Abstract
There is a constitutive production of water in brain. The efflux routes of this excess water remain to be identified. We used basal brain water content as a proxy for the capacity of water exit routes. Basal brain water content was increased in mice with a complete loss of aquaporin-4 (AQP4) water channels (global Aqp4−/− mice), but not in mice with a selective removal of perivascular AQP4 or in a novel mouse line with a selective deletion of ependymal AQP4 (Foxj1-Cre-Aqp4flox/flox mice). Unique for the global Aqp4−/− mice is the loss of the AQP4 pool subjacent to the pial membrane. Our data suggest that water accumulates in brain when subpial AQP4 is missing, pointing to a critical role of this pool of water channels in brain water exit.
Keywords: alpha-syntrophin, aquaporin, AQP4, astrocytes, brain edema, cerebrospinal fluid, CSF, endfeet, extracellular space, ependyma, Foxj1, glia, glymphatic, interstitial fluid, neuron-glial, paravascular, water homeostasis
Introduction
There are several unresolved questions regarding the handling of water in the central nervous system (Hladky and Barrand, 2014). One important question that still needs to be resolved is how water is drained from the brain. There is a constitutive production of cerebrospinal fluid through the choroid plexus and of interstitial fluid from the brain microvasculature, and this production has to be balanced by an efflux of fluid to blood or subarachnoid space. There is also a net production of water from the brain’s glucose metabolism that must be offset by water efflux (MacAulay and Zeuthen, 2010).
The predominant brain aquaporin – aquaporin-4 (AQP4) – is a bidirectional water channel that plays a critical role as an influx route of water in brain edema (Manley et al., 2000;Amiry-Moghaddam et al., 2003). An increasing body of evidence suggests that AQP4 doubles as an exit route for excess water (Papadopoulos and Verkman, 2013;Nagelhus and Ottersen, 2013). We have addressed this issue in a glial conditional Aqp4 knockout mouse and found that this line showed a delayed clearance of brain water in the postnatal phase and increased basal brain water content in the adult phase (Haj-Yasein et al., 2011). The latter study left an important question unresolved: which of the several AQP4 pools in brain is responsible for water exit? Glial-conditional Aqp4 knockout animals are deficient in AQP4 in three different locations: 1, in astrocytic membrane domains facing the basal lamina of brain capillaries; 2, in astrocytic membrane domains facing pia; and 3, in the basolateral membrane domains of ependymal cells. A priori, each of these three pools of AQP4 could have been responsible for the effects observed in our glial conditional Aqp4−/− animals. Further, it was not ruled out that glial conditional Aqp4−/− mice could have had developmental anomalies that impeded drainage of water downstream of the subarachnoidal space. To resolve these questions we compared brain water contents in three different mouse lines: 1, a global Aqp4 knockout line, lacking each of the three AQP4 pools described above (Thrane et al., 2011); 2, α-syntrophin−/− mice, lacking the AQP4 pool normally present in pericapillary astrocytic membranes (Neely et al., 2001); 3, Foxj1-Cre:Aqp4f/f mice, a mouse line that was specifically generated for the present study and that harbored a selective deletion of the ependymal pool of AQP4. In addition, we measured the intracranial pressure (ICP) response after intracisternal liquid infusions to determine whether a genetically induced increase in brain water content could be due to inadvertent obstructions of outflow routes from the subarachnoidal space.
Materials and methods
Animals
The experiments were conducted on male adult (12–26 weeks, weighing 21–35 g) constitutive Aqp4−/− (Thrane et al., 2011) and α-syntrophin−/− mice (Adams et al., 2000) with C57BL/6J mice as controls, and novel ependymal-conditional Aqp4−/− mice. The latter mice were generated by crossing Aqp4 floxed mice (Haj-Yasein et al., 2011) with Foxj1-Cre mice (Zhang et al., 2007) and subsequently breeding offspring with each other. Breeders were homozygous for the Aqp4 floxed allele (Aqp4f/f) and either non-carrier or heterozygous carrier of Foxj1-Cre. We obtained similar amounts of ependymal-conditional Aqp4−/− mice (“Foxj1-Cre:Aqp4f/f mice”) as Foxj1-Cre negative litter controls (“Aqp4f/f mice”). The animals were allowed ad libitum access to food and drinking water. All animal experiments were approved by the Animal Care and Use Committee of the Institute for Basic Medical Sciences, University of Oslo.
Light microscopic immunocytochemistry
For all fixation protocols, the animals were deeply anesthetized by an i.p. injection of a Zoletil-Xylazine-Fentanyl cocktail (zolazepam 188 mg/kg, tiletamine 188 mg/kg, xylazine 4.5 mg/kg, fentanyl 26 µg/kg) before transcardiac perfusion with 0.1 M phosphate buffer (PB; pH 7.4) with 2% dextran for 15 sec and fixative for 20 min (flow rate 8 ml/min). The perfused animals were stored at 4°C overnight in situ. The brain was removed and cryoprotected by undergoing sucrose step (10, 20, and 30% in PB), and coronal sections were cut at 16 µm thickness on a cryostat. The sections were either used for hematoxylin-eosin staining or immunocytochemistry. Light microscopic immunocytochemistry was carried out using a method of indirect fluorescence, as described previously (Nagelhus et al., 1998). We used rabbit affinity-purified isolated antibody against AQP4 (Sigma A5971; 1mg/ml; 1:500), diluted in 0.01 M PB with 3% normal goat serum, 1% bovine serum albumin, 0.5% Triton X-100, and 0.05% sodium azide, pH 7.4, and revealed by donkey secondary antibodies with indocarbocyanine (Cy3) (1:1,000: Jackson ImmunoResearch Laboratories, West Grove, PA). The secondary antibody was diluted in the same solution as the primary antibody with the omission of sodium azide. The cortical sections were mounted with ProLong Gold antifade reagent with DAPI (Life Technologies), and viewed and photographed with a model "LSM 510 META" microscope (Zeiss) equipped with a model "Imager.M1 AX10" epifluorescence unit (Zeiss), using 561 nm for Cy3 visualization and 408 nm for DAPI visualization. For cortical sections, a 20×/0.8 Plan-Apochromat objective was used, and for the ventricles we used a 40×/1.3 Oil Plan-Neofluar objective.
Measurement of brain water content and weight
Brain water content and brain weight was measured with the wet/dry mass method (Haj-Yasein et al., 2011). Animals were sacrificed by cervical dislocation and the brains were immediately dissected out intact and in a standardized fashion to ensure a non-biased brain mass sample collection. All mice were prepared at the same circadian time. The pia mater was kept in place and a transverse cut was made by a rectangular razor blade through the brainstem just posterior to the cerebellum and perpendicular to the axis of the brainstem. Each brain sample was massed in a pre-weighed 10 ml glass vial, before being manually homogenized with a spatula against the inside of same vial. The vial was then massed with the brain sample once again, and wet brain sample mass was calculated from the difference. The samples were dried in a vacuum oven (Fistreem International) for 24 h at 80 °C and −1000 mbar. After drying, each vial with dried brain was again massed. Percentage brain water content was calculated as (wet mass − dry mass) × 100/(wet mass).
Measurements of intracranial pressure
Mice were anesthetized with an i.p. injection of a Zoletil-Xylazine-Fentanyl cocktail (zolazepam 188 mg/kg, tiletamine 188 mg/kg, xylazine 4.5 mg/kg, fentanyl 26 µg/kg). Body temperature was monitored with a rectal probe and kept at 37 °C by a temperature-controlled heating pad (Harvard Apparatus). Tracheostomy was performed and mice mechanically ventilated (model “SAR-1000” ventilator, CWE Inc.) with room air at 100 breaths per minute (bpm), volume 0.25–0.35 ml/min (depending on size of the mouse). Blood gases, blood pressure and oxygen saturation were monitored using MouseOx (StarrLife Sciences) with thigh sensor for mice. Oxygen saturation was at all times above 90%. An incision in the neck region was made and muscle was bluntly dissected to localize the cisterna magna. A 30 GA needle connected to tubing and a 50 µl Hamilton syringe was inserted into the cisterna magna and fixed with tissue adhesive (LiquiVet Adhesive, Oasis). A small craniotomy (1 mm in diameter, -3 mm bregma, -2 mm lateral from midline) was made in the skull and the ICP catheter (SPR-1000, Millar) connected to a pressure transducer (PCU 2000) inserted 2 mm under the dura towards bregma. When a stabile pressure was measured, baseline values were recorded for 1 min, before artificial cerebrospinal fluid (aCSF, containing (in mM): 124 NaCl, 2 KCl, 1.25 KH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, and 12 glucose, pH 7.3) was infused at 2 µl/min for 5 min by a pump (kdScientific, model 100 series), as previously described (Iliff et al., 2012).
Data analyses
The genotypes of the mice were for practical reasons known to the investigator when collecting samples and performing measurements. Genotypes were confirmed by re-genotyping biopsies after completion of data analysis. Data on basal brain water content and weight was analyzed using Prism (Version 6 for PC, GraphPad Software). Unpaired t test was used for comparison between mutant mice and the respective controls.
Data on ICP was recorded in Clampex 10.4, imported into Matlab and maximum values calculated. Further processing and analysis of data were made in Excel. Unpaired t test was used for comparison between mutants and the respective controls. For all comparisons p<0.05 was considered statistically significant.
Results
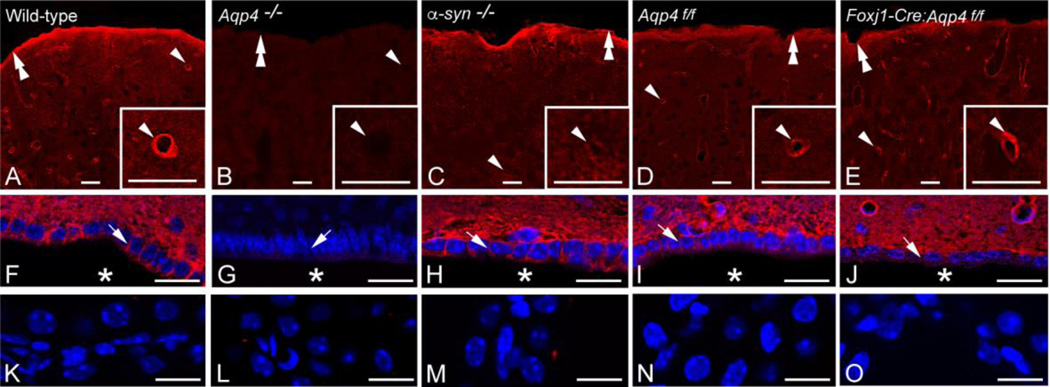
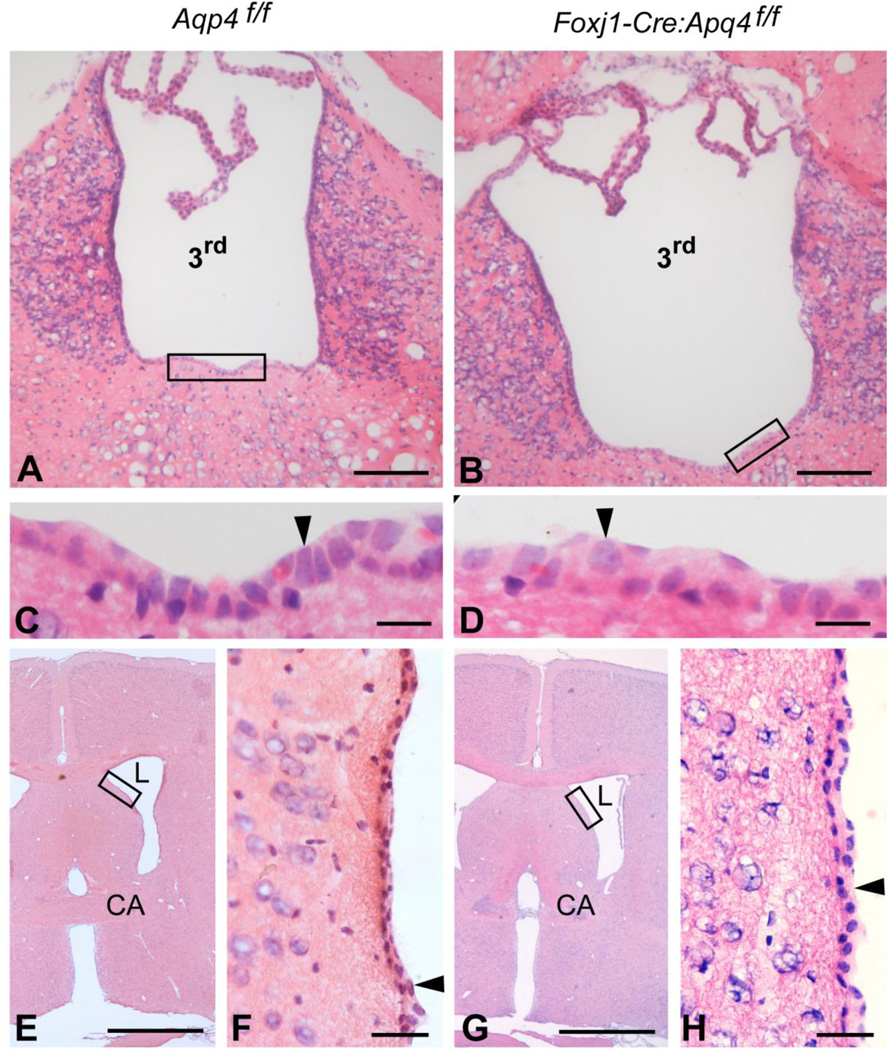
AQP4 immunofluorescence differed between genotypes. In wild-type mice (Fig. 1A,F), AQP4 immunolabeling was most intense subjacent to pia and around blood vessels, with lower intensities in parenchymal membranes and basolaterally in ependymal cells. Global Aqp4−/− mice were devoid of AQP4 in all these locations (Fig.1B,G), while α-syntrophin−/− mice showed a selective loss of perivascular AQP4 (Fig.1C,H). Aqp4 floxed mice (Fig. 1D,I) showed similar AQP4 labeling as wild-types. The Foxj1-Cre:Aqp4f/f mice, the novel ependymal-conditional Aqp4 knockouts (Fig.1E,J), lacked AQP4 in basolateral ependymal membranes but retained AQP4 both perivascularly and subpially. None of the genotypes showed AQP4 immunofluorescence labeling over the choroid plexus epithelium (Fig.1K-O), in line with previous reports (Nielsen et al., 1997;Yang et al., 2011). Hematoxylin-eosin staining of coronal brain sections from ependymal-conditional Aqp4 knockouts and controls (n=3 for each genotype) did not reveal apparent abnormalities in the cellular architecture in the vicinity of the third and lateral ventricles (Fig. 2). Specifically, the ependymal cells displayed similar morphology and organization in mutant (Fig. 2B, D, G and H) and control mice (Fig. 2A, C, E and F). These findings contrast those of Li et al., (2009), who reported disorganized ependymal layer in global Aqp4−/− mice.
Figure 1.
Effect of gene knockout on AQP4 distribution. AQP4 immunofluorence labeling (red) of the cortex (A-E), periventricular area (F-J; nuclear labeling in blue) and choroid plexus (K-O; nuclear labeling in blue) of wild-type (A, F, K), global Aqp4−/− mice (B, G, L), α-syntrophin−/− mice (C, H, M), homozygous Aqp4 floxed mice (“Aqp4f/f”) (D, I, N) and ependymal-conditional Aqp4−/− mice (”Foxj1-Cre:Aqp4f/f mice”) (E, J, O). A, F, K: In wild-type mice AQP4 labeling is most pronounced around cortical blood vessels (arrowhead; same vessel shown at higher magnification in inset) and along the pial surface (double arrowhead). Distinct AQP4 immunolabeling is evident in the basolateral membrane (arrow) of the ependyma covering the third ventricle (asterisk). The choroid plexus epithelium is devoid of AQP4 immunolabeling. B, G, L: Global Aqp4 deletion abolishes AQP4 immunolabeling. Labels as in A, F. C, H, M: In α-syntrophin−/− mice AQP4 labeling is lost around cortical blood vessels but still prominent along the pial surface and in the basolateral ependymal membrane. Labels as in A, F. D, I, N: AQP4 labeling in Aqp4f/f mice is indiscriminately from that of wild-types. Labels as in A, F. E, J, O: In Foxj1-Cre:Aqp4f/f mice the AQP4 immunofluorescence signal is lost from ependymal membranes but otherwise similar to that of litter controls (Aqp4f/fl mice) and wild-types. Labels as in A,F. Scale bars: 20 µm.
Figure 2.
Ependymal-conditional Aqp4−/− mice do not reveal apparent abnormalities in the ependymal architecture. A–H: Hematoxylin-eosin staining of coronal brain sections revealed comparable morphology of the ependyma (arrowheads) lining the third (3rd) and lateral (L) ventricles in control (“Aqp4f/f”; A, C, E and F) and ependymal-conditional Aqp4−/− (”Foxj1-Cre:Aqp4f/f”; B, D, G and H) mice. Boxed areas in A, B, E and G are enlarged in C, D, F and H, respectively. CA, commisura anterior. Scale bars: A, B: 100 µm; C, D: 10 µm; E, G: 1 mm; F, H: 30 µm.
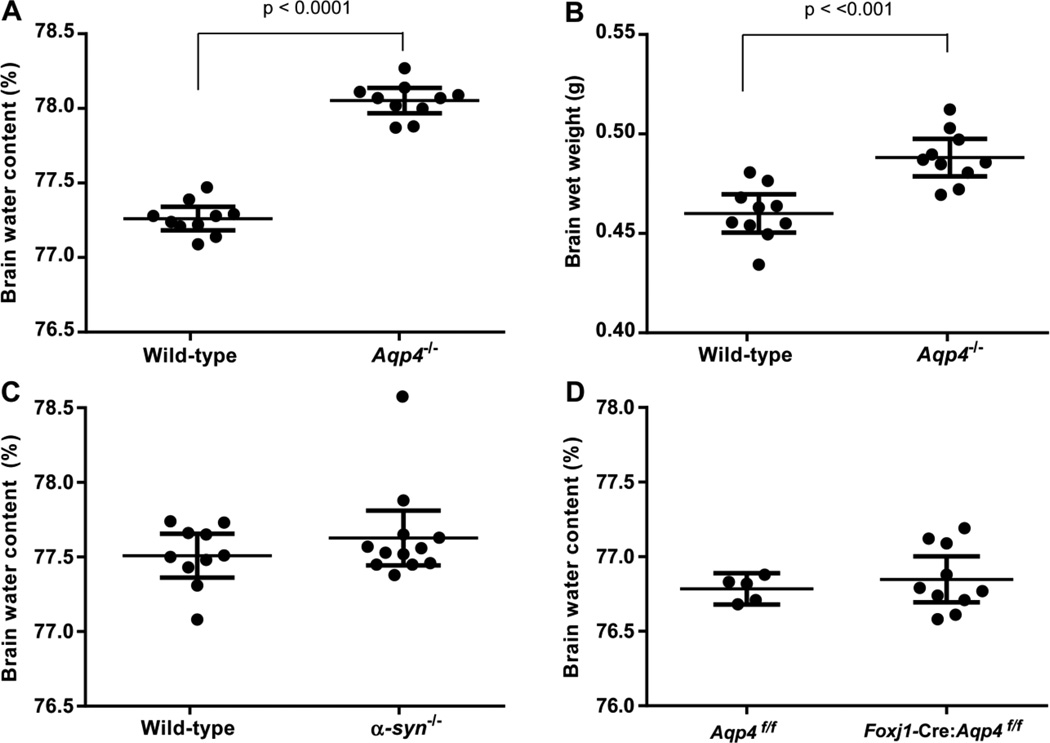
Basal brain water content was significantly higher in global Aqp4−/− mice than in wild-types (Fig. 3A). Whereas brain dry weight did not significantly differ between the genotypes (0.1057 ± 0.0009 g vs. 0.1030 ± 0.0009 g in Aqp4−/− mice and wild-types, respectively, n=10 in each group, p=0.0572, unpaired t test), brain wet weight was 6% higher in Aqp4−/− compared to wild-type mice (0.4882 ± 0.004 g vs. 0.4601 ± 0,004 g, respectively, p<0.001, unpaired t test; Fig.3B). Neither α-syntrophin−/− nor Foxj1-Cre:Aqp4f/f mice differed from their controls in basal brain water content (Fig. 3 C,D) or brain wet weight (not shown).
Figure 3.
Impact of gene deletion on basal brain water content and brain wet weight. A, B: In Aqp4−/− mice (n=10) basal brain water content and brain wet weight are significantly elevated compared to wild-types (n=10; p values are indicated, unpaired t test). C, D: Neither α-syntrophin−/− mice (n=12) nor Foxj1-Cre:Aqp4f/f mice (n=10) differed significantly from their controls (n=10 and n=5, respectively) in basal brain water content (p>0.05 for both comparisons, unpaired t test).
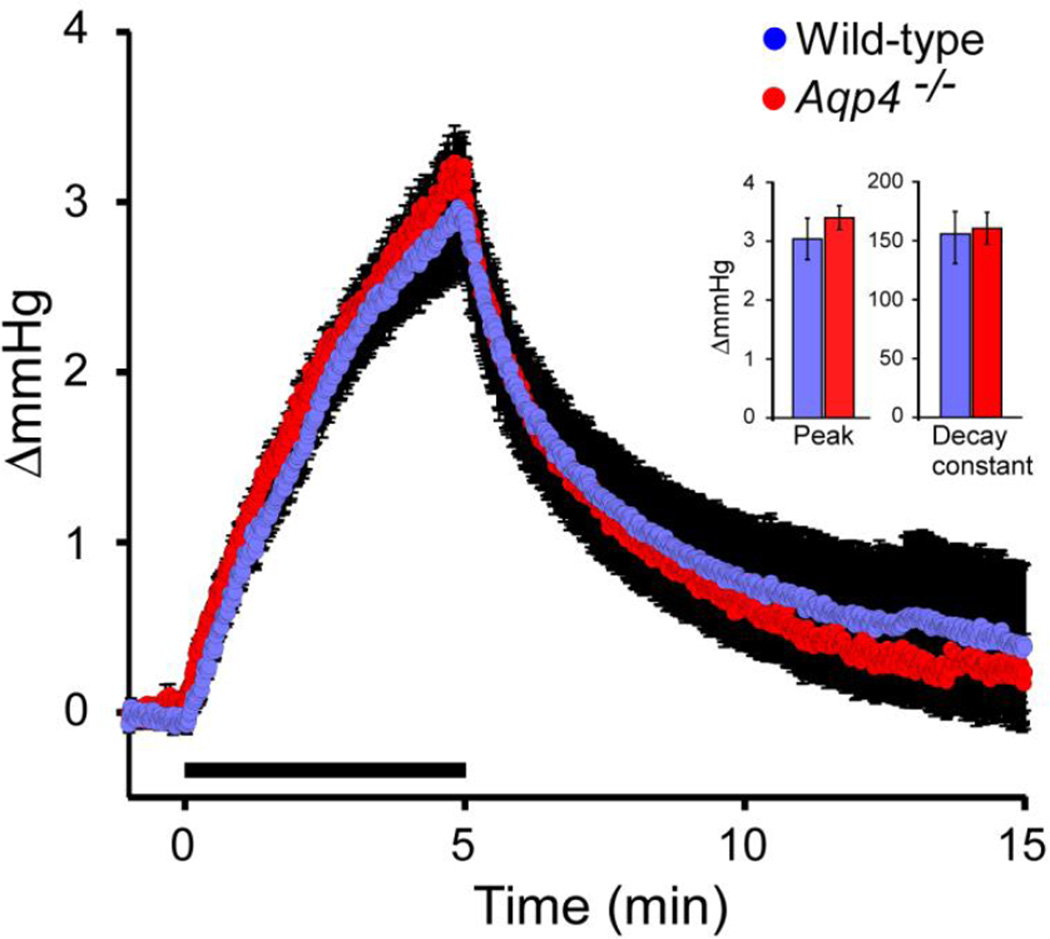
Intracisternal infusion of aCSF at 2 µl/min for 5 min was associated with a temporary elevation of ICP in both Aqp4−/− and wild-type mice (Fig. 4). Neither peak value (3.40 ± 0.21 and 3.25 ± 0.32 mm Hg in Aqp4−/− and wild-type mice, respectively, n=8 in both groups, p=0.62, unpaired t test) nor decay (160.5 ± 14 and 152.7 ± 22, p=0.09, unpaired t test) differed between the genotypes. This is in line with the idea that the gene deletion interferes with water drainage upstream of, rather than downstream of, the subarachnoidal space.
Figure 4.
Effect of intracisternal infusion of aCSF on ICP. Infusion of aCSF at 2 µl/min for 5 min (infusion period indicated by bar) elevates ICP in both Aqp4−/− and wild-types. Neither peak ICP increase nor ICP decay differed between the genotypes (p>0.05, unpaired t test for both comparisons).
Discussion
To identify the exit routes for brain water is a matter of utmost importance, given the clinical implications of impaired water drainage. A failing balance between brain water production and brain water efflux will cause the intracranial pressure to increase to levels that might ultimately be detrimental to brain function.
Several lines of evidence suggest that the water channel AQP4 serves as an exit route for excess brain water. Notably, intraparenchymal infusion of isotonic solution leads to a more pronounced ICP elevation in mice lacking AQP4 (Papadopoulos et al., 2004). Moreover, deletion of Aqp4 exacerbates vasogenic brain edema in multiple disease models (Papadopoulos and Verkman, 2013). Direct evidence for the role of glial AQP4 water channels in draining brain water came from our study using glial-conditional Aqp4−/− mice (Haj-Yasein et al., 2011). Specifically, we showed that glial-conditional Aqp4−/− mice displayed a delayed clearance of brain water in the postnatal phase and increased basal brain water content in the adult stage. The latter work indicates that brain water contents can be seen as a proxy for the capacity of water efflux routes. The rationale is that in the presence of relative impediments to water efflux, the equilibrium between cerebral and extracerebral water pools will be skewed towards more brain water. Hence we assessed brain water contents in three different mouse lines, with differential deletions of AQP4, in order to identify the AQP4 pools that are critical for water efflux.
Obviously, much would be gained by having access to more direct ways of differentiating between putative efflux routes for water. Pending relevant tracers and considering the limitations of the use of tritiated water (Hladky and Barrand, 2014), we have to make do with the current proxy. ICP changes following intracerebral water injections are indirect measures of water flux and will be confounded by changes in volume reserve capacities (due to increased brain weight and hence an increased brain volume). Thus, such measurements do not provide an advance over the present experimental approach.
The most salient observation was that global Aqp4−/− animals showed an increase in basal brain water contents and brain wet weight while knockout of the α-syntrophin dependent pool or ependymal pool of AQP4 failed to show such changes. If one looks at the interfaces between brain and extracerebral liquid spaces – i.e., candidate sites for brain water drainage – the difference between Aqp4−/− mice and α-syntrophin−/− mice is that the latter line contains a sizeable residual pool of AQP4 subjacent to the pial membrane (Amiry-Moghaddam et al., 2003). By inference, this result suggests that water clearance is critically dependent on the subpial pool of AQP4 – i.e., that this pool must be fully or partially intact for efficient drainage to occur. Conversely, the absence of water accumulation in brains with selective loss of perivascular AQP4 (the α-syntrophin−/− mice) suggests that the latter pool of AQP4 is not critical for water efflux. Unfortunately, no animal line exists that has a selective depletion of the subpial pool of AQP4. Such a line could help provide quantitative data on the relative contribution of the perivascular vs. subpial pool of AQP4. An alternative explanation of our findings is that the brain water accumulation in Aqp4−/− animals reflects loss of water channels or developmental abnormalities downstream of the subarachnoidal space. This possibility is not consistent with our ICP measurements following intracisternal aCSF injections. These injections produced the same pressure increase in Aqp4−/− mice as in wild-types.
An ependymal-conditional Aqp4−/− mouse line was generated specifically for this study in order to resolve whether elimination of ependymal AQP4 would cause water retention. No difference was found between ependymal knockouts and control mice, neither for basal brain water content nor for brain wet weight. This suggests that AQP4-dependent water movement through the ependymal layer is not critical for water clearance from brain.
Conclusion
The present study suggests that depletion of astrocytic AQP4 causes water to accumulate in brain. An accumulation of brain water does not occur when deletion is restricted to the perivascular pool of AQP4, indicating that the other major pool of astrocytic AQP4 (the subpial pool) is the one that is critical for water efflux. That the subpial pool of AQP4 may become limiting for water efflux adds a new aspect to current concept of brain water circulation. The current concept has largely neglected the role of transpial water transport. Our finding that depletion of perivascular AQP4 failed to affect brain water contents must not be taken to suggest that this pool is not involved in water transport between blood and brain. Indeed, a series of studies provide convincing evidence that this AQP4 pool serves as an influx route of water in cerebral edema. Since AQP4 allows bidirectional transport of water, the inference is that perivascular AQP4 must double as an efflux route. What the present study indicates is that the deletion of this route can be compensated for by the sizeable pool of subpial AQP4. This conclusion stands to be corroborated once knockouts are available that offer selective removal of the latter pool.
Highlights.
-
-
Aquaporin-4 (AQP4) water channels in astrocytes regulate basal brain water content
-
-
AQP4 is not involved in draining CSF through arachnoid villi or lymphatic vessels
-
-
Ependymal AQP4 does not have any major effect on basal brain water content
Acknowledgments
We thank Mr. Gaute Nesse, Institute of Medical Microbiology, Oslo University Hospital, Rikshospitalet, Oslo, for performing the genotyping and Mrs. Carina V. S. Knudsen for technical assistance. This work was supported by the Research Council of Norway (NevroNor and FRIMEDBIO Grants to E.A.N.), the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 601055, the National Institutes of Health (NIH) grants HL121791 and HL120153, and the Letten Foundation. The sponsors had no involvement in study design, data collection, analysis and interpretation of data, and writing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams ME, Kramarcy N, Krall SP, Rossi SG, Rotundo RL, Sealock R, Froehner SC. Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol. 2000;150:1385–1398. doi: 10.1083/jcb.150.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci U S A. 2003;100:2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, Gundersen GA, Skare O, Laake P, Klungland A, Thoren AE, Burkhardt JM, Ottersen OP, Nagelhus EA. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci U S A. 2011;108:17815–17820. doi: 10.1073/pnas.1110655108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kong H, Wu W, Xiao M, Sun X, Hu G. Aquaporin-4 maintains ependymal integrity in adult mice. Neuroscience. 2009;162:67–77. doi: 10.1016/j.neuroscience.2009.04.044. [DOI] [PubMed] [Google Scholar]
- MacAulay N, Zeuthen T. Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience. 2010;168:941–956. doi: 10.1016/j.neuroscience.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013;93:1543–1562. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelhus EA, Veruki ML, Torp R, Haug FM, Laake JH, Nielsen S, Agre P, Ottersen OP. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Muller cells and fibrous astrocytes. J Neurosci. 1998;18:2506–2519. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci U S A. 2001;98:14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14:265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrane AS, Rappold PM, Fujita T, Torres A, Bekar LK, Takano T, Peng W, Wang F, Rangroo TV, Enger R, Haj-Yasein NN, Skare O, Holen T, Klungland A, Ottersen OP, Nedergaard M, Nagelhus EA. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc Natl Acad Sci U S A. 2011;108:846–851. doi: 10.1073/pnas.1015217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Gao F, Liu H, Yu WH, He GQ, Zhuo F, Qiu GP, Sun SQ. Immunolocalization of aquaporins in rat brain. Anat Histol Embryol. 2011;40:299–306. doi: 10.1111/j.1439-0264.2011.01070.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang G, Shornick LP, Roswit WT, Shipley JM, Brody SL, Holtzman MJ. A transgenic FOXJ1-Cre system for gene inactivation in ciliated epithelial cells. Am J Respir Cell Mol Biol. 2007;36:515–519. doi: 10.1165/rcmb.2006-0475RC. [DOI] [PMC free article] [PubMed] [Google Scholar]