ABSTRACT
One of the fundamental plant growth substances, indole-3-acetic acid (IAA), belongs to a class of phytohormones known as auxins. The main IAA biosynthesis pathway involves the conversion of tryptophan to indole-3-pyruvic acid, which is in turn converted to IAA. The two enzymes responsible for these conversions, members of the TAA1 and YUCCA gene families, respectively, have recently been implicated in the synthesis of another auxin, phenylacetic acid (PAA). While there is some evidence to support this theory, there are also some concerns. Here we address the question: to what extent does the TAA1/YUCCA system contribute to the biosynthesis of PAA? In addition, we highlight the importance of measuring auxin metabolites and conjugates in addressing such questions.
KEYWORDS: Aminotransferase, amino acid, biosynthesis, metabolism, phytohormone
Auxin biosynthesis remains a developing field, although substantial progress has been made in recent years.1-7 The main auxin in plants is indole-3-acetic acid (IAA), and it is now widely accepted that most IAA is derived from tryptophan (Trp) via a 2-step pathway. Trp is initially converted to indole-3-pyruvic acid (IPyA) by members of the TAA1 protein family,1,2,8 and IPyA is subsequently converted to IAA by proteins encoded by the YUCCA gene family (Fig. 1).3,4,6 While other pathways may exist for IAA biosynthesis, these are not necessarily ubiquitous, often have specific tissue expression, and/or are thought to make only minor contributions to the overall levels of IAA.9-12
Figure 1.
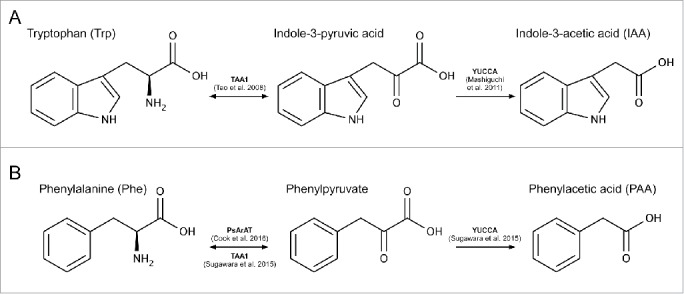
Overview of auxin biosynthetic pathways for (A) IAA and (B) PAA. (A) It is widely accepted that Trp is converted to indole-3-pyruvic acid by the TAA1 family of amino-transferases.2,10 Indole-3-pyruvic acid is then converted to IAA by the YUCCA family of flavin-containing mono-oxygenases.4 (B) The available evidence indicates that PAA is biosynthesised from phenylalanine via phenylpyruvate.5,7 However, while Sugawara5 suggested that TAA1 enzymes catalyze the first step, and YUCCAs the second, we suggest that these enzymes play at most only minor roles.
Recently, some attention has shifted to the biosynthesis of another auxin, phenylacetic acid, PAA.5,7 It has been suggested that the PAA biosynthesis pathway involves a TAA1-mediated conversion of phenylalanine (Phe) to phenylpyruvate, followed by the YUCCA-catalyzed conversion of phenylpyruvate to PAA (Fig. 1).5 This is an important suggestion, because if the IAA biosynthetic machinery is also responsible for the production of PAA, IAA biosynthesis mutants might be deficient in PAA as well as IAA. Since PAA has rarely been quantified in auxin mutants in the past, this begs the question, could changes in PAA be partly responsible for the phenotypes we see in auxin biosynthesis mutants?
There is currently some debate about the extent to which the TAA1 and YUCCA families actually play a role in PAA biosynthesis.7 In Cook et al (2016),7 we agreed with Sugawara et al (2015)5 that the pathway, Phe to phenylpyruvate to PAA, can operate in plants, and presented evidence from metabolism studies supporting these steps. Importantly, however, we questioned whether the same TAA1 enzymes that catalyze the transamination of Trp to IPyA also catalyze the conversion of Phe to phenylpyruvate, given that non-TAA1 aminotransferases have been identified for the reversible conversion of Phe to phenylpyruvate.13,14 Similarly, we questioned whether the same YUCCA enzymes that catalyze the conversion of IPyA to IAA also catalyze the conversion of phenylpyruvate to PAA in vivo.
Here we assess the evidence presented in the 2 recent papers,5,7 and in so doing we discuss the possible shortcomings associated with the main approaches used - in particular, overexpression of the genes concerned, and the measurement of hormone levels in loss of function mutants.
After the discovery of the TAA1 and YUCCA gene families, some of the members were expressed in E. coli, to determine the molecular function of the encoded proteins. Both AtTAA1 and AtYUC2 were shown to possess catalytic activity using this technique.2,4 Additional analyses on PsTAR1, PsTAR2 and AtYUC6 have further confirmed the involvement of these gene families in IAA biosynthesis.3,5 8
The AtTAA1 enzyme is reportedly capable of utilizing all 3 aromatic amino acids (Trp, Phe and tyrosine) in vitro, as well as other amino acids.2 Similarly, 2 Arabidopsis YUCCAs, AtYUC2 and AtYUC6, have been shown to convert phenylpyruvate to PAA in vitro, in addition to their role in IAA biosynthesis.3,5
Consistent with this, Sugawara et al (2015)5 also showed that transgenic Arabidopsis lines, overexpressing YUC1, YUC2 or YUC6, accumulated high levels of IAA and PAA conjugates (those bound to aspartate [Asp] and glutamate [Glu]), which suggests an increase in the synthesis of both auxins. This is not surprising as the expression of YUC1, YUC2 and YUC6 was dramatically elevated (by 41-, 142-, and 29- fold, respectively) in these lines, and the substrate, phenylpyruvate, is present in plants.7,14,15
The results of YUCCA overexpression were interpreted as evidence that YUCCAs play a role in PAA biosynthesis.5 On the other hand, we have pointed out that if YUC proteins are involved in this way, knocking out the corresponding genes should affect PAA levels, provided that IAA levels are also affected. However, analysis of an Arabidopsis higher order mutant containing non-functional AtYUC1, AtYUC2 and AtYUC6 (yuc1yuc2yuc6) showed that PAA levels were normal (Fig. 2)5 even though IAA levels were markedly reduced (Fig. 2). The same is seen in the maize mutant de-18, where a non-functional ZmYUC1 results in endosperm with a massive reduction in IAA, but normal PAA levels.7,16
Figure 2.
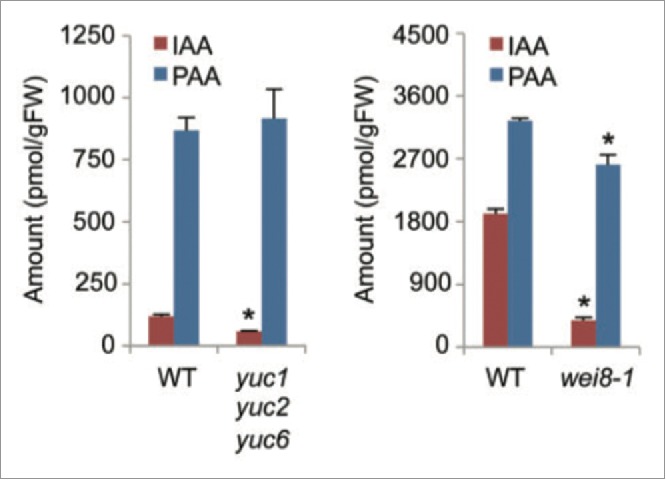
Auxin levels in the high order YUC mutant (yuc1yuc2yuc6) and TAA1 mutant (wei8-1) of Arabidopsis. IAA levels are decreased in both mutants, although PAA is only slightly decreased in the former, and not at all in the latter. Adapted from Sugawara et al (2015).5
Similarly, the AtTAA1 mutation, wei8-1, caused only a slight reduction in PAA levels, even though there was a large drop in IAA content (Fig 2.).5 Again, in the analogous pea mutant tar2-1 (PsTAR2), the level of PAA was unaffected despite large reductions in the content of both IAA and chlorinated IAA.7 These endogenous levels data indicate that the TAA1 and YUCCA enzymes play at most a minor role in PAA biosynthesis.
Yet, how definitive are endogenous levels for discriminating between major and minor pathways? Several papers have ascribed a major role to a pathway on the basis that a mutation known to affect that pathway causes a major drop in auxin content.5,8,16,17 However, is this justified? To answer that, we can consider a hypothetical model where two taps deliver water into a bucket – one at a much faster rate than the other (Fig. 3). These taps are analogous to major and minor pathways of auxin biosynthesis in the organ or tissue of interest. The combined rate of the input is identical to the rate at which this bucket is draining into a second bucket (analogous to the production of auxin metabolites/conjugates, Fig. 3A). Under normal circumstances, a constant level is maintained in the first bucket (free auxin), while the contents of the second accumulate. This situation reflects the accumulation of auxin end products that occurs in some plant systems, e.g.7
Figure 3.
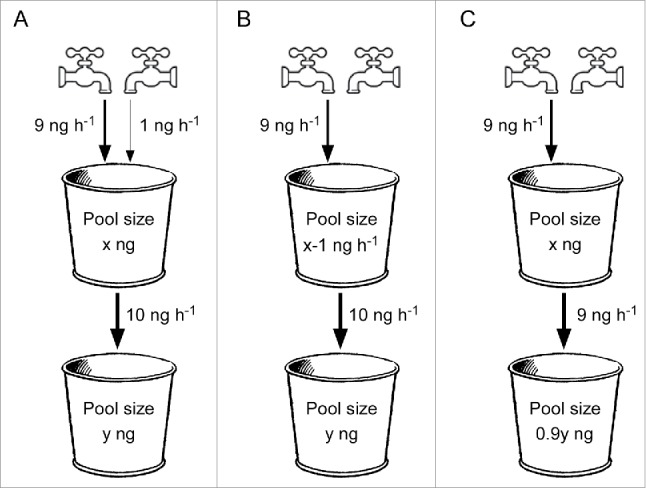
A “Bucket Model,” showing (A) a steady-state system with major and minor inputs (2 taps) into the first bucket and an output that equals the combined input; (B) a system with reduced input (one tap turned off) and normal output, and (C) a system under homeostatic control with reduced input (minor) and output. Arrows indicate direction of flow and associated values represent hypothetical rates (per hour) and theoretical pool sizes are shown within each bucket.
Turning off either of the taps, which would be analogous to genetically blocking auxin biosynthesis, will eventually result in a low level in the first bucket (Fig. 3B), given that the flow into the second bucket remains constant. Thus, simply determining the contents of the first bucket (free auxin) does not allow us to discriminate between the blockage of either a major or minor input, as impairing a minor pathway can still have a large effect on free auxin content.
Therefore, to make this distinction, it is necessary to measure the contents of the second bucket. If the flux into the second bucket becomes limited by the reduced free auxin content, we would expect a reduction in the content of metabolites/conjugates. The extent of this reduction will be proportional to the contribution normally made by the blocked pathway. Therefore, if we find large drops in both buckets, this would indicate a blockage to a major biosynthetic pathway.
It is important to note, however, that auxin biosynthesis is under homeostatic control18-21 and if the rate at which the first bucket drains to the second were homeostatically reduced (Fig. 3C), the level of free auxin can be maintained. Once again, the measurement of only the endogenous auxin levels would not be instructive. However, in this case, we would see a clear reduction in the contents of the second bucket (auxin metabolites/conjugates), one proportional to the contribution normally made by the blocked pathway.
In summary, the measurement of auxin metabolites/conjugates is crucial for discriminating between major and minor pathways. In Cook et al (2016),7 we showed that tar2-1 strongly affects both free acid and conjugate levels for IAA and 4-Cl-IAA in pea seeds. Therefore, the IPyA pathway is the major pathway for the synthesis of these 2 auxins in this tissue. However, the levels of PAA and PAA conjugates were not affected by the tar2-1 mutation; nor were they affected in the maize defective endosperm (de-18) mutant.7 This further strengthens our case that the same enzymes do not function in both IAA and PAA biosynthesis.
Conclusions
Both of the recent papers5,7 agree that PAA can be synthesized from phenylalanine via phenylpyruvate. The point of controversy concerns the extent to which the TAA1 and YUCCA proteins catalyze the steps Phe to phenylpyruvate to PAA, respectively. We argue that there is little evidence that these enzymes play any more than a minor role in PAA biosynthesis in vivo. The observation that overexpression of YUCCAs leads to elevated flux through the PAA pathway5 simply confirms that YUCCAs can utilize phenylpyruvate in some circumstances, but the normal PAA levels in YUCCA mutants is not consistent with a major role in vivo.
It is possible that other previously proposed pathways for IAA biosynthesis9 may function for PAA. Yet, previously, we were unable to detect phenylethylamine, phenylacetonitrile or phenylacetamide in pea.7 As each of these represents an analogous component of an alternative IAA biosynthetic pathway, the presence or function of these pathways remains unknown. We previously noted, however, that phenylacetaldehyde may be a precursor to PAA in pea apical tissue.7
Interestingly, an aromatic aminotransferase from petunia catalyzes the reversible transamination of phenylpyruvate to Phe.14 Based on the in vivo ability of pea extracts to carry out this conversion, we have identified a similar gene in pea with strong sequence similarity.7 We suggest that there may be separate enzymes or protein families for the conversion of Trp to IPyA and for Phe to phenylpyruvate. In evolutionary terms, this has likely been favored, as increasing body plan complexity undoubtedly requires an increasingly fine-tuned control system. Such a system would allow the discrete synthesis and control of auxins on the one hand, and phenylalanine and its derivatives on the other.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie D-Y, Dolezal K, Schlereth A, Jürgens G, Alonso JM. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 2008; 133:177-91; PMID:18394997; http://dx.doi.org/ 10.1016/j.cell.2008.01.047 [DOI] [PubMed] [Google Scholar]
- 2.Tao Y, Ferrer J-L, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al.. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 2008; 133:164-76; PMID:18394996; http://dx.doi.org/ 10.1016/j.cell.2008.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai X, Mashiguchi K, Chen Q, Kasahara H, Kamiya Y, Ojha S, DuBois J, Ballou D, Zhao Y. The biochemical mechanism of auxin biosynthesis by an Arabidopsis YUCCA flavin-containing monooxygenase. J Biol Chem 2013; 288:1448-57; PMID:23188833; http://dx.doi.org/ 10.1074/jbc.M112.424077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, et al.. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA 2011; 108:18512-7; PMID:22025724; http://dx.doi.org/ 10.1073/pnas.1108434108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugawara S, Mashiguchi K, Tanaka K, Hishiyama S, Sakai T, Hanada K, Kinoshita-Tsujimura K, Yu H, Dai X, Takebayashi Y, et al.. Distinct characteristics of indole-3-acetic acid and phenylacetic acid, two common auxins in plants. Plant Cell Physiol 2015; 56:1641-54; PMID:26076971; http://dx.doi.org/ 10.1093/pcp/pcv088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci USA 2011; 108:18518-23; PMID:22025721; http://dx.doi.org/ 10.1073/pnas.1108436108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook SD, Nichols DS, Smith J, Chourey PS, McAdam EL, Quittenden L, Ross JJ. Auxin biosynthesis: Are the indole-3-acetic acid and phenylacetic acid biosynthesis pathways mirror images? Plant Physiol 2016; 171:1230-41; PMID:27208245; http://dx.doi.org/ 10.1104/pp.16.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tivendale ND, Davidson SE, Davies NW, Smith JA, Dalmais M, Bendahmane AI, Quittenden LJ, Sutton L, Bala RK, Le Signor C, et al.. Biosynthesis of the halogenated auxin, 4-chloroindole-3-acetic acid. Plant Physiol 2012; 159:1055-63; PMID:22573801; http://dx.doi.org/ 10.1104/pp.112.198457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugawara S, Hishiyama S, Jikumaru Y, Hanada A, Nishimura T, Koshiba T, Zhao Y, Kamiya Y, Kasahara H. Biochemical analyses of indole-3-acetaldoxime-dependent auxin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA 2009; 106:5430-5; PMID:19279202; http://dx.doi.org/ 10.1073/pnas.0811226106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y. Auxin biosynthesis: a simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol Plant 2012; 5:334-8; PMID:22155950; http://dx.doi.org/ 10.1093/mp/ssr104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tivendale ND, Ross JJ, Cohen JD. The shifting paradigms of auxin biosynthesis. Trends Plant Sci 2014; 19:44-51; PMID:24524164; http://dx.doi.org/ 10.1016/j.tplants.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Chu J, Yu T, Xu Q, Sun X, Yuan J, Xiong G, Wang G, Wang Y, Li J. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc Natl Acad Sci USA 2015; 112:4821-6; PMID:25831515; http://dx.doi.org/ 10.1073/pnas.1503998112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonda I, Bar E, Portnoy V, Lev S, Burger J, Schaffer AA, Tadmor Y, Gepstein S, Giovannoni JJ, Katzir N, et al.. Branched-chain a nd aromatic amino acid catabolism into aroma volatiles in Cucumis melo L. fruit. J Exp Bot 2010; 61:1111-23; PMID:20065117; http://dx.doi.org/ 10.1093/jxb/erp390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo H, Widhalm JR, Qian Y, Maeda H, Cooper BR, Jannasch AS, Gonda I, Lewinsohn E, Rhodes D, Dudareva N. An alternative pathway contributes to phenylalanine biosynthesis in plants via a cytosolic tyrosine: phenylpyruvate aminotransferase. Nat Commun 2013; 4:2833; PMID:24270997; http://dx.doi.org/ 10.1038/ncomms3833 [DOI] [PubMed] [Google Scholar]
- 15.Manela N, Oliva M, Ovadia R, Sikron-Persi N, Ayenew B, Fait A, Galili G, Perl A, Weiss D, Oren-Shamir M. Phenylalanine and tyrosine levels are rate-limiting factors in production of health p romoting metabolites in Vitis vinifera cv. Gamay Red cell suspension. Front Plant Sci 2015; 6(538): 1-13; PMID:26236327; http://dx.doi.org/22961134 10.3389/fpls.2015.00538. [DOI] [Google Scholar]
- 16.Bernardi J, Lanubile A, Li Q-B, Kumar D, Kladnik A, Cook SD, Ross JJ, Marocco A, Chourey PS. Impaired auxin biosynthesis in the defective endosperm18 mutant is due to mutational loss of expression in the ZmYuc1 gene encoding endosperm-specific YUCCA1 protein in maize. Plant Physiol 2012; 160:1318-28; PMID:22961134; http://dx.doi.org/ 10.1104/pp.112.204743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips KA, Skirpan AL, Liu X, Christensen A, Slewinski TL, Hudson C, Barazesh S, Cohen JD, Malcomber S, McSteen P. vanishing tassel2 encodes a grass-specific tryptophan aminotransferase required for vegetative and reproductive development in maize. Plant Cell 2011; 23:550-66; PMID:21335375; http://dx.doi.org/ 10.1105/tpc.110.075267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellor N, Band LR, Pěnčík A, Novák O, Rashed A, Holman T, Wilson MH, Voß U, Bishopp A, King JR, et al.. Dynamic regulation of auxin oxidase and conjugating enzymes AtDAO1 and GH3 modulates auxin homeostasis. Proc Natl Acad Sci USA 2016; 113:11022-7; PMID:27651495; http://dx.doi.org/ 10.1073/pnas.1604458113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porco S, Pěnčík A, Rashed A, Voß U, Casanova-Sáez R, Bishopp A, Golebiowska A, Bhosale R, Swarup R, Swarup K, et al.. Dioxygenase-encoding AtDAO1 gene controls IAA oxidation and homeostasis in Arabidopsis. Proc Natl Acad Sci USA 2016; 113:11016-21; PMID:27651491; http://dx.doi.org/ 10.1073/pnas.1604375113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Lin JE, Harris C, Pereira FCM, Wu F, Blakeslee JJ, Peer WA. DAO1 catalyzes temporal and tissue-specific oxidative inactivation of auxin in Arabidopsis thaliana. Proc Natl Acad Sci USA 2016; 113:11010-5; PMID:27651492; http://dx.doi.org/ 10.1073/pnas.1604769113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stepanova AN, Alonso JM. Auxin catabolism unplugged: Role of IAA oxidation in auxin homeostasis. Proc Natl Acad Sci USA 2016; 113:10742-4; PMID:27651487; http://dx.doi.org/ 10.1073/pnas.1613506113 [DOI] [PMC free article] [PubMed] [Google Scholar]


