H. RAP2.6L overexpression delays waterlogging induced premature senescence by increasing stomatal closure more than antioxidant enzyme activity. Plant Mol Biol 2012; 79:609-22; PMID:22661072; http://dx.doi.org/ 10.1007/s11103-012-9936-8 [DOI] [PubMed] [Google Scholar]
- 34.Lubovska Z, Dobra J, Storchova H, Wilhelmova N, Vankova R. Cytokinin oxidase/dehydrogenase overexpression modifies antioxidant defense against heat, drought and their combination in Nicotiana tabacum plants. J Plant Physiol 2014; 171:1625-33; PMID:25171514; http://dx.doi.org/ 10.1016/j.jplph.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 35.Bandurska H, Niedziela J, Chadzinikolau T. Separate and combined responses to water deficit and UV-B radiation. Plant Sci 2013; 213:98-105; PMID:24157212; http://dx.doi.org/ 10.1016/j.plantsci.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 36.Tossi V, Lamattina L, Jenkins GI, Cassia RO. Ultraviolet-B-induced stomatal closure in Arabidopsis is regulated by the UV RESISTANCE LOCUS8 photoreceptor in a nitric oxide-dependent mechanism. Plant Physiol 2014; 164:2220-30; PMID:24586043; http://dx.doi.org/ 10.1104/pp.113.231753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rakitin VY, Karyagin VV, Rakitina TY, Prudnikova ON, Vlasov PV. UV-B stress-induced ABA production in Arabidopsis thaliana mutants defective in ethylene signal transduction pathway. Russ J Plant Physiol 2008; 55:854-6; http://dx.doi.org/ 10.1134/S1021443708060174 [DOI] [Google Scholar]
- 38.Wang F, Kong W, Wong G, Fu L, Peng R, Li Z, Yao Q. AtMYB12 regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis thaliana. Mol Gen Genom 2016; 291:1545-59; PMID:27033553; http://dx.doi.org/22291200 10.1007/s00438-016-1203-2 [DOI] [PubMed] [Google Scholar]
- 39.Kumar D, Datta R, Hazra S, Sultana A, Mukhopadhyay R, Chattopadhyay S. Transcriptomic profiling of Arabidopsis thaliana mutant pad2.1 in response to combined cold and osmotic stress. PloS ONE 2015; 10:e0122690; PMID:25822199; http://dx.doi.org/22291200 10.1371/journal.pone.0122690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishibashi Y, Tawaratsumida T, Kondo K, Kasa S, Sakamoto M, Aoki N, Zheng SH, Yuasa T, Iwaya-Inoue M. Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol 2012; 158:1705-14; PMID:22291200; http://dx.doi.org/ 10.1104/pp.111.192740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang YP, E ZG, Jiang H, Wang L, Zhou J, Zhu DF. A comparative study of stress-related gene expression under single stress and intercross stress in rice. Gen Mol Res 2015; 14:3702-17; PMID:25966139; http://dx.doi.org/27135514 10.4238/2015.April.17.20 [DOI] [PubMed] [Google Scholar]
- 42.Rejeb IB, Pastor V, Mauch-Mani B. Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants 2014; 3:458-75; PMID:27135514; http://dx.doi.org/ 10.3390/plants3040458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Torres-Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Rodriguez Egea P, Bögre L, Grant M. Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J 2007; 26:1434-43; PMID:17304219; http://dx.doi.org/ 10.1038/sj.emboj.7601575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Andrade J, Ramirez V, Flors V, Vera P. Arabidopsis ocp3 mutant reveals a mechanism linking ABA and JA to pathogen-induced callose deposition. Plant J 2011; 67:783-94; PMID:21564353; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04633.x [DOI] [PubMed] [Google Scholar]
- 45.Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J. Callose deposition: a multifaceted plant defense response. Mol Plant-Microb Int 2011; 24:183-93; PMID:20955078; http://dx.doi.org/25546584 10.1094/MPMI-07-10-0149 [DOI] [PubMed] [Google Scholar]
- 46.Ramegowda V, Senthil-Kumar M. The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic understanding from drought and pathogen combination. J Plant Physiol 2015; 176:47-54; PMID:25546584; http://dx.doi.org/ 10.1016/j.jplph.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 47.Gupta A, Sarkar AK, Senthil-Kumar M. Global transcriptional analysis reveals unique and shared responses in Arabidopsis thaliana exposed to combined drought and pathogen stress. Front Plant Sci 2016; 7:686; PMID:27252712; http://dx.doi.org/ 10.3389/fpls.2016.00686 [DOI] [PMC free article] [PubMed] [Google Scholar]


