Abstract
Background
Liver fibrosis and its sequel cirrhosis represent a major health care burden, and assessment of fibrosis by biopsy is gradually being replaced by noninvasive methods. In clinical practice, the determination of fibrosis stage is important, since patients with advanced fibrosis have faster progression to cirrhosis and antiviral therapy is indicated in these patients.
Aims
To assess the role of transient elastography (TE) and compare it with APRI and FIB4 for predicting liver fibrosis and assessing the effect of host and viral factors on fibrosis and treatment outcome in CHC patients.
Methods
In a retrospective analysis, 330 CHC patients underwent liver stiffness measurement (LSM) by TE and tests needed for calculating APRI and FIB4 scores at baseline. 228 patients received a combination of Pegylated IFN-based antiviral therapy and were analyzed for therapeutic response.
Results
The study included 330 patients (median age 39 years [range 18–67]), predominantly males (n = 227, 68.8%) with baseline LSMs. The median liver stiffness was 7.8 kPa (range 3.2–69.1 kPa). LSMs and its thresholds for severe fibrosis progression (≥9.5 kPa) and cirrhosis (≥12.5 kPa) were significantly higher in patients with age ≥40 years, diabetes mellitus, and patients with significant alcohol intake (P = 0.003 to P < 0.001). By taking TE as a reference, the diagnostic accuracy of FIB4 scores for predicting cirrhosis (AUROC 0.896) was good (+LR 13.4) compared to APRI (AUROC 0.823) with moderate likelihood ratio (+LR 6.9). Among 228 treated patients the SVR rate in genotype 3 was 70% versus 57.8% in genotype 1. Fibrosis score F4 (P = 0.023) and HCV genotype (P = 0.008) were independent predictors of SVR.
Conclusion
The study shows that LSM by TE and fibrosis assessment by FIB4/APRI scores can be used with fair reliability to predict fibrosis and treatment response in patients with CHC infection.
Abbreviations: LF, liver fibrosis; HCV, hepatitis C; TE, transient elastography; LSM, liver stiffness measurement; APRI, AST to Platelet ratio index; FIB4, fibrosis-4 score; kPa, kilopascals; IQR/M, interquartile range/median; LB, liver biopsy; CHB, chronic hepatitis B; CLD, chronic liver disease; PEG INF, Pegylated Interferon; RBV, Ribavarin; BMI, body mass index; RVR, rapid virological response; EVR, early virological response; ETR, end of treatment response; SVR, sustained virological response; RGT, response guided treatment; DM, diabetes mellitus; AST, aspartate transaminases; ALT, alanine transaminases; ROC, receiver operating characteristic; PPV, positive predictive value; NPV, negative predictive value
Keywords: noninvasive markers, transient elastography, chronic hepatitis C, liver fibrosis, liver biopsy
Liver fibrosis and its sequel cirrhosis represent a major health care burden.1 Progressive liver fibrosis is a characteristic feature of chronic liver diseases, and its implication is evolution toward cirrhosis, liver failure, and hepatocellular carcinoma with advancement of the primary disease with time.2 The major causes of liver fibrosis are chronic hepatitis C (CHC) and chronic hepatitis B (CHB), autoimmune liver disease, alcohol and nonalcoholic steatohepatitis.3 Approximately, 170 million people are infected with CHC worldwide and most of these patients generally show an asymptomatic onset and slow progression of fibrosis.4, 5 The mechanisms of fibrogenesis in CHC infection have not been explored in great detail; however, there is a possible direct profibrogenic and procarcinogenic mechanisms of certain HCV proteins.6 As the degree of fibrosis affects both prognosis and treatment, the prediction of fibrosis is critical for management decisions in patients with CHC.
Liver biopsy (LB) still remains the gold standard for the diagnosis of liver fibrosis; however, it is invasive and has a finite risk of major complications.7 Hence, many noninvasive tests for assessment of liver fibrosis have been proposed and have been used in the past.8 These tests rely on distinct but complementary approach and include a biologic method, which quantifies serum levels of biomarkers, and a physical method that measures liver stiffness by ultrasound or magnetic resonance imaging.9 Although no single noninvasive test or model developed to date can match the information obtained from actual histology, combination of two modalities can be used to reduce the need for liver biopsy. Liver stiffness measurement (LSM) by transient elastography (TE) is a new upcoming, noninvasive and attractive alternative for staging of fibrosis by noninvasive tests. Several studies have assessed the diagnostic performance of TE for significant fibrosis and cirrhosis in CHC patients and confirmed the excellent diagnostic performance for advanced fibrosis and cirrhosis.9 In light of its accuracy, simplicity and rapid results, TE has gained widespread use in many countries.10 APRI and FIB4 are other widely used first-line tests for the prediction of significant fibrosis and cirrhosis. There is a sparse literature on fibrosis assessment by noninvasive methods and prediction of response to Pegylated Interferon and Ribavarin in CHC from India. Hence this study was carried out to assess the role of TE and compare it with APRI and FIB4 to predict liver fibrosis and assess the effect of host and viral factors on fibrosis and treatment outcome in CHC patients.
Patients and Methods
Study Design and Assessments
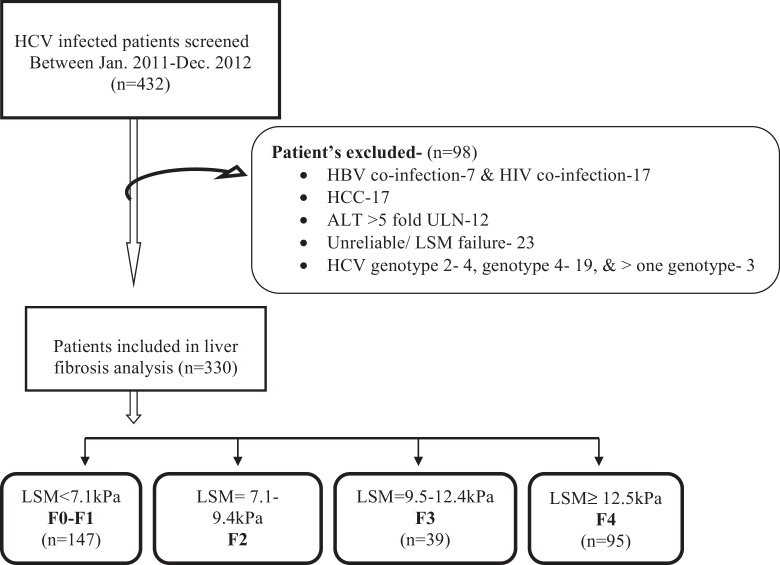
In a retrospective analysis from January 2011 to December 2012, a total of 432 patients with CHC were consecutively screened; in whom baseline LSM and APRI/FIB4 for liver fibrosis assessment was indicated. HCV infection was diagnosed by the presence of serum antibodies against HCV and detectable HCV RNA by quantitative real-time polymerase chain reaction (RT-PCR) assay (Roche Diagnostic), with a limit of detection of ≥15 IU/ml. The exclusion criteria used were: a co-infection with hepatitis B virus (n = 7) or human immunodeficiency virus (n = 17), Hepatocellular carcinoma (17), ALT flare [value five fold the upper limit normal (45 U/ml)] (n = 12), and a failed or unreliable LSM (n = 23). Four patients with genotype 2, nineteen patients with genotype 4 and three patients co-infected with more than one HCV genotype were also excluded because of small number for analysis. Therefore the total number of patients enrolled in the study was 330 as shown in flow Diagram 1.
The study was approved by institutional ethics committee for assessing the anonymous routine clinical data without written informed consent from patients. A detailed clinical history using a precoded questionnaire and biochemical parameters were taken from liver clinic file database. BMI was considered normal within range of 18.5–22.9, overweight from 23 to 24.9, and obese ≥25 according to Indian guidelines for obesity 11. Alcoholics were defined as those who were consuming ≥30 g of alcohol per day in the last year or more.
TE and Serum Biomarkers Assay
TE was carried out with a Fibroscan (Echosens, Paris, France), which provides a quantifiable estimate of liver stiffness in kilopascals (kPa). Measurements of liver stiffness was performed on the right lobe of liver through intercostal space while the patients was lying in the dorsal decubitus position with the right arm in maximum abduction. Ten successful measurements were performed on each patient and the median value was considered representative of elastic module of the liver. LSM was considered reliable when it included ≥10 valid measurements with success rate ≥60% and IQR/M < 0.3 as per usual definition.12 In most of studies in CHC, the proposed cut-off for cirrhosis ranged from 11.9 to 14.8 kPa. However a recent study by Bousier et al.12 have shown that cut off published by Castera et al.13 provided highest accuracy for significant fibrosis and LSM classification. So LSM were classified in the METAVIR system according to validated cutoffs published by Castera et al.13 for no or minimal fibrosis [F0–F1] < 7.1 kPa, moderate fibrosis [F2] = 7.1–9.4 kPa, severe fibrosis [F3] = 9.5–12.4 kPa, and for cirrhosis [F4], cutoff of ≥12.5 (Figure 1).
Figure 1.
Study enrollment and disposition of HCV infected patients.
Serum biomarker scores were calculated for APRI (AST to platelet ratio) and FIB4 by using standard formulae as described: APRI = AST (/ULN)/platelet (109/L) × 100 with AST (ULN) taken as 35 in our study population. FIB4 = age (years) × AST (U/L)/platelets (109/L) × ALT (U/L)1/2. The recommended cut-offs14 for significant fibrosis and cirrhosis (APRI—0.5 and 2, FIB4—1.25 and 3.25, respectively) were used to define the positive tests.
Treatment Outcomes
Amongst 330 CHC patients in whom LSM was assessed at baseline, 228 (69.1%) patients received Pegylated IFN-based antiviral therapy. Genotype 1 was present in 64 (28%) whereas 164 (72%) patients were having genotype 3. Antiviral therapy was prescribed as per the response guided therapy guidelines,15 and the patients were assessed on an outpatient basis as described in our previous study.16 Patients were evaluated for treatment response during therapy (e.g. rapid virological response [RVR], early virological response [EVR], and end of treatment response [ETR]) and sustained virological response [SVR] at 6 months after stopping therapy. Patients with undetectable HCV RNA at 4 and 12 weeks after the commencement of treatment were considered to have RVR and EVR respectively. As SVR was the primary outcome in this study, patients with relapse were considered along with nonresponders who were patients who experienced suboptimal virological response during therapy period.
Statistical Analysis
The statistical analysis was carried out using the Statistical Package for Social Sciences version 16 for Windows (SPSS Inc., Chicago, IL). Quantitative variables were expressed as medians (range). All quantitative variables were changed to qualitative trait for analysis to maintain the uniformity of Data. Qualitative or categorical variables were described as frequencies and proportions. Proportions were compared using the Chi-square or Fisher's exact test, whichever was applicable. Multiple logistic regression models were used to assess the relationship of fibrosis and with patient's age, gender, BMI, alcohol intake, DM, ALT, platelets, HCV RNA and genotype. In fibrosis model, the dependent variables were significant fibrosis progression, coded as 0 = F1 or 1 = ≥F2 in LSM; cirrhosis coded as 0 ≤ F4 or 1 = F4 in LSM. For assessment of SVR (dependent variable), we selected the same independent variables as included in fibrosis model and added fibrosis levels as an independent variable. Variables found to be associated with the dependent variable(s) at univariate analysis were included in all multivariate regression models. All statistical tests were two-sided and performed at a significance level of a = 0.05.
Receiver operating characteristic (ROC) curves were applied to identify the area under ROC curves of APRI and FIB4 to discriminate ≥F2 stage and cirrhosis (F4). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using cutoffs for significant fibrosis and cirrhosis.
Results
A total of 330 CHC patients were enrolled for fibrosis analysis in this study. The median age was 39 years (range 18–67) and male gender was predominant (n = 227, 68.8%). The median BMI was 23.14 kg/m2 (range 17.2–28.6). Overall, 161 (48.5%) had normal BMI, 63 (19.4%) were overweight, and 106 (32.1%) were obese. The history of alcohol consumption (≥30 g/day) was observed in 36 (10.9%) cases of male gender only. Diabetes was seen in 40 (12.1%) patients and past history of hypertension was seen in 69 (20.9%) patients. The predominant HCV genotype being HCV-3 (n = 214; 64.8%) followed by HCV-1 (n = 116; 35.2%). The baseline characteristics of patients are shown in Table 1,
Table 1.
Baseline Characteristics of 330 HCV Patients and LSM.
| Variable | Number (%) | LSM-Median (25–75th percentiles), (IQR/M) | P value |
|---|---|---|---|
| Age (range 18–67 years) | |||
| <40 | 168 (50.9) | 6.5 (5.10–9.20) (0.146 ± 0.066) | |
| ≥40 | 162 (49.1) | 11.75 (6.8–26.08) (0.153 ± 0.09) | <0.001 |
| Gender | |||
| Male | 227 (68.78) | 7.9 (6.1–16.6) (0.148 ± 0.073) | 0.180 |
| Female | 103 (31.21) | 7.3 (5.3–14.7) (0.153 ± 0.083) | |
| BMI, kg/m2 (range 17.2–28.6) | |||
| <23 | 161 (48.5) | 7.1 (5.6–12.4) (0.149 ± 0.069) | |
| ≥23 to <25 | 63 (19.4) | 8.5 (5.6–17.5) (0.138 ± 0.072) | 0.228 |
| 25 | 106 (32.1) | 8.8 (6.1–21.1) (0.157 ± 0.088) | |
| DM | 40 (12.1) | 17.5 (6.65–28.23) (0.154 ± 0.106) | <0.001 |
| HTN | 69 (20.9) | 8.5 (6.1–16.7) (0.153 ± 0.101) | 0.309 |
| Alcoholic (≥30 g/day) | 36 (10.9) | 14.05 (8.7–32.08) (0.152 ± 0.069) | <0.001 |
| HCV RNA | |||
| ≤600,000 IU/ml | 184 (55.8%) | 7.7 (5.6–20.2) (0.152 ± 0.080) | |
| >600,000 IU/ml | 146 (44.2%) | 7.85 (5.9–13.95) (0.147 ± 0.073) | 0.875 |
| HCV genotype | |||
| 1 | 116 (35.2%) | 7.1 (5.43–12.0) (0.151 ± 0.080) | 0.088 |
| 3 | 214 (64.8%) | 8.1 (6.0–19.80) (0.150 ± 0.075) | |
Risk Factors for Fibrosis
The median liver stiffness was 7.8 kPa (range 3.2–69.1 kPa) and IQR/M was 0.147 (range 0.009–0.310). On the basis of the validated cut offs by Castera et al.,13 95 (28.8%) patients had cirrhosis (≥12.5 kPa), 39 (11.8%) had severe fibrosis but no cirrhosis (9.5–12.4 kPa), 49 (14.8%) had moderate fibrosis (7.1–9.4 kPa) and no or minimal fibrosis (≤7 kPa) was seen in 147 (44.5%) patients. Table 2 displays the characteristics of patients according to the severity of liver fibrosis. Lack or minimal fibrosis (F0–F1) was predominant in younger patients (<40 years) where as advanced liver fibrosis (≥F3) and cirrhosis (F4) were significantly higher (P < 0.001) in those above 40 years. Higher grade of liver fibrosis was also seen in patients with diabetes mellitus, history of significant alcohol intake, male gender, higher BMI and high HCV RNA levels. The distinct viral genotypes did not have significant impact on liver stiffness. By multivariate analysis, age ≥ 40 years (OR 3.465, 95%Cl 2.116–5.674; P < 0.001), diabetes mellitus (OR 2.909, 95%Cl 1.329–6.367; P = 0.008) and alcohol (OR 3.345, 95%Cl 1.478–7.571; P = 0.004) were independently associated with advanced fibrosis. However, age ≥ 40 years (OR 5.597, 95%Cl 3.115–10.058; P < 0.001) and diabetes mellitus (OR 2.656, 95%Cl 1.269–5.563; P = 0.001) were associated with cirrhosis in this cohort.
Table 2.
Characteristics of Patients According to METAVIR System with Proposed Cutoffs by Castera et al.13
| Variable | Number (%) mild fibrosis F0–F1 | Moderate fibrosis F2 | Severe fibrosis F3 | Cirrhosis F4 | Significant fibrosis progression ≥F2 | Severe fibrosis progression ≥F3 | P value (univariate analysis) |
|---|---|---|---|---|---|---|---|
| Age | <0.001 | ||||||
| <40 | 102 (60.7) | 26 (15.5) | 21 (12.5) | 19 (11.3) | 66 (39.3) | 40 (23.8) | <0.001a |
| ≥40 | 45 (27.8) | 23 (14.2) | 18 (11.1) | 76 (46.9) | 117 (72.2) | 94 (58.0) | <0.001b |
| <0.001c | |||||||
| Gender | 0.466 | ||||||
| Male (227) | 98 (43.2) | 31 (13.7) | 30 (13.2) | 68 (30.0) | 129 (56.8) | 98 (43.2) | 0.456a |
| Female (103) | 49 (47.6) | 18 (17.5) | 9 (8.7) | 27 (26.2) | 54 (52.4) | 36 (35.0) | 0.159b |
| 0.487c | |||||||
| BMI, kg/m2 | 0.458 | ||||||
| <23 | 80 (49.7) | 25 (15.5) | 17 (10.6) | 39 (24.2) | 81 (50.3) | 56 (34.8) | 0.186a |
| ≥23 to < 25 | 25 (39.7) | 11 (17.5) | 8 (12.5) | 19 (30.2) | 38 (60.3) | 27 (42.9) | 0.087b |
| ≥25 | 42 (39.6) | 13 (12.3) | 14 (13.2) | 37 (34.9) | 64 (60.4) | 51 (48.1) | 0.163c |
| <0.001 | |||||||
| DM | 11 (27.5) | 0 (0) | 5 (12.5) | 24 (60.0) | 29 (72.5) | 29 (72.5) | 0.021a |
| <0.001b | |||||||
| <0.001c | |||||||
| 0.555 | |||||||
| HTN | 29 (42.0) | 8 (11.6) | 11 (15.9) | 21 (30.4) | 40 (58.0) | 32 (46.4) | 0.636a |
| 0.272b | |||||||
| 0.734c | |||||||
| Alcoholic | <0.001 | ||||||
| (≥30 g/day) | 5 (13.9) | 5 (13.9) | 8 (22.2) | 18 (50.0) | 31 (86.1) | 26 (72.2) | <0.001a |
| <0.001b | |||||||
| 0.003c | |||||||
| HCV genotype | 0.565 | ||||||
| 1 | 57 (49.1) | 18 (15.5) | 15 (12.9) | 26 (22.4) | 59 (50.9) | 41 (35.3) | 0.217a |
| 3 | 90 (42.1) | 31 (14.5) | 24 (11.2) | 69 (32.2) | 124 (57.9) | 93 (43.5) | 0.152b |
| 0.60c | |||||||
| HCV RNA | 0.540 | ||||||
| ≤600,000 IU/ml | 82 (44.6) | 27 (14.7) | 18 (9.8) | 57 (31.0) | 102 (55.4) | 75 (40.8) | 0.994a |
| >600,000 IU/ml | 65 (44.5) | 22 (15.1) | 21 (14.4) | 38 (26.0) | 81 (55.5) | 59 (40.4) | 0.949b |
| 0.324c |
P denotes comparisons between F0–F1 vs F2 vs F3 vs F4.
Comparisons between F0–F1 vs F2 + F3 + F4.
Comparisons between F0–F1 + F2 vs F3 + F4.
Comparisons between F0–F1 + F2 + F3 vs F4.
Comparative Diagnostic Accuracy of Biomarkers with TE
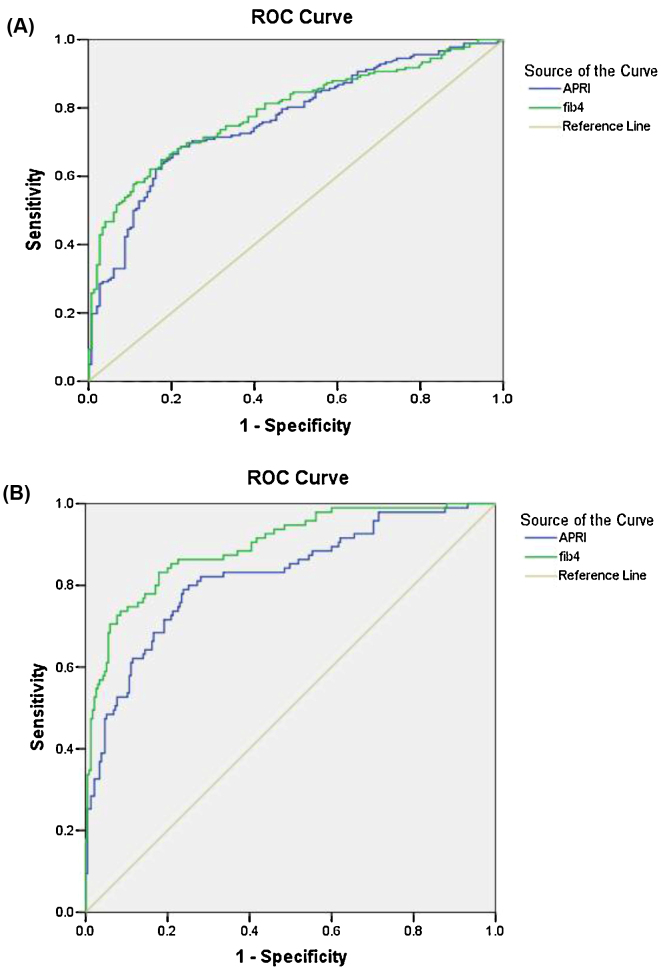
Since APRI and FIB4 are simple noninvasive tests with excellent diagnostic accuracy for significant and severe fibrosis, they were chosen for comparison with TE by using complex patented formulas with standard cut offs (APRI score—0.5 and 2, FIB4 score—1.25 and 3.25, respectively). Figure 2 shows ROCs curves for significant fibrosis (Figure 2A) and cirrhosis (Figure 2B) according to APRI versus FIB4 scores. The corresponding AUROC are, respectively, 0.77 and 0.79 for the diagnosis of significant fibrosis, as well as 0.82 and 0.90 for the diagnosis of cirrhosis. Corresponding values of sensitivity, specificity, positive predictive value (PPV), NPV, positive likelihood ratio (+LR), and negative likelihood ratio (−LR) are shown in Table 3. For prediction of significant fibrosis, APRI has shown higher sensitivity (91.2%) as compared to FIB4 (73.6%) but had lowered specificity (32.4% vs 68.3%). The likelihood ratio of APRI and FIB4 scores for predicting ≥F2 fibrosis was lower (1.2 and 2.3, respectively) thus both have poor predictive values. For the detection of cirrhosis, both APRI and FIB4 had low sensitivity (49.5% and 57.9% respectively) however had high specificity (92.8% and 95.7%, respectively). FIB4 has better likelihood ratio (+LR, 13.4) compared to APRI with moderate likelihood ratio (+LR, 6.9). In order to improve the diagnostic performance, biomarkers were also tested in combinations (APRI + FIB4). Combination of both further increases the usefulness of these biomarkers in diagnosing cirrhosis as shown in Table 3.
Figure 2.
(A) AUROC analysis for diagnosis of ≥F2 fibrosis using APRI and FIB4 scores. (B): AUROC analysis for the diagnosis of cirrhosis using APRI and FIB4 scores.
Table 3.
Diagnostic Performances of APRI and FIB4 Scores for Predicting Significant Fibrosis and Cirrhosis.
| Significant fibrosis (LSM ≥ F2) | |||||
|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | +LR/−LR | |
| FIB4 (cutoff 1.25) | 73.6 | 68.3 | 74.0 | 67.9 | 2.3/0.4 |
| APRI (cutoff 0.5) | 91.2 | 32.4 | 62.4 | 75.0 | 1.2/0.3 |
| Combination (FIB4 + APRI) | 73.0 | 70.2 | 75.2 | 68.0 | 2.5/0.4 |
| Advanced fibrosis/cirrhosis (LSM = F4) | |||||
| FIB4 (cutoff 3.25) | 57.9 | 95.7 | 84.6 | 84.9 | 13.4/0.4 |
| APRI (cutoff 2) | 49.5 | 92.8 | 73.4 | 81.9 | 6.9/0.5 |
| Combination (FIB4 + APRI) | 48.4 | 96.6 | 85.2 | 85.4 | 14.2/0.5 |
PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; −LR, negative likelihood ratio.
Treatment Responses
The treatment outcomes of 228 treated CHC patients (intention to treat analysis) are summarized in Table 4. Treatment response was categorized as SVR in 155 (68%) patients; SVR response was observed in 114 (70%) in CHC-3 versus 37 (57.8%) in those infected by CHC-1. To determine the predictors of SVR, we compared the baseline characteristics and treatment response between SVR and nonSVR groups. In the univariate analysis, the confounding variables that contributed to the achievement of SVR included age (<40 years) (P < 0.001), fibrosis levels (LSM) (P = 0.006) and infection with genotype 3 (P = 0.04). By multivariate analysis, LSM F4 (odd ratio 2.542, 1.137–5.681) (P = 0.023) and genotype 3 (odd ratio 2.454, 1.262–4.771) (P = 0.008) were independently associated with increased rates of SVR. In addition, RVR and EVR were strong predictors of SVR (P < 0.001).
Table 4.
Baseline Characteristics of 228 Patients (Treated with Peg IFN Plus Ribavirin) and Treatment Response.
| Variable | Total |
aGenotype 1 |
bGenotype 3 |
(P value) | |||
|---|---|---|---|---|---|---|---|
| SVR | No SVR | SVR | No SVR | SVR | No SVR | ||
| Age | <0.001 | ||||||
| <40 | 96 (78.0%) | 27 (22.0%) | 23 (69.7%) | 10 (30.3%) | 73 (81.1%) | 17 (18.9%) | a0.047 |
| ≥40 | 59 (56.2%) | 46 (43.8%) | 14 (45.2%) | 17 (54.8%) | 45 (60.8%) | 29 (39.2%) | b0.004 |
| Gender | 0.089 | ||||||
| Male | 104 (64.6%) | 57 (35.4%) | 25 (55.6%) | 20 (44.4%) | 79 (68.1%) | 37 (31.9%) | a0.574 |
| Female | 51 (76.1%) | 16 (23.9%) | 12 (63.2%) | 7 (36.8%) | 39 (81.3%) | 9 (18.8%) | b0.088 |
| BMI | 0.314 | ||||||
| <23 | 78 (69.6%) | 34 (30.4%) | 19 (57.6%) | 14 (42.4%) | 59 (74.7%) | 20 (25.3%) | a0.242 |
| ≥23 to <25 | 29 (67.4%) | 14 (32.6%) | 6 (85.7%) | 1 (14.3%) | 23 (63.9%) | 13 (36.1%) | b0.471 |
| ≥25 | 48 (65.8%) | 25 (34.2%) | 12 (50.0%) | 12 (50.0%) | 36 (73.5%) | 13 (26.5%) | 0.301 |
| DM | 16 (59.3%) | 11 (40.7%) | 3 (42.9%) | 4 (57.1%) | 13 (65.0%) | 7 (35.0%) | a0.443 |
| Non diabetic | 139 (69.2%) | 62 (30.8%) | 34 (59.6%) | 23 (40.4%) | 105 (72.9%) | 39 (27.1%) | b0.449 |
| Alcohol intake | 0.037 | ||||||
| ≥30 g/d | 13 (50.0%) | 13 (50.0%) | 1 (33.3%) | 2 (66.7%) | 12 (52.2%) | 11 (47.8%) | a0.379 |
| No alcohol intake | 142 (70.3%) | 60 (29.7%) | 36 (59.0%) | 25 (41.0%) | 106 (75.2%) | 35 (24.8%) | b0.023 |
| Fibrosis | |||||||
| F1 | 80 (76.2%) | 25 (23.8%) | 24 (64.9%) | 13 (35.1%) | 56 (82.4%) | 12 (17.6%) | 0.006 |
| F2 | 23 (76.7%) | 7 (23.3%) | 6 (75.0%) | 2 (25.0%) | 17 (77.3%) | 5 (22.7%) | a0.155 |
| F3 | 21 (65.6%) | 11 (34.4%) | 3 (33.3%) | 6 (66.7%) | 18 (78.3%) | 5 (21.7%) | b0.004 |
| F4 | 31 (50.8%) | 30 (49.2%) | 4 (40.0%) | 6 (60.0%) | 27 (52.9%) | 24 (47.1%) | |
| HCV RNA | 0.336 | ||||||
| ≤600,000 IU/ml | 87 (70.7%) | 36 (29.3%) | 17 (60.7%) | 11 (39.3%) | 70 (73.7%) | 25 (26.3%) | a0.678 |
| >600,000 IU/ml | 68 (64.8%) | 37 (35.2%) | 20 (55.6%) | 16 (44.4%) | 48 (69.6%) | 21 (30.4%) | b0.562 |
| RVR (n = 151) | 126 (83.4%) | 25 (16.6%) | 25/(78.1%) | 7 (21.9%) | 101 (84.9%) | 18 (15.1%) | 0.001 |
| NoRVR (n 77) | 29 (37.7%) | 48 (62.3%) | 12 (37.5%) | 20 (62.5%) | 17 (37.8%) | 28 (62.2%) | |
| EVR (n = 192) | 153 (79.7%) | 39 (20.3%) | 37 (77.1%) | 11 (22.9%) | 115 (78.2%) | 32 (21.8%) | 0.001 |
| NoEVR (n 36) | (8.3%) | 33 (91.7%) | 0/(%) | 16 (100%) | 3 (17.6%) | 14 (82.4%) | |
Bold signifies p< 0.05
Discussion
In clinical practice, the determination of fibrosis stage is important to decide whether the patient has mild or advanced liver disease 9. Since patients with advanced fibrosis have faster progression to cirrhosis, antiviral therapy must be given to these patients. Liver biopsy is often limited by its invasiveness, sampling error, and intra/inter-observer variability in histological interpretation. Over the past two decades, a number of noninvasive approaches have been validated to provide such information. In the present study, we used TE to access fibrosis, because it has been shown to provide a reproducible (intra- and interobserver variability approximately 3%) and accurate prediction of liver fibrosis in CHC patients compared to biopsy with higher predictive values for more advanced stages (Metavir F3–F4).17
Apart from indication to treatment, advanced stages require interventions to control negative co-factors for disease progression. In this study, we explored the possible risk factors associated with moderate to severe fibrosis and studied their impact on treatment outcomes. Currently, several risk factors are reported to be associated with the development of fibrosis in CHC, including age at onset of infection, obesity, metabolic syndrome, significant alcohol consumption and genotype 3.18, 19, 20 In the present study, we found that an age ≥40, diabetes and alcohol consumption ≥30 g/day were associated with significantly higher fibrosis levels among CHC patients. A study by Serste et al.21 suggested faster fibrosis progression when the HCV is acquired after 40 years. Hence it is important that treatment should not be delayed in patient's ≥40 years. Liver fibrosis is common pathway for a multitude of liver injuries (viral, hereditary, metabolic, and toxin). Alcohol, which itself can cause liver disease and fibrosis, may affect liver stiffness and worsen fibrosis.22 We consistently found higher LSMs (14.05 kPa vs. 7.2 kPa) in alcoholic versus nonalcoholic group in our study. Similarly patients with diabetes were associated with higher LSM (LSM 17.5 kPa vs. 7.4 kPa) suggesting the role of diabetes in liver fibrosis progression. HCV and diabetes have important interactions and the processes seem to involve direct viral effects, insulin resistance, proinflammatory cytokines, chemokines, and other immune-mediated mechanisms.23 In our study, we found a trend of higher fibrosis levels in HCV-3 (P = 0.088) compared to CHC-1; however, it was not statistically significant. This may be related to higher steatosis in genotype 3 and small number of genotype-1 patients (35.2%) in our study. We also found higher LSM in patients with higher BMI ≥23 as compared to BMI < 23; however, it was not statistically significant. Since most of invalid LSMs occurred in overweight/obese patients, on-significant difference in fibrosis in our study may be due to exclusion of those with invalid LSMs.
Serum biomarkers have also been used as an alternative to liver biopsy for the staging of liver fibrosis.24 We compared the accuracy of the serum biomarker assay in predicting fibrosis, taking LSM by TE as reference. This was done since TE more accurately detects cirrhosis and significant fibrosis.9 APRI and FIB-4 were used as a first-line test with virtually no cost and appeared to have fair diagnostic accuracy for significant fibrosis and cirrhosis. Several studies in CHC have shown that the major strength of the APRI is its ability to exclude significant fibrosis, but a recent large meta-analysis suggested that APRI could identify CHC related fibrosis with moderate degree of accuracy also.25 In our study, we found that the sensitivity of APRI for diagnosis of significant fibrosis was high (91.2%); however, specificity was low (32.4%) (AUROC = 0.77) which is consistent with the recently published meta-analysis. For cirrhosis, the sensitivity and specificity were 49.5% and 92.8% respectively with AUROC of 0.82.
FIB-4 is another simple and cheap index validated in numerous studies of CHC infected patients. It uses cutoff values of 1.45 and 3.25 to rule-out or rule-in significant fibrosis respectively. In a study of 832 patients, FIB-4 >3.25 had a specificity of 97% for diagnosis of cirrhosis (AUROC 0.76).26 In another study, AUROC for cirrhosis was 0.91.27 In our study, we found that the sensitivity of FIB4 for diagnosis of cirrhosis was 57.9%; however, specificity was consistently high (95.7%) (AUROC = 0.90). For significant fibrosis, the sensitivity and specificity of FIB4 were 73.6% and 68.3%, respectively, (AUROC 0.79) and added no significant benefit over APRI as a tool for fibrosis assessment. When we used combination of APRI + FIB4, overall sensitivity decreased but specificity increased for diagnosis of fibrosis and cirrhosis. This suggests that this combination can help in decision making algorithms and in decreasing the need of invasive procedures such as liver biopsy in clinical practice.
Our study confirms the high prevalence of CHC genotype 3 in North India, which has been reported in most of the other studies from India.28, 29 The virological response to peg IFN plus Ribavirin in our study revealed similar or even higher SVR rates in CHC-1 than those in main registration trials of peg IFN/RBV therapy (SVR 40–50%). Our higher SVR rate for CHC-1 could be explained by more common CC genotype (IL-28 polymorphisms) in Asians30 and lesser degree of fibrosis (Table 3) as observed in previous studies also. We found that response to therapy in genotype 1 was significantly lower than genotype 3 (SVR 70%) which is concordant with the results of our previous study (SVR 67%).16 Among risk factors, we found significant effects of age ≥40 years on SVR rates for poor response to therapy. This may be explained by the fact that higher age is often accompanied by concomitant diseases (psychiatric, vascular and metabolic, including insulin resistance and diabetes mellitus) or cirrhosis that makes IFN-based antiviral treatment more difficult to tolerate due to side effects.31 Our study also showed lower response rates to treatment in patients having history of significant alcohol intake and higher fibrosis levels which are in accordance with previous studies.32 The SVR rates were significantly higher in patients without cirrhosis (LSM < 12.5 kPa) as compared to those with cirrhosis (LSM ≥ 12.5 kPa), as documented universally.15 We found a trend of lower response rate and higher LSMs with increasing BMI, but it was not statistically significant. Other studies also showed male sex and high BMI had lower response rate to peg INF therapy and Ribavarin. The baseline viral load did not predict treatment response in this study similar to our previous study.16
In the present study, patients with RVR achieve higher SVR (83.4%) compared to those without RVR (37.7%), regardless of genotypes. For CHC-1, patients with RVR achieved higher SVR (78.1% versus no RVR 37.5%), which is consistent with the established literature on the positive predictive value of RVR.33 The limitation of our study is the retrospective design and lower number of female patients (29.3%). Liver biopsy was also not done in these patients, which still remain the gold standard for assessment of liver fibrosis in CHC patients though the data on noninvasive markers have shown promise.
Conclusion
The study shows that LSM by TE and fibrosis assessment by FIB4/APRI scores can be used with fair reliability to predict fibrosis in patients with CHC infection. LSM by TE suggests that age ≥40 years, daily alcohol intake (≥30gm) and diabetes mellitus have a significant impact on fibrosis progression in CHC patients. Fibrosis stage and HCV genotype play an important role in determining treatment outcome with Pegylated IFN plus Ribavirin.
Conflicts of interest
The authors have none to declare.
References
- 1.Henderson N.C., Iredale J.P. Liver fibrosis: cellular mechanisms of progression and resolution. Clin Sci (Lond) 2007;112:265–280. doi: 10.1042/CS20060242. [DOI] [PubMed] [Google Scholar]
- 2.Iwaisako K., Brenner D.A., Kisseleva T. What's new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol. 2012;27:65–68. doi: 10.1111/j.1440-1746.2011.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bataller R., Brenner D.A. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ydreborg M., Westin J., Rembeck K. Impact of IL28B-related single nucleotide polymorphisms on liver transient elastography in chronic hepatitis C infection. PLOS ONE. 2013;8:e80172. doi: 10.1371/journal.pone.0080172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masuzaki R., Tateishi R., Yoshida H. Assessment of disease progression in patients with transfusion-associated chronic hepatitis C using transient elastography. World J Gastroenterol. 2012;18:1385–1390. doi: 10.3748/wjg.v18.i12.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai M.M. Hepatitis C virus proteins: direct link to hepatic oxidative stress, steatosis, carcinogenesis and more. Gastroenterology. 2002;122:568–571. doi: 10.1053/gast.2002.31474. [DOI] [PubMed] [Google Scholar]
- 7.Loko M.A., Bani-Sadr F., Valantin M.A. Antiretroviral therapy and sustained virological response to HCV therapy are associated with slower liver fibrosis progression in HIV-HCV-coinfected patients: study from the ANRS CO 13 HEPAVIH cohort. Antivir Ther. 2012;17:1335–1343. doi: 10.3851/IMP2419. [DOI] [PubMed] [Google Scholar]
- 8.Bruno R., Sacchi P., Cima S. Correlation between FIB4, liver stiffness and metabolic parameters in patients with HIV and hepatitis C virus co-infection. Dig Liver Dis. 2011;43:575–578. doi: 10.1016/j.dld.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293–1302. doi: 10.1053/j.gastro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Pradhan F., Ladak F., Tracey J., Crotty P., Myers R.P. Feasibility and reliability of the FibroScan S2 (pediatric) probe compared with the M probe for liver stiffness measurement in small adults with chronic liver disease. Ann Hepatol. 2013;12:100–107. [PubMed] [Google Scholar]
- 11.http://www.igovernment.in/site/india-reworks-obesity-guidelines-BMI-lowered.
- 12.Boursier J., Zarski J.P., de Ledinghen V. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182–1191. doi: 10.1002/hep.25993. [DOI] [PubMed] [Google Scholar]
- 13.Castéra L., Vergniol J., Foucher J. Prospective comparison of transient elastography, fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Degos F., Perez P., Roche B. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study) J Hepatol. 2010;53:1013–1021. doi: 10.1016/j.jhep.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Tohra S.K., Taneja S., Ghosh S. Prediction of sustained virological response to combination therapy with pegylated interferon alfa and ribavirin in patients with genotype 3 chronic hepatitis C. Dig Dis Sci. 2011;56:2449–2455. doi: 10.1007/s10620-011-1770-3. [DOI] [PubMed] [Google Scholar]
- 17.Coppola A., Di Capua M., Conca P. Noninvasive assessment of liver fibrosis in patients with chronic hepatitis C (and congenital bleeding disorders): where do we stand. Semin Thromb Hemost. 2013;39:803–815. doi: 10.1055/s-0033-1354421. [DOI] [PubMed] [Google Scholar]
- 18.Sebastiani G., Gkouvatsos K., Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J Gastroenterol. 2014;20:11033–11053. doi: 10.3748/wjg.v20.i32.11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Premoli A., Paschetta E., Hvalryg M., Spandre M., Bo S., Durazzo M. Characteristics of liver diseases in the elderly: a review. Minerva Gastroenterol Dietol. 2009;55:71–78. [PubMed] [Google Scholar]
- 20.Kirk G.D., Mehta S.H., Astemborski J. HIV, age, and the severity of hepatitis C virus-related liver disease: a cohort study. Ann Intern Med. 2013;158:658–666. doi: 10.7326/0003-4819-158-9-201305070-00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sersté T., Bourgeois N. Ageing and the liver. Acta Gastroenterol Belg. 2006;69:296–298. [PubMed] [Google Scholar]
- 22.Mueller S., Millonig G., Sarovska L. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol. 2010;16:966–972. doi: 10.3748/wjg.v16.i8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonelli A., Ferrari S.M., Giuggioli D. Hepatitis C virus infection and type 1 and type 2 diabetes mellitus. World J Diabetes. 2014;5:586–600. doi: 10.4239/wjd.v5.i5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crisan D., Radu C., Lupsor M., Sparchez Z., Grigorescu M.D., Grigorescu M. Two or more synchronous combination of noninvasive tests to increase accuracy of liver fibrosis assessement in chronic hepatitis C; results from a cohort of 446 patients. Hepat Mon. 2012;12:177–184. doi: 10.5812/hepatmon.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T., Wang X., Karsdal M.A., Leeming D.J., Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105–117. doi: 10.4137/BMI.S10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterling R.K., Lissen E., Clumeck N. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 27.Vallet-Pichard A., Mallet V., Nalpas B. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 28.Hazari S., Panda S.K., Gupta S.D., Batra Y., Singh R., Acharya S.K. Treatment of hepatitis C virus infection in patients of northern India. J Gastroenterol Hepatol. 2004;19:1058–1065. doi: 10.1111/j.1440-1746.2004.03405.x. [DOI] [PubMed] [Google Scholar]
- 29.Hissar S.S., Goyal A., Kumar M. Hepatitis C virus genotype 3 predominates in North and Central India and is associated with significant histopathologic liver disease. J Med Virol. 2006;78:452–458. doi: 10.1002/jmv.20561. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen N.H., McCormack S.A., Vutien P. Meta-analysis: superior treatment response in asian patients with hepatitis C virus genotype 6 versus genotype 1 with Pegylated Interferon and Ribavirin. Intervirology. 2015;58:27–34. doi: 10.1159/000369097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vukotic R., Gamal N., Andreone P. Prospective, observational real-life study on eligibility for and outcomes of antiviral treatment with peginterferon α plus ribavirin in chronic hepatitis C. Dig Liver Dis. 2015;47:151–156. doi: 10.1016/j.dld.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Grebely J., Dore G.J. What is killing people with hepatitis C virus infection. Semin Liver Dis. 2011;31:331–339. doi: 10.1055/s-0031-1297922. [DOI] [PubMed] [Google Scholar]
- 33.Fried M.W., Shiffman M.L., Reddy K.R. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]