Abstract
Background
Overnutrition of saturated fats and fructose is one of the major factors for the development of nonalcoholic fatty liver disease. Because omega-3 polyunsaturated fatty acids (n-3fa) have established lipid lowering properties, we tested the hypothesis that n-3fa prevents high-fat and fructose-induced fatty liver disease in mice.
Methods
Male C57BL/6J mice were randomly assigned to one of the following diet groups for 14 weeks: normal diet (ND), high-fat lard-based diet (HFD), HFD with fructose (HFD + Fru), high-fat fish-oil diet (FOD), or FOD + Fru.
Results
Despite for the development of obesity and insulin resistance, FOD had 65.3% lower (P < 0.001) hepatic triglyceride levels than HFD + Fru, which was blunted to a 38.5% difference (P = 0.173) in FOD + Fru. The lower hepatic triglyceride levels were associated with a lower expression of lipogenic genes LXRα and FASN, as well as the expression of genes associated with fatty acid uptake and triglyceride synthesis, CD36 and SCD1, respectively. Conversely, the blunted hypotriglyceride effect of FOD + Fru was associated with a higher expression of CD36 and SCD1.
Conclusions
During overnutrition, a diet rich in n-3fa may prevent the severity of hepatic steatosis; however, when juxtaposed with a diet high in fructose, the deleterious effects of overnutrition blunted the hypolipidemic effects of n-3fa.
Abbreviations: ACC1, acetyl-CoA carboxylase-1; CPT1a, carnitine palmitoyltransferase 1a; ChREBP, carbohydrate response element binding protein; FASN, fatty acid synthase; FFA, free fatty acid; LPL, lipoprotein lipase; LXRα, liver-X-receptor; MTTP, microsomal triglyceride transfer protein; n-3fa, omega-3 polyunsaturated fatty acids; NAFLD, nonalcoholic fatty liver disease; PPARα, peroxisome proliferator activated receptor α; PPARγ, peroxisome proliferator activated receptor γ; SCD1, stearoyl-CoA desaturase 1; SREBP1c, sterol response element binding protein; T2DM, type 2 diabetes mellitus; TRL, triglyceride-rich lipoproteins; VLDL, very low-density lipoprotein
Keywords: lipotoxicity, lipid metabolism, overnutrition, fructose, omega-3 polyunsaturated fatty acids
For the past decade, the growing prevalence of overweight and obesity is a major global health problem responsible for the rapid rise in the incidence of nonalcoholic fatty liver disease (NAFLD).1 NAFLD is a multifactorial progressive liver disorder that is the result of hepatic insulin resistance combined with increased fatty acid uptake and de novo lipogenesis.2 NAFLD is quickly becoming one of the most common liver diseases present in 20–30% of the Western population and increasing up to approximately 90% in morbidly obese individuals. The prevalence of NAFLD parallels the increase in obesity and type 2 diabetes mellitus (T2DM) with hepatic steatosis present in approximately 70% of patients with T2DM.3, 4
Overnutrition, defined as the chronic consumption of a calories consisting of 40–60% fat and 15–20% fructose to mimic the prevailing diet in the western society,5 is one of the major contributing factors for the increased incidence of obesity and related comorbidities. The American Heart Association's dietary guidelines recommend the consumption of a low saturated fat/high complex carbohydrate diet for weight loss and to reduce the risk for coronary heart disease.6 In contrast to these recommendations, during the past 40 years, the consumption of fructose and rapidly absorbed carbohydrates has increased in most industrialized countries.7 Moreover, low-fat diets that are high in refined carbohydrates have been tied to dyslipidemia, insulin resistance, and hypertension in comparison to high-fat diets that are low in refined carbohydrates or high in complex carbohydrates.8, 9
The use of omega-3 polyunsaturated fatty acids (n-3fa), such as docosahexaenoic acid (DHA; C22:6) and eicosapentaenoic acid (EPA; C20:5), may provide health professionals a nonpharmacological therapeutic strategy for the treatment of NAFLD. EPA and DHA are commonly consumed through either diet (e.g., fatty fish) or marine and synthetic-based supplements.10 The American Heart Association recommends the consumption of an average of 1 g/day of EPA/DHA or two servings of fatty fish weekly.11 In contrast, the World Health Organization recommends an intake of about 400–1000 mg/day.12 A definitive therapeutic dosage of n-3fa for the treatment of NAFLD remains controversial, in part because an unclear understanding of the bioactive properties of n-3fa during overnutrition and its interaction with other bioactive nutrients.
The primary treatment strategy for patients with NAFLD is lifestyle modification with an emphasis on weight loss, increased physical activity, and dietary modifications. Diet appears to be central to development of obesity-induced hepatic steatosis; however, the causal relationship remains to be elucidated. Presently, there are no specific dietary guidelines for the treatment of NAFLD due to an incomplete understanding of the pathophysiology of this condition; however, the development of safe, nontoxic therapeutic options is needed to reverse the metabolic disturbances observed in NAFLD.10 We hypothesized that a high-fat fish-oil diet will prevent hepatic steatosis induced by overnutrition of a diet high in fat and fructose. To test our hypotheses, we examined the contrasting dietary effects of fish oils and fructose on hepatic steatosis by examining the expression of hepatic genes that regulate lipid metabolism. Our specific aims for this project were to determine the therapeutic potential of fish oils for the treatment of NAFLD during continued overnutrition, as well as to develop a better understanding of the bioactive properties of fish oils and fructose when combined on genes central to development of NALFD during an overnutrition state.
Materials and Methods
Experimental Animals and Protocols
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Southern Illinois University Edwardsville. All animal experimentations were conducted in accordance with accepted standards of humane use and care of laboratory animals for biomedical research published by the National Institutes of Health (No. 85-23, Revised 1996). Male C57BL/6J mice were acquired from Jackson Laboratory (Bar Harbor, ME) at 6 weeks of age and maintained at a 12-h light:12-h dark cycle. Animals (n = 2–3/cage) were housed in an individually ventilated cage system and fed ad libitum on a standard low-fat diet (D12450B) until 8 weeks of age. Research Diets, Inc. (New Brunswick, NJ) formulated all diets for this study.
At 8 weeks of age, mice were randomly placed in one of the three dietary treatment groups for 14 weeks. Diet formulations are shown in Table 1. The following are the three diets: a normal diet (D12450B, 10% of fat calories from lard and soybean oil; ND; n = 8), a high-fat diet (D12492, 60% of fat calories from lard and soybean oil; HFD; n = 10), and a high-fat Menhaden fish-oil diet (D09020505, 60% of fat calories at a ratio of 63.0:27.7:9.3 from Menhaden fish oil:lard:soybean oil; FOD; n = 8). FOD was composed of approximately 22.4% n-3 polyunsaturated fatty acids. The fatty acid composition of each diet is shown in Table 2. To determine the effects of fructose on both high-fat diets, mice (n = 8 per group) were randomly assigned to either a HFD or FOD diet with a 20% w/v fructose (Fru) solution (Alfa Aesar, Ward Hill, MA), whereas the remaining mice were given regular drinking water. The ND, and HFD and HFD + Fru groups served as negative and positive controls, respectively. The energy content of the ND was 3.85 kcal/g, whereas the HFD and FOD diets were matched at 5.24 kcal/g. The energy content of the high-fructose drinking solution was 0.8 kcal/ml. Fructose in solution was used in this study due to evidence that most adults aged 6–50 years consume the majority of fructose in sugar-sweetened nonalcoholic beverages (e.g., soft drinks).7 Fructose solutions, ranging between 10% and 30%, have been commonly used in rodent models to induce hepatic steatosis with lower concentrations showing marginal changes in hepatic lipid content.13, 14, 15 Hence, we choose a 20% fructose solution with the intent to best replicate fructose consumption in humans who consume diets that result in metabolic diseases.14, 16
Table 1.
Diet Ingredients and Relative Composition of the Low-fat and High-fat Diets.
| Variable | ND |
HFD |
FOD |
|||
|---|---|---|---|---|---|---|
| Grams | % kcal | Grams | % kcal | Grams | % kcal | |
| Kcal/g | 3.85 | 5.24 | 5.24 | |||
| Protein | 20.0 | 20.0 | 20.0 | |||
| Casein, 30 Mesh | 200 | 98.5 | 200 | 98.5 | 200 | 98.5 |
| l-Cystine | 3 | 1.5 | 3 | 1.5 | 3 | 1.5 |
| Carbohydrate | 70.0 | 20.0 | 20.0 | |||
| Corn starch | 315 | 45.0 | 0 | 0 | 0 | 0 |
| Maltodextrin | 35 | 5.0 | 125 | 64.5 | 125 | 64.5 |
| Sucrose | 350 | 50.0 | 68.8 | 35.5 | 68.8 | 35.5 |
| Fat | 10.0 | 60.0 | 60.0 | |||
| Soybean oil | 25 | 55.6 | 25 | 9.3 | 25 | 9.3 |
| Lard | 20 | 44.4 | 245 | 90.7 | 75 | 27.7 |
| Menhaden oil | 0 | 0 | 0 | 0 | 170 | 63.0 |
| Saturated | 22.7 | 32.1 | 30.7 | |||
| Monounsaturated | 29.9 | 35.9 | 29.4 | |||
| Polyunsaturated | 47.4 | 32.0 | 45.2 | |||
| Mineral Mix S10026 | 10 | 0 | 10 | 0 | 10 | 0 |
| Dicalcium phosphate | 13 | 0 | 13 | 0 | 13 | 0 |
| Calcium carbonate | 5.5 | 0 | 5.5 | 0 | 5.5 | 0 |
| Potassium citrate | 16.5 | 0 | 16.5 | 0 | 16.5 | 0 |
| Vitamin mix V10001 | 10 | <1% | 10 | <1% | 10 | <1% |
| Choline bitartrate | 2 | 0 | 2 | 0 | 2 | 0 |
Note. Macronutrient composition of diets as provided by the manufacturer. ND, normal diet; HFD, high-fat diet; FOD, high-fat Menhaden fish-oil diet.
Table 2.
Fatty Acid Composition of Low-fat and High-fat Diets.
| Fatty acid, % weight | ND | HFD | FOD |
|---|---|---|---|
| 14:0 (Myristic) | 0.5 | 1.1 | 5.8 |
| 16:0 (Palmitic) | 14.9 | 19.6 | 18.2 |
| 16:1 (Palmitoleic) | 0.6 | 1.3 | 7.6 |
| 18:0 (Stearic) | 7.1 | 10.6 | 5.6 |
| 18:1 (Oleic) | 28.8 | 34.0 | 20.3 |
| 18:2 (Linoleic) | 41.9 | 28.7 | 16.2 |
| 18:3 (α-Linolenic) | 5.0 | 2.0 | 3.5 |
| 18:4; n-3 (Stearidonic) | 0.0 | 0.0 | 4.3 |
| 20:0 (Arachidic) | 0.0 | 0.2 | 0.2 |
| 20:1 (Eicosenoic) | 0.2 | 0.5 | 1.3 |
| 20:4 (Arachidonic) | <0.1 | <0.1 | 1.4 |
| 20:5; n-3 (Eicosapentaenoic) | 0.0 | 0.0 | 10.4 |
| 22:5; n-3 (Docosapentaenoic) | 0.0 | 0.0 | 0.1 |
| 22:6; n-3 (Docosahexaenoic) | 0.0 | 0.0 | 7.6 |
Fatty acid composition of diets as provided by the manufacturer. ND, normal diet; HFD, high-fat diet; FOD, high-fat Menhaden fish-oil diet.
Animal weights were recorded weekly, as well as the average fluid and food intake for each cage. Twelve hours prior to the collection of blood and tissue samples, food was removed and fresh drinking water was provided for each cage. At the end of the experiment, mice were euthanized by humane methods and exsanguinated by cardiac puncture. Collected whole blood was treated with EDTA, centrifuged at 1500 × g for 15 min, and plasma was collected and stored at −80 °C until analysis. Following the collection of blood, mice were perfused with ice-cold 0.9% (w/v) NaCl solution and the liver was removed, blotted dry, weighed, and frozen in liquid nitrogen.
Plasma Chemistry
Plasma total cholesterol, triglyceride, and glucose concentrations were measured enzymatically using colorimetric assay kits (Thermo Fisher Scientific, Middletown, VA). Plasma insulin concentrations were determined using an ELISA assay kit (Mercodia AB, Uppsala, Sweden). HOMA-IR was calculated from fasting glucose and insulin levels (fasting glucose * fasting insulin/22.5).
Analyses of Tissue Lipids
Hepatic lipid content was determined by using a modified Hara and Radin protocol.17 Triglyceride and cholesterol concentrations in lipid extracts were determined enzymatically with colorimetric assay kits (Thermo Fisher Scientific, Middletown, VA).
Quantitative Real-Time PCR
RNA was isolated using Trizol RNA Isolation Reagent (Life Technologies, Grand Island, NY) according to the manufacturer's protocol. RNA concentrations were quantified using the Qubit 2.0 Fluorometer (Life Technologies, Grand Island, NY). RNA samples were digested with DNase I (Life Technologies, Grand Island, NY) followed by reverse transcription of RNA to cDNA using the Applied Biosystems High Capacity Reverse Transcriptase kit (Life Technologies). Briefly, equal volumes of 2 μg RNA and 2× reverse transcriptase and random primers reaction mix were combined and placed in a Veriti Thermal Cycler (Applied Biosystems, Foster City, CA) under the following conditions: 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 5 min. Subsequently, real-time quantitative PCR (qPCR) was performed on the resulting cDNA in a final volume of 20 μL, which contained 50 ng of reverse-transcribed cDNA, 10 μL of 2× Kapa Probe Fast PCR Master Mix (Kapa Biosystems, Wilmington, MA), 4 μL of DEPC-treated dH2O, and 1 μL TaqMan assay primers predesigned by Life Technologies for the detection of carbohydrate response element binding protein (ChREBP, Mm02342723_m1), SREPB1c (Mm00550338_m1), liver-X-receptor (LXRα, Rn00581185_m1), peroxisome proliferator activated receptor α (PPARα, Mm00440939_m1), PPARγ (Mm01184322_m1), carnitine palmitoyltransferase 1a (CPT1a, Mm01231183_m1), FASN (Mm00662319_m1), SCD1 (Mm00772290_m1), ACC1 (Mm01304257_m1), cluster of differentiation 36 (CD36, Mm01135198_m1), microsomal triglyceride transfer protein (MTTP, Mm00435015_m1), lipoprotein lipase (LPL, Mm00434764_m1) genes, and β-actin (Mm00607939_s1) serving as the reference housekeeping gene. The reaction conditions were 95 °C for 20 s, followed by 40 cycles of 95 °C for 3 s and 60 °C for 30 s. The mRNA expression for each gene and sample was calculated using the recommended ΔΔCt method, in which the ND group served as the referent group. All reactions were carried out in duplicate in 96-well plates using the 7500 fast real-time PCR system (Applied Biosystems, Foster City, CA).
Statistical Analyses
Tests for normality, homogeneity, and sphericity were performed to determine data quality. In cases of nonsphericity, corrected F-values from the Greenhouse–Geisser test were used. Descriptive data are presented as mean ± standard errors (SEM). Differences between treatment groups were identified by one-way analysis of variance (ANOVA). Pairwise differences were located using Tukey's post hoc test. All analyses were performed using SPSS v21.0 software package (IBM Corp., Armonk, NY). Statistical significance was set at P < 0.05.
Results
Body Weight and Plasma Markers
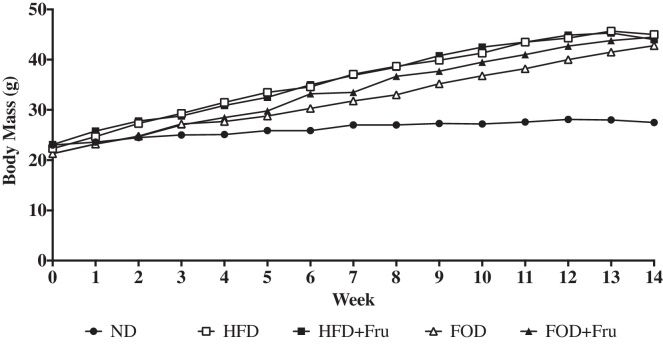
Mean body mass changes for mice from each dietary treatment group over the 14-week intervention are shown in Figure 1. At the end of 4-weeks, HFD, HFD + Fru, FOD, and FOD + Fru were significantly (P < 0.05) heavier than the ND group (Table 3). No significant differences in body mass were observed between the mice that fed the different types of high-fat diets independent of fructose consumption. Additionally, the liver mass of the mice fed HFD, HFD + Fru, and FOD + Fru were significantly (P < 0.05) greater than ND; however, no significant difference (P = 0.099) was observed between ND and FOD.
Figure 1.
Mean body mass changes for mice from each dietary treatment group. At the conclusion of the 14-week dietary intervention, HFD, HFD + Fru, FOD, and FOD + Fru were on average 60% heavier than the ND group.
Table 3.
Total Body and Tissue Mass Following 14 Weeks of Diet Treatments.
| Variable | ND | HFD | HFD + Fru | FOD | FOD + Fru |
|---|---|---|---|---|---|
| Body mass (g) | 27.5 ± 0.6 | 45.0 ± 1.1* | 44.0 ± 1.5* | 42.8 ± 1.2* | 44.5 ± 1.9* |
| Liver mass (g) | 1.2 ± 0.1 | 2.2 ± 0.1* | 2.1 ± 0.2* | 1.8 ± 0.2 | 2.5 ± 0.3* |
Data are presented as mean ± SEM. Normal diet and regular drinking water (ND; n = 8); high-fat diet and regular drinking water (HFD; n = 10); HFD and high-fructose solution (HFD + Fru; n = 8); high-fat Menhaden fish-oil diet and regular drinking water (FOD; n = 8); high-fat Menhaden fish-oil diet and high-fructose solution (FOD + Fru; n = 8).
P < 0.05 compared to ND.
As expected, the caloric consumption in the high-fat diet groups with and without fructose consumption was significantly (P < 0.05) greater that ND (Table 4). When examining differences between mice in the high-fat diet groups, no difference in caloric consumption was observed between HFD and FOD. In contrast, the addition of the fructose solution to HFD resulted in a caloric intake that was 16.6%, 15.8%, and 10.4% greater than the HFD, FOD, and FOD + Fru groups, respectively. Interestingly, the fructose consumption was not different between the HFD + Fru and FOD + Fru groups; however, the total caloric consumption was significantly different between these two groups (P = 0.049).
Table 4.
Dietary Consumption by Mice Following 14 Weeks of Treatment.
| Variable | ND | HFD | HFD + Fru | FOD | FOD + Fru |
|---|---|---|---|---|---|
| Total kcal (kcal/wk) | 65.7 ± 3.8 | 86.0 ± 3.8* | 103.1 ± 2.1*,† | 86.8 ± 0.8*,‡ | 92.4 ± 5.2*,‡ |
| Fructose solution (kcal/wk) | – | – | 24.1 ± 1.1 | – | 21.0 ± 1.9 |
Data are presented as mean ± SEM. Normal diet and regular drinking water (ND; n = 8); high-fat diet and regular drinking water (HFD; n = 10); HFD and high-fructose solution (HFD + Fru; n = 8); high-fat Menhaden fish-oil diet and regular drinking water (FOD; n = 8); high-fat Menhaden fish-oil diet and high-fructose solution (FOD + Fru; n = 8).
P < 0.05 compared to ND.
P < 0.05 compared to HFD.
P < 0.05 compared to HFD + Fru.
Plasma and hepatic characteristics following the 14-week dietary intervention are shown in Table 5. When examining differences between groups for fasting plasma glucose concentrations, the HFD group had significantly (P < 0.05) higher fasting plasma glucose concentrations when compared to HFD + Fru and FOD; however, the difference between HFD and FOD + Fru did not reach the level of statistical significance (P = 0.398). Interestingly, no differences in plasma glucose concentrations were observed between the ND, HFD + Fru, and FOD groups. Fasting plasma insulin concentrations were significantly (P < 0.05) elevated in mice on the high-fat diets independent of fructose consumption when compared to the ND. As expected, no significant differences were observed between high-fat diet groups. Using HOMA-IR as a measure of insulin resistance, HFD, HFD + Fru, and FOD + Fru groups reported significantly (P < 0.05) elevated basal measures of insulin resistance when compared to the ND group. Interestingly, FOD displayed lower HOMA-IR than the other high-fat groups such that it was not significantly different than ND (P = 0.073) or high-fat diet groups.
Table 5.
Plasma and Hepatic Characteristics Following 14 Weeks of Diet.
| Variable | ND | HFD | HFD + Fru | FOD | FOD + Fru |
|---|---|---|---|---|---|
| Plasma | |||||
| Glucose (mg/dL) | 158.2 ± 24.3 | 367.9 ± 38.9* | 241.9 ± 32.2† | 249.7 ± 10.1† | 281.2 ± 15.2* |
| Insulin (μg/L) | 0.61 ± 0.12 | 1.71 ± 0.26* | 2.14 ± 0.31* | 1.99 ± 0.57* | 2.46 ± 0.66* |
| HOMA-IR | 5.9 ± 1.3 | 41.5 ± 10.6* | 34.5 ± 8.0* | 30.7 ± 9.5 | 41.8 ± 11.3* |
| Tg (mg/dL) | 103.8 ± 5.9 | 82.5 ± 15.8 | 142.3 ± 15.0*,† | 134.2 ± 9.7*,† | 142.8 ± 9.4*,† |
| TC (mg/dL) | 131.3 ± 6.4 | 147.1 ± 10.7 | 193.2 ± 10.9* | 146.4 ± 12.3 | 228.7 ± 12.8*,†,‡,§ |
| Liver | |||||
| Total lipid (%) | 4.5 ± 0.3 | 9.4 ± 1.8* | 10.7 ± 1.3* | 5.3 ± 1.2‡ | 9.8 ± 2.8* |
| Triglyceride (μg/mg tissue) | 10.5 ± 1.6 | 31.9 ± 9.6* | 69.4 ± 5.7*,† | 24.1 ± 3.6‡ | 39.2 ± 10.4*,‡ |
| Cholesterol (μg/mg tissue) | 10.4 ± 0.9 | 2.7 ± 0.2* | 8.0 ± 0.6*,† | 10.2 ± 0.7†,‡ | 12.9 ± 0.8*,†,‡,§ |
| Glycogen (μg/mg tissue) | 2.6 ± 0.6 | 4.9 ± 0.7 | 4.5 ± 1.1 | 12.4 ± 2.9*,†,‡ | 7.4 ± 2.9*,§ |
Data are presented as mean ± SEM. Normal diet and regular drinking water (ND; n = 8); high-fat diet and regular drinking water (HFD; n = 10); HFD and high-fructose solution (HFD + Fru; n = 8); high-fat Menhaden fish-oil diet and regular drinking water (FOD; n = 8); high-fat Menhaden fish-oil diet and high-fructose solution (FOD + Fru; n = 8). Tg, plasma triglyceride; TC, total plasma cholesterol.
P < 0.05 compared to ND.
P < 0.05 compared to HFD.
P < 0.05 compared to HFD + Fru.
P < 0.05 compared to FOD.
Fasting plasma triglyceride and total cholesterol concentrations were not significantly different between the ND and HFD groups; however, HFD + Fru showed significantly (P < 0.01) greater plasma triglyceride and total cholesterol when compared to the ND and HFD groups. Plasma triglyceride concentrations in the high-fat FOD and FOD + Fru were also significantly (P < 0.05) greater than the ND and HFD groups, but did not differ from HFD + Fru. In contrast, plasma total cholesterol in FOD was not significantly different than ND and HFD, but was significantly different than both HFD + Fru (P = 0.006) and FOD + Fru (P < 0.001). Interestingly, the addition of fructose to HFD and FOD resulted in 23.9% (P = 0.002) and 36.0% (P < 0.001) greater plasma total cholesterol concentrations than the high fructose-free HFD and FOD groups, respectively.
Hepatic Lipids
Despite overnutrition and the presence of obesity and basal insulin resistance, total hepatic lipid in FOD was not statistically different than ND (P = 0.758); however, total hepatic lipid was 50.5% (P = 0.026) lower than the HFD + Fru group. Total hepatic lipid in FOD was 43.6% (P = 0.079) and 45.9% (P = 0.078) lower than HFD and FOD + Fru, respectively, but failed to meet the criteria for statistical significance. The lower total hepatic lipid in FOD was due largely in part to lower hepatic triglyceride levels. Similar to total hepatic lipid, hepatic triglyceride levels in FOD was 65.3% (P < 0.001) lower than the HFD + Fru group, but did not significantly differ than ND (P = 0.188). Despite that FOD was 24.4% (P = 0.444) and 38.5% (P = 0.173) lower than HFD and FOD + Fru, respectively, the differences did not meet the criteria for statistical significance. Interestingly, the addition of the fructose solution to the HFD and FOD diets resulted in a significant (P < 0.05) increase in hepatic cholesterol levels when compared to HFD and FOD with regular drinking water. No difference was observed between the ND and FOD groups; however, the addition of fructose to FOD resulted in hepatic cholesterol levels greater than all groups.
Hepatic Gene Expression of Markers for Lipid Metabolism
Following the 14-week dietary intervention, genes associated with de novo lipogenesis (Figure 2A) were reduced in the diets high in fish oils and fructose when compared to the ND group. No significant difference in the expression of either LXRα or SREBP-1c, nuclear transcription factors that regulate de novo lipogenesis, was observed between ND and HFD. Interestingly, the addition of fructose to HFD + Fru significantly reduced the expression of LXRα by 28.9% (P < 0.001). The addition of fructose to FOD + Fru also reduced the expression of LXRα by 22.7% (P = 0.070); however, the change did not reach the level of statistical significance. Similar trends in the data were observed with SREBP-1c where fructose consumption in HFD + Fru reduced its expression by 37.4% (P < 0.001) and in FOD + Fru by 21.4% (P = 0.143). The downregulation of LXRα and SREBP-1c likely explains the observed 51.4% (P = 0.004) lower expression of FASN in HFD + Fru when compared to HFD. The expression of ChREBP, a glucose-regulated transcription factor that regulates the expression of glycolytic and lipogenic enzymes, was significantly lower in HFD by 63.7% (P = 0.005) when compared to the ND group. Interestingly, the expression of ChREBP in the HFD + Fru, FOD, and FOD + Fru groups was significantly (P < 0.05) higher than HFD, but not differs than the ND group. Also, as observed with LXRα and SREBP-1c, the 31.6% (P = 0.131) lower expression in FASN when comparing FOD and FOD + Fru did not reach the level of statistical significance. The effect of fructose on the high-fat diets was not observed in the expression of ACC1, which reported no significant difference between the high-fat HFD and FOD diet groups independent of fructose consumption. In contrast, the expression of SCD1 was significantly upregulated following the addition of fructose to HFD by 1.4-fold (P = 0.047) and approached the criteria of significance in FOD with a 1.4-fold increase (P = 0.129).
Figure 2.
The expression of genes associated with proteins that regulate (A) de novo lipogenesis and (B) oxidation, uptake, and release of lipids following 14 weeks of dietary treatment. Data are presented as mean ± SEM. Normal diet (ND; n = 8) and high-fat diet (HFD; n = 10) with regular drinking water, HFD, and high-fructose solution (HFD + Fru; n = 8); high-fat Menhaden fish-oil diet and regular drinking water (FOD; n = 8); high-fat Menhaden fish-oil diet and high-fructose solution (FOD + Fru; n = 8). *P < 0.05 compared to ND; †P < 0.05 compared to HFD; ‡P < 0.05 compared to HFD + Fru; and §P < 0.05 compared to FOD.
The effects of the different dietary treatments on the expression of genes associated with proteins that regulate fatty acid oxidation, fatty acid uptake, and lipoprotein-mediated triglyceride/fatty acid uptake and release are shown in Figure 2B. Carnitine palmitoyl transferase 1a (CPT1a), a marker of fatty acid β-oxidation, and its regulatory protein PPARα were both significantly (P < 0.05) lower in the high-fat HFD and FOD diets independent of fructose consumption when compared to the ND group. A 30.9% (P = 0.025) increase in PPARα expression was observed in the FOD group, which was eliminated in the FOD + Fru group (P = 0.010) when compared to HFD. Despite the significant difference in PPARα expression between the high-fat HFD and FOD diet groups, the consumption of FOD did not have an effect on CPT1a expression. In contrast, PPARγ expression was significantly (P < 0.05) higher in the HFD + Fru, FOD, and FOD + Fru groups when compared to both the ND and HFD groups. Interestingly, we observed a higher expression of PPARγ within the different high-fat diet groups HFD + Fru and FOD + Fru when fructose consumption was added to the diet. Following addition of fructose to HFD + Fru, PPARγ was increased 1-fold (P = 0.002), whereas the addition of fructose to FOD + Fru resulted in a blunted increase of 29.7% (P = 0.169). A similar trend in the data was observed in CD36, associated with fatty acid uptake and regulated by PPARγ, where the addition of fructose to HFD + Fru and FOD + Fru resulted in a 1-fold (P = 0.041) and 2.1-fold (P = 0.160) increase in expression when compared to the HFD and FOD groups, respectively.
To evaluate the role of the liver in the uptake and release of lipid by lipoprotein, we evaluated the gene expression of MTTP and LPL. Despite differences between the high-fat diet groups for both plasma and hepatic lipids, no significant difference in the expression of MTTP was observed between HFD and FOD groups independent of fructose consumption. In contrast, the expression of LPL was responsive to fructose consumption in HFD + Fru and FOD + Fru showing a 1-fold (P = 0.001) and 63.6% (P = 0.018) increase when compared to HFD and FOD, respectively.
Discussion
For this study, our specific aims were to determine the effect of a high-fat fish-oil diet on the development of NAFLD during continued overnutrition, as well as to develop a better understanding of the bioactive properties of fish oils and fructose when combined on genes central to the development of NAFLD. Despite overnutrition and the development of obesity and insulin resistance, mice that fed FOD displayed total hepatic lipid levels that were similar to the ND group. Moreover, the lower total hepatic lipid levels in the FOD group were attributed to lower hepatic triglyceride levels. Based on the expression of hepatic genes, the reduction in hepatic triglycerides was due in part to the lower expression of lipogenic genes LXRα and FASN paired with the lower expression of genes that mediate fatty acid uptake and synthesis of triglyceride, CD36 and SCD1, respectively. Interestingly, fructose consumption in the FOD + Fru group resulted in a blunted effect of FOD on total hepatic lipids. The higher hepatic triglyceride in FOD + Fru was associated with a higher expression of genes associated with fatty acid uptake (PPARγ and CD36) and triglyceride synthesis (SCD1) paired with a reduced expression of MTTP, which mediates the synthesis and release of triglyceride-rich VLDL. In contrast, FOD provided no significant lowering effects on plasma triglyceride and cholesterol concentrations when compared to ND and HFD groups. Interestingly, fructose consumption in the HFD + Fru and FOD + Fru mice resulted in greater plasma and hepatic cholesterol levels than the other dietary treatment groups.
De novo lipogenesis is an integrated metabolic pathway comprised of the glycolytic conversion of glucose to acetyl-CoA, the synthesis of saturated fatty acids from malonyl-CoA followed by its desaturation, and the final formation of triglyceride.18 The first, primary rate-limiting enzyme of glycolysis is glucokinase, which catalyzes the first reaction in hepatic glucose disposal. Following glycolysis, acetyl-CoA is carboxylated to malonyl-CoA by ACC1 and the rise in malonyl-CoA promotes lipogenesis, esterification of fatty acyl-CoA, and inhibition of CPT1a reducing mitochondrial β-oxidation.19 De novo lipogenesis is regulated by the transcription factors SREBP1c and ChREBP. Lipogenic enzymes ACC1, FASN, and SCD1 are targets for the transcription factors SREBP1c and ChREBP.20, 21
SREBP1c, regulated and activated by LXRα,22, 23 has a central role in the regulation of lipogenic enzymes ACC1, FASN, and SCD1. Evidence suggests that LXRα can target PPARγ, ChREBP, and SCD1, all of which can contribute to pathogenesis of NAFLD.24, 25, 26, 27 In this study, the HFD group did not demonstrate a higher LXRα or SREP1c expression when compared to the ND group; however, the HFD + Fru group did demonstrate a 29–36% lower expression of both LXRα and SREBP1c when compared to the ND and HFD groups. It is hypothesized that the reduction in LXRα and SREBP1c expression was due to the addition of fructose to the HFD + Fru diet. A similar but smaller effect was noticed when comparing FOD + Fru to the FOD groups. Dong and colleagues28 showed reductions in LXRα, LXRβ, and retinoid-X-receptor β (RXRβ) following 4 weeks of a 60% high-fructose diet in male Syrian golden hamsters that were matched by a 60% increase in serum triglycerides and 27% higher hepatic triglycerides. The rise in serum and hepatic triglycerides was attributed to a reduction in long-chain acyl-CoA synthetase 3 (ACSL3) expression, an enzyme regulated by LXRα. This hypothesis can be supported by observations made by Bu and colleagues29 who selectively knocked down ACSL3 by approximately 70% using siRNA in male Sprague-Dawley rats, which reduced the transcriptional activities of ChREBP, SREBP1c, and LXRα and lipogenic and glycolytic genes ACCα/β, FASN, SCD1, and L-pyruvate kinase (L-PK). Moreover, the data by Bu and colleagues29 suggest that the decreased expression of these target genes might be due to a unique pool of fatty acids, acyl-CoAs, or metabolites created by ACSL3 that act as important signaling molecules to regulate hepatic lipogenesis. The role that PUFAs have on hepatic ACSL3 expression remains unclear; however, mice fed FOD without fructose in this study demonstrated a small recovery in SREBP1c expression, which were matched by a normalization of both plasma and hepatic triglyceride levels. A better understanding of the role of dietary PUFA on ACSL3 in models of NAFLD that incorporates a lipidomics approach is required to determine if the upregulation ACSL3 is a possible therapeutic target of interest.
The suppression of lipogenic enzymes ACC1, FASN, and SCD1 by PUFAs has been observed by several other studies30, 31, 32; however, this observation is often paired by a reduction in both the expression of LXRα and SREBP1c. In this study, we observed a small recovery in the expression of SREBP1c in the FOD group, but no changes were observed in the lipogenic genes ACC1, FASN, and SCD1. This observation is novel given that Xu and colleagues33 reported a suppression of SREBP1c ex vivo in primary rat hepatocytes supplemented with 150 μM of eicosapentaenoic acid (C20:5, n-3). It remains unclear as to why we observed a recovery of SREBP1c gene expression in FOD mice; however, it may be due to the fact that FOD was a mixed diet and not a pure source of n-3fa that was used by Xu and colleagues. Furthermore, Pawar and colleagues34 observed no significant suppression in LXRα and its regulation of transcripts involved in bile acid synthesis suggesting that PUFA-induced suppression of SREBP1c and its targeted lipogenic genes are independent of LXRα.
The n-3fa-induced reduction in the hepatic expression SREPB1c and lipogenic genes is often matched by an increased expression of both PPARα35, 36 and PPARγ.37, 38 In various murine models of NAFLD, PPARα expression is often reduced, whereas PPARγ expression is elevated.39 PPARα has broad regulatory roles associated with not only mitochondrial β-oxidation by upregulating CPT1a, but also may directly or indirectly regulate glucose metabolism39, 40 and reduce hepatic inflammation and fibrosis.41 Conversely, PPARγ promotes the uptake of free fatty acids through CD36. Normally the expression of CD36 is low in healthy livers,42 however, its expression has been observed to increase during NAFLD often paralleling the hepatic triglyceride content.43 In this study, we observed a significant suppression of the expression of PPARα in all high-fat dietary groups that were matched by a similar reduced expression of CPT1a when compared to the ND group. Conversely, PPARγ expression was significantly elevated in all high-fat dietary groups, except HFD, and matched by increased CD36 expression. Despite the elevated CD36 expression in the high-fat diets, the small reduction in CD36 expression in FOD likely played a part in the lower hepatic lipid and triglyceride levels. In contrast, mice fed the combined FOD + Fru diet showed greater CD36 expression than the ND and HFD diets, but did not meet the level of statistical significance when compared to FOD despite a 2.1-fold (P = 0.160) increase in expression. This dramatic increase in CD36 expression paired with increased expression of SCD1 best explains the reversal of the hypotriglyceridemic effect of FOD with FOD + Fru.
Increased VLDL secretion44, 45 and the development of an atherogenic profile characterized by an increase in circulating small, dense LDL and reduced plasma HDL-cholesterol concentrations45 are commonly observed in patients with NAFLD and insulin resistance. Interestingly as NAFLD progresses to NASH, VLDL synthesis and secretion becomes impaired, exemplified by the reduced expression of MTTP accelerating the development of steatosis.46 MTTP is essential for the synthesis of VLDL in the liver. In this study, MTTP was significantly reduced in all of the high-fat diet groups compared to the ND group. Based on this observation, the lower expression of MTTP and higher expression of CD36 and SCD1 may have played a synergistic role in increasing hepatic triglyceride levels in the HFD + Fru and FOD + Fru diets.
There are several limitations to the study. First, insulin resistance was characterized by using HOMA-IR versus glucose and insulin tolerance tests. Using HOMA-IR does not fully demonstrate the complete diet-induced physiologically dysfunction; however, given that that HOMA-IR was approximately 5–7-fold greater in the high-fat fed mice, it is likely that they were insulin resistant. Second, the use of only male mice was another limitation of this study; however, we choose to use only male mice due to the identification of sex-specific differences in the development of NAFLD.47 Finally, conclusions were drawn from only gene expression profiles of the mice following the 14-week dietary interventions. It is important that future investigations include loss of function experiments, specifically related to CD36; however, these types of experiments were beyond the aims of this study. Despite these limitations, the study provides several different lines of evidence that during a positive caloric dietary state, fish oils can have significant hypolipidemic effects, although the bioactive properties of a high-fructose solution appear to diminish these benefits through increased fatty acid uptake and triglyceride synthesis.
Conclusions
In conclusion, the 14 weeks of a high-fat fish-oil diet reduced total hepatic lipid and triglyceride levels despite the concomitant development of obesity and insulin resistance. The reduction in hepatic triglyceride may be due in part to the lower expression of CD36 and SCD1 paired with a reduced de novo lipogenesis as observed with lower expression of LXRα and FASN. In contrast, supplementing fructose with FOD + Fru blunted the reduction in hepatic triglycerides, which may be due in part to the higher expression of CD36 and SCD1. This study supports the use of fish oils for the treatment of NAFLD; however, the continued consumption of dietary sources high in fructose may impair the benefits derived from fish oils and n-3fa.
Author Contributions
JSW, KBS and TNN designed the research; JSW, TNN, AS, KEP, KBS performed the research and analyzed the data; JSW and TNN wrote the paper; and AS, KEP and KBS critically revised the paper.
Conflicts of Interest
The authors have none to declare.
Acknowledgments
This study was supported by the Seed Grants for Translational and Exploratory Projects (J.S.W.) and the Undergraduate Research and Creative Arts Program (K.E.P., A.S., T.N.N., and K.B.S.) at Southern Illinois University Edwardsville, Edwardsville, IL.
References
- 1.Tarantino G., Scopacasa F., Colao A. Serum Bcl-2 concentrations in overweight-obese subjects with nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17:5280–5288. doi: 10.3748/wjg.v17.i48.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravussin E., Smith S.R. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2002;967:363–378. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 3.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–1058. doi: 10.1016/s1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 4.de Alwis N.M., Day C.P. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48(suppl 1):S104–S112. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Whaley-Connell A., Sowers J.R. Indices of obesity and cardiometabolic risk. Hypertension. 2011;58:991–993. doi: 10.1161/HYPERTENSIONAHA.111.180406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckel R.H., Jakicic J.M., Ard J.D. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 7.Tappy L., Le K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 8.Appel L.J., Sacks F.M., Carey V.J. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer E.J., Gleason J.A., Dansinger M.L. The effects of low-fat, high-carbohydrate diets on plasma lipoproteins, weight loss, and heart disease risk reduction. Curr Atheroscler Rep. 2005;7:421–427. doi: 10.1007/s11883-005-0058-5. [DOI] [PubMed] [Google Scholar]
- 10.Bouzianas D.G., Bouziana S.D., Hatzitolios A.I. Potential treatment of human nonalcoholic fatty liver disease with long-chain omega-3 polyunsaturated fatty acids. Nutr Rev. 2013;71:753–771. doi: 10.1111/nure.12073. [DOI] [PubMed] [Google Scholar]
- 11.Kris-Etherton P.M., Harris W.S., Appel L.J., American Heart Association, Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser. 2003;916:i–viii. 1–149, backcover. [PubMed] [Google Scholar]
- 13.Spruss A., Kanuri G., Wagnerberger S., Haub S., Bischoff S.C., Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 14.Charlton M., Krishnan A., Viker K. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301:G825–G834. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basaranoglu M., Basaranoglu G., Sabuncu T., Senturk H. Fructose as a key player in the development of fatty liver disease. World J Gastroenterol. 2013;19:1166–1172. doi: 10.3748/wjg.v19.i8.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Buul V.J., Tappy L., Brouns F.J. Misconceptions about fructose-containing sugars and their role in the obesity epidemic. Nutr Res Rev. 2014;27:119–130. doi: 10.1017/S0954422414000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara A., Radin N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978;90:420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- 18.Towle H.C., Kaytor E.N., Shih H.M. Regulation of the expression of lipogenic enzyme genes by carbohydrate. Annu Rev Nutr. 1997;17:405–433. doi: 10.1146/annurev.nutr.17.1.405. [DOI] [PubMed] [Google Scholar]
- 19.McGarry J.D. Glucose-fatty acid interactions in health and disease. Am J Clin Nutr. 1998;67:500S–504S. doi: 10.1093/ajcn/67.3.500S. [DOI] [PubMed] [Google Scholar]
- 20.Stoeckman A.K., Towle H.C. The role of SREBP-1c in nutritional regulation of lipogenic enzyme gene expression. J Biol Chem. 2002;277:27029–27035. doi: 10.1074/jbc.M202638200. [DOI] [PubMed] [Google Scholar]
- 21.Uyeda K., Repa J.J. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Joseph S.B., Laffitte B.A., Patel P.H. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 23.Repa J.J., Liang G., Ou J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cha J.Y., Repa J.J. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–751. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 25.Chu K., Miyazaki M., Man W.C., Ntambi J.M., Stearoyl-coenzyme A. desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol. 2006;26:6786–6798. doi: 10.1128/MCB.00077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo J.B., Moon H.M., Kim W.S. Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol. 2004;24:3430–3444. doi: 10.1128/MCB.24.8.3430-3444.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Repa J.J., Gauthier K., Mangelsdorf D.J. Regulation of lipoprotein lipase by the oxysterol receptors, LXRalpha and LXRbeta. J Biol Chem. 2001;276:43018–43024. doi: 10.1074/jbc.M107823200. [DOI] [PubMed] [Google Scholar]
- 28.Dong B., Kan C.F., Singh A.B., Liu J. High-fructose diet downregulates long-chain acyl-CoA synthetase 3 expression in liver of hamsters via impairing LXR/RXR signaling pathway. J Lipid Res. 2013;54:1241–1254. doi: 10.1194/jlr.M032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bu S.Y., Mashek M.T., Mashek D.G. Suppression of long chain acyl-CoA synthetase 3 decreases hepatic de novo fatty acid synthesis through decreased transcriptional activity. J Biol Chem. 2009;284:30474–30483. doi: 10.1074/jbc.M109.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jump D.B., Botolin D., Wang Y., Xu J., Christian B., Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 31.Xu J., Christian B., Jump D.B. Regulation of rat hepatic L-pyruvate kinase promoter composition and activity by glucose, n-3 polyunsaturated fatty acids, and peroxisome proliferator-activated receptor-alpha agonist. J Biol Chem. 2006;281:18351–18362. doi: 10.1074/jbc.M601277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jump D.B. Fatty acid regulation of hepatic lipid metabolism. Curr Opin Clin Nutr Metab Care. 2011;14:115–120. doi: 10.1097/MCO.0b013e328342991c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J., Teran-Garcia M., Park J.H., Nakamura M.T., Clarke S.D. Polyunsaturated fatty acids suppress hepatic sterol regulatory element-binding protein-1 expression by accelerating transcript decay. J Biol Chem. 2001;276:9800–9807. doi: 10.1074/jbc.M008973200. [DOI] [PubMed] [Google Scholar]
- 34.Pawar A., Botolin D., Mangelsdorf D.J., Jump D.B. The role of liver X receptor-alpha in the fatty acid regulation of hepatic gene expression. J Biol Chem. 2003;278:40736–40743. doi: 10.1074/jbc.M307973200. [DOI] [PubMed] [Google Scholar]
- 35.Goto T., Kim Y.I., Takahashi N., Kawada T. Natural compounds regulate energy metabolism by the modulating the activity of lipid-sensing nuclear receptors. Mol Nutr Food Res. 2013;57:20–33. doi: 10.1002/mnfr.201200522. [DOI] [PubMed] [Google Scholar]
- 36.Videla L.A., Pettinelli P. Misregulation of PPAR functioning and its pathogenic consequences associated with nonalcoholic fatty liver disease in human obesity. PPAR Res. 2012;2012:107434. doi: 10.1155/2012/107434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Periz A., Planaguma A., Gronert K. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto K., Itoh T., Abe D. Identification of putative metabolites of docosahexaenoic acid as potent PPARgamma agonists and antidiabetic agents. Bioorg Med Chem Lett. 2005;15:517–522. doi: 10.1016/j.bmcl.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 39.Bandsma R.H., Van Dijk T.H., Harmsel At A. Hepatic de novo synthesis of glucose 6-phosphate is not affected in peroxisome proliferator-activated receptor alpha-deficient mice but is preferentially directed toward hepatic glycogen stores after a short term fast. J Biol Chem. 2004;279:8930–8937. doi: 10.1074/jbc.M310067200. [DOI] [PubMed] [Google Scholar]
- 40.Peeters A., Baes M. Role of PPARalpha in hepatic carbohydrate metabolism. PPAR Res. 2010;2010 doi: 10.1155/2010/572405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tailleux A., Wouters K., Staels B. Roles of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta. 2012;1821:809–818. doi: 10.1016/j.bbalip.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Inoue M., Ohtake T., Motomura W. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 2005;336:215–222. doi: 10.1016/j.bbrc.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 43.Buque X., Martinez M.J., Cano A. A subset of dysregulated metabolic and survival genes is associated with severity of hepatic steatosis in obese Zucker rats. J Lipid Res. 2010;51:500–513. doi: 10.1194/jlr.M001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bugianesi E., Gastaldelli A., Vanni E. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 45.Adiels M., Olofsson S.O., Taskinen M.R., Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 46.Raabe M., Veniant M.M., Sullivan M.A. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103:1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spruss A., Henkel J., Kanuri G. Female mice are more susceptible to nonalcoholic fatty liver disease: sex-specific regulation of the hepatic AMP-activated protein kinase-plasminogen activator inhibitor 1 cascade, but not the hepatic endotoxin response. Mol Med. 2012;18:1346–1355. doi: 10.2119/molmed.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]