ABSTRACT
Previously, we described a priming effect of α-linolenic acid (LnA) on anti-herbivore defense response in maize seedlings.1 We showed that exogenous application of LnA stimulated higher jasmonic acid (JA) accumulation and herbivore-induced plant volatile (HIPV) emission after treatment with insect elicitor (IE). To further investigate the specificity of LnA's priming effect, we incubated maize seedlings in palmitoleic acid (PeicA), γ-linolenic acid (γ LnA) and stearic acid (StA) solutions, and analyzed HIPV emission in response to IE. Seedlings incubated in PeicA and γ LnA had 3 and 1.8 times higher HIPV release when compared to controls. In contrast, treatment with StA did not up-regulate HIPV release. We propose that the elevated level and/or the presence of unsaturated fatty acids sensitize the defense signaling system, which in turn augments the defense response of maize when under insect herbivore attack.
KEYWORDS: Free fatty acid, green leaf volatiles, herbivore-induced plant volatiles, insect herbivory, priming
The deployment of defense response in a plant generally comes at the expense of growth.2 Priming of defense response offers an alternative, where the major cost for defense can be forgone; however, when under attack by pests and pathogens primed plants are more efficient in the activation of defenses resulting in a stronger and/or faster response.3 Green leaf volatiles (GLV) are a group of plant volatiles that are rapidly released in quantity from leaf tissue upon damage.4,5 GLV's ability to prime defense response in maize was first described by Engelberth et al.6 There, GLV exposure augmented the defense response of maize seedlings challenged with IE by enhancing JA and HIPV production relative to controls. Since then, similar results have been reported for several plant species,7-10 suggesting a universal role of GLVs in defense priming.
JA plays a key role in regulating plant defense against insect herbivore attack by activating the biosynthesis of toxic secondary compounds,11 protease inhibitors12 and HIPV.13 Produced from LnA (18:3) through the octadecanoid pathway,11 JA is the key regulator in the synthesis and release of various HIPV,13,14 which can function as attractants for the enemies of attacking insect herbivores or as a repellant for other insects.15,16 Therefore, the accumulation of JA and/or up-regulation of HIPV emission can be considered as reliable biomarkers for priming.1,6
Recently, we reported an increase in unsaturated free fatty acids in both monocot and dicot plants after GLV exposure.1 These results suggested that increases in unsaturated free fatty acids were a common response to GLV treatment among different plant species. Using maize as model, we established a positive relationship between free LnA levels and defense priming, whereby LnA-treated plants had higher JA accumulation and HIPV emission after treatment with IE when compared to their respective controls.1 In contrast, physical movements such as a shaking reduced free LnA levels thereby decreasing JA accumulation and compromising the priming effect. To further characterize the role of free fatty acids on IE-induced defense responses we selected 3 different fatty acids and tested them for their effects on plant defense responses at concentrations corresponding to those used for LnA1 and monitored IE-induced HIPV release as our biomarker.
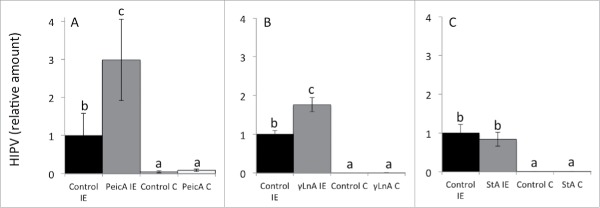
To verify that exogenous LnA does not serve as additional substrate for the octadecanoid pathway, we tested 3 different fatty acids, which cannot easily be transformed into LnA, for their priming effects in maize. We selected PeicA and γLnA, 2 unsaturated fatty acids, and StA, a saturated fatty acid, and treated maize seedlings as described previously.1 After treatment with IE HIPV were collected and analyzed by GC/MS. Maize seedlings incubated in unsaturated fatty acids (PeicA and γLnA) showed up-regulated HIPV release, with PeicA treated plants 3 times and γLnA group 1.8 times higher than their respective controls (Tukey's test, p < 0.05) (Fig. 1). By contrast, seedlings incubated in StA did not show a priming effect when compared with the control group and HIPV release was similar to that observed in control plants after treatment with IE.
Figure 1.
Herbivore-induced plant volatiles (HIPV) released from maize seedlings pre-treated with (A) palmitoleic acid (PeicA), (B) γ-linolenic acid (γ LnA) and (C) stearic acid (StA) and challenged with insect elicitor (IE), volicitin. Plants seedlings were incubated in 300mM aqueous solutions of the respective fatty acid overnight. Application of volicitin on wounded site mimicked insect herbivore feeding, as described by Li et al.1 Controls incubated in water and wounded mechanically, without the application of volicitin on the damage sites. For better comparison HIPV are showed as relative amounts with HIPV released from water incubated and volicitin treated seedlings set as 1 (h−1*gFW−1). Plant volatiles were collected 4 hours after volicitin treatments for 1h. Different letters above each bar indicate statistical difference determined by ANOVA analysis followed by Tukey tests where appropriate (p < 0.05). Error bar represent standard deviation. N = 6.
The increased emission of HIPV demonstrated a priming effect stimulated by non-JA producing unsaturated fatty acids. These results 1) validate our previous findings that the JA produced after LnA priming was not synthesized from exogenous LnA, and 2) supported our hypothesis that a priming effect may arise given the presence of unsaturated fatty acids that are not precursors of JA. Taken together, these suggest the elevated level/presence of unsaturated fatty acid, like LnA, PeicA and γLnA, may sensitize the defense response of plants and facilitated stronger signaling, leading to a priming effect. Free fatty acids, in particular unsaturated ones, have previously been shown to affects defense-related processes. For example, LnA was shown to inhibit callose synthase17 and may thus alter long-distance signaling events by keeping plasmodesmata open. Also, certain free fatty acids can modulate the activity of specific ion channels,18 thereby potentially changing IE-induced signaling events resulting in increased responses. However, to date we have no evidence for any of these potential activities of free fatty acids in maize, and further research is required to elucidate the role of these alterations in free fatty acid levels in this and other plants.
We have shown previously that treatment of maize seedling with volatile GLV rapidly changes the free fatty acid composition in these plants. We have further demonstrated that these changes in free fatty acids correlate well with IE-induced responses and concluded that mainly long distance signaling events are affected by free fatty acids and that they do not appear to serve as substrates for the biosynthetic pathway leading to the production of JA as the most important signaling compound in herbivore-induced defenses. This priming effect was further supported by the results presented herein showing that unsaturated free fatty acids that cannot be transformed easily into a precursor for JA biosynthesis show the same priming effect on IE-induced defense responses, while treatment with a saturated fatty acid did not. Therefore, other forms of modulations of signaling pathways by unsaturated free fatty acids, in particular those affecting long distance signaling and resulting in enhanced defense responses must therefore be considered. The elucidation of these pathways is likely to be key for our understanding of how plants prime their defense responses.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Li T, Cofer T, Engelberth MJ, Engelberth J. Defense priming and jasmonates: a role for free fatty acids in insect elicitor-induced long distance signaling. Plants 2016; 5(1):5; PMID:27135225; http://dx.doi.org/ 10.3390/plants5010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huot B, Yao J, Montgomery BL, He SY. Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Molecular Plant 2014; 7:1267-87; PMID:24777989; http://dx.doi.org/ 10.1093/mp/ssu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrath U, Beckers G, Flor V, Garcia-Agustin P, Jakab G, Mauch F, Newman M, Pieterse C, Poinssot B, Pozo M, Pugin A et al.. Priming: getting ready for battle. Mol Plant-Microbe Interact 2006; 19:1062-71; PMID:17022170; http://dx.doi.org/ 10.1094/MPMI-19-1062 [DOI] [PubMed] [Google Scholar]
- 4.Baldwin I. Plant volatiles. Curr Biol 2010; 20:R392-R397; PMID:20462477; http://dx.doi.org/ 10.1016/j.cub.2010.02.052 [DOI] [PubMed] [Google Scholar]
- 5.Mastui K, Koeduka T. Green leaf volatiles in plant signaling and response. Subcelluar Chem 2016; 86:427-43; PMID:27023245; http://dx.doi.org/14749516 10.1007/978-3-319-25979-6_17 [DOI] [PubMed] [Google Scholar]
- 6.Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci 2004; 101:1781-5; PMID:14749516; http://dx.doi.org/ 10.1073/pnas.0308037100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytologist 2008; 180:722-34; PMID:18721163; http://dx.doi.org/ 10.1111/j.1469-8137.2008.02599.x [DOI] [PubMed] [Google Scholar]
- 8.Gomi K, Satoh M, Ozawa R, Shinonaga Y, Sanada S, Sasaki K, Matsumura M, Ohashi Y, Kanno H, Akimitsu K, Takabayashi J. Role of hydroperoxide lyase in white-backed planthopper (Sogatella furcifera Horváth)-induced resistance to bacterial blight in rice, Oryza sativa L. Plant J 2010; 61:46-57; PMID:19891707; http://dx.doi.org/ 10.1111/j.1365-313X.2009.04031.x [DOI] [PubMed] [Google Scholar]
- 9.Ameye M, Audenaert K, Zutter ND, Steppe K, Meulebroek LV, Vanhaecke L, Vleesschauwer DD, Haesaert G, Smagghe G. Priming of wheat with the green leaf volatile Z-3-hexenyl acetate enhances defense against Fusarium graminearum but boots deoxynivalenol production. Plant Physiol 2015; 167:1671-84; PMID:25713338; http://dx.doi.org/ 10.1104/pp.15.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirao T, Okazawa A, Harada K, Kobayashi A, Muranaka T, Hirata K. Green leaf volatiles enhance methyl jasmonate response in Arabidopsis. J Biosci Bioeng 2012; 114:540-5; PMID:22795666; http://dx.doi.org/ 10.1016/j.jbiosc.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 11.Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 2007; 100:681-97; PMID:17513307; http://dx.doi.org/ 10.1093/aob/mcm079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koiwa H, Bressan RA, Hasegawa PM. Regulation of protease inhibitors and plant defense. Trends Plant Sci 1997; 2:379-84. http://dx.doi.org/ 10.1016/S1360-1385(97)90052-2 [DOI] [Google Scholar]
- 13.Paré PW, Tumlinson JH. Plant volatiles as a defense against insect herbivores. Plant Physiol 1999; 121-325-332; PMID:10517823; http://dx.doi.org/12569409 10.1104/pp.121.2.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmelz EA, Alborn HT, Banchio E, Tumlinson JH. Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 2003; 216:665-73; PMID:12569409; http://dx.doi.org/ 10.1007/s00425-002-0898-y [DOI] [PubMed] [Google Scholar]
- 15.Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the “cry for help”. Trends Plant Sci 2010; 15:167-75; PMID:20047849; http://dx.doi.org/ 10.1016/j.tplants.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 16.Thaler J. Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 1999; 399:686-8; http://dx.doi.org/ 10.1038/21420 [DOI] [Google Scholar]
- 17.Koh E-J, Zhou L, Williams DS, Park J, Ding N, Duan Y-P, Kang B-H. Callose deposition in the phloem plasmodesmata and inhibition of phloem transport in citrus leaves infected with Candidatus Liberibacter asiaticus. Protoplasma 2012; 249:687-97; PMID:21874517; http://dx.doi.org/ 10.1007/s00709-011-0312-3 [DOI] [PubMed] [Google Scholar]
- 18.Lee Y, Lee HJ, Crain RC, Lee A, Korn SJ. Polyunsaturated fatty acids modulate stomatal aperture and two distinct K+ channel currents in guard cells. Cell Signal 1994; 6:181-6; PMID:8086281; http://dx.doi.org/ 10.1016/0898-6568(94)90042-6 [DOI] [PubMed] [Google Scholar]