Abstract
Cecal ligation and perforation (CLP) is a common technique for studying sepsis in mice. Because of the invasiveness of the procedure and its effects on clinical condition, many animal care and use committees require the use of analgesics with CLP. However, some analgesics have immunomodulatory effects and thus can hinder the overall research outcomes of a project. Here we sought to determine the effects of buprenorphine hydrochloride (Bup HCl) compared with sustained-release buprenorphine (Bup SR) on clinical condition, plasma concentrations of monocyte chemoattractant protein (MCP) 1 and IL6, and overall mortality in a murine CLP model of sepsis. Male C57/BL6 mice underwent CLP surgery and received Bup HCl or Bup SR as a component of an IACUC-approved analgesic dosing regimen. Mice were observed twice daily for clinical condition scoring by the same blinded investigator for the duration of the study. MCP1 and IL6 levels and mortality did not differ significantly between the 2 groups. Scoring of clinical condition revealed a significant decrease in behaviors associated with perceived pain at 12 and 24 h postoperatively in mice in the Bup SR group compared with the Bup HCl group. Because of the lack of significant effect on MCP1 and IL6 levels and mortality and the superior analgesic effects of Bup SR, we recommend the use of Bup SR for analgesia during the murine CLP model of sepsis.
Abbreviations: Bup HCl, buprenorphine hydrochloride; Bup SR, sustained-release buprenorphine; CLP, cecal ligation and perforation; TINT, time-to-integrate-to-nest test
The incidence of sepsis, a systemic inflammatory response to infection that results in multiorgan failure, has increased in recent years.31,39,42 This complex, multifactorial syndrome is characterized by oxidative stress and a systemic inflammatory response to an infective insult. Although the complete pathophysiology of human sepsis is still poorly understood,39,42 the consequences are severe and include widespread tissue injury, multiple-organ failure, and death.10,30,47 The murine cecal ligation and perforation (CLP) model is considered the ‘gold standard’ of sepsis models, mimicking the clinical progression observed during human sepsis.10,12,13,20,42 The murine CLP procedure involves a laparotomy to induce peritonitis and subsequent polymicrobial sepsis.10-12,21,39,42 Researchers who intend to translate findings of sepsis research from the murine model to human medicine often use the CLP procedure.
In the literature, the use of analgesics in the murine model of sepsis is a topic of debate. One of the greatest concerns raised is the potential effect of an analgesic on the immune response of the animal.10,21 Previous studies have examined the murine immune response to several common analgesics. One study examining the effect of buprenorphine on CLP in C57BL/6 mice demonstrated that buprenorphine causes few perturbations of inflammatory parameters in male mice undergoing the procedure.10 Another group compared the use of tramadol and buprenorphine and found that mice given a high dose of tramadol after CLP had significantly greater mortality and higher levels of cytokines than did those treated with buprenorphine.21 These concerns alone do not outweigh the benefits of using appropriate analgesics in septic mice. The Public Health Service Policy on the Humane Care and Use of Laboratory Animals requires that animals used in biomedical and behavioral research are provided with appropriate treatment, including the appropriate use of tranquilizers, analgesics, and postsurgical veterinary medical and nursing care.34 The Government Principles for the Utilization and Care of Vertebrate Animals used in Testing, Research, and Training states that procedures with animals that may cause more than momentary or slight pain or distress should be performed with appropriate sedation, analgesia, or anesthesia.34 In addition to remaining in compliance with government policies, it is in the researchers’ best interest to provide appropriate analgesia, given that unrelieved pain interferes with metabolic and immunologic functions.21-24
A common analgesic used in research is the synthetic opiate buprenorphine. Buprenorphine is classified as a partial μ agonist and κ antagonist and has few immunomodulatory effects.10,15 Previous studies have shown that buprenorphine did not have significant effects on mortality or several immune parameters.10,21 The dosage recommended is 0.05 to 0.1 mg/kg at a frequency ranging from every 6 h to every 12 h.7,15 Buprenorphine SR (Bup SR) is a sustained-release injectable formulation of buprenorphine that was developed by a United States veterinary compounding pharmacy. Reports have claimed that Bup SR provides analgesic relief for 12 to 72 h duration in murine species.6,8,9 Previous studies have shown few effects of short-acting buprenorphine (Bup HCl) on inflammatory parameters in a murine model of sepsis.10,21 However, whether Bup SR has a similar lack of immunomodulary effects when administered at doses that would alleviate clinical signs of pain in a murine model of sepsis is unknown.
The purpose of this study was to determine whether Bup HCl and Bup SR differed in their effects on mortality, plasma concentrations of the inflammatory cytokines monocyte chemoattractant protein (MCP) 1 and IL6, and clinical signs of pain in a murine model of sepsis. In this study, mice undergoing the CLP procedure received either 0.1 mg/kg Bup HCl every 6 to 12 h for a total of 48 h or 1.0 mg/kg Bup SR once at time of anesthetic induction. Compared with the Bup HCl group, Bup SR mice exhibited significantly fewer signs of pain during the first 24 h after surgery, according to clinical condition scoring, with no measurable differences in mortality or circulating levels of MCP1 and IL6 between groups.
Materials and Methods
Animals.
Male C57/BL6 mice (weight, 24 to 28 g) were purchased from Charles River Laboratories (Wilmington, MA). Mice were acclimated for at least 3 d prior to experimental use. Animals were housed at a maximum of 5 per cage on autoclaved corncob bedding (Alpha-dri–Cob Blend, Shepherd Specialty Papers, WF Fisher and Son, Somerville, NJ) in individually ventilated microisolation cages. After undergoing CLP, mice were assigned to cages according to treatment with a maximum of 5 mice per cage; mice were not single-housed during the experiment. Enrichment was provided in the form of social housing and cotton nesting pads (Cotton squares, Ancare, Bellmore, NY). Mice had unrestricted access to irradiated feed (Purina Lab Diet 5053, PMI, St Louis, MO) and water treated by reverse osmosis. Mice were housed in a SPF facility and were SPF for pathogens including Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, mouse parvovirus, Theiler mouse encephalomyelitis virus, reovirus type 3, epizootic diarrhea of infant mice virus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus, ectromelia virus, K virus, polyomavirus, and endo- and ectoparasites. Mice were maintained in accordance with the Guide for the Care and Use of Laboratory Animals22 in an AAALAC-accredited facility. All procedures were approved by the Columbia University IACUC and followed applicable governmental policies and regulations.
CLP procedure.
All mice underwent the CLP procedure under isoflurane anesthesia. Prior to the initial incision, mice were given a dose of either Bup HCl (0.1 mg/kg SC) or Bup SR (1 mg/kg SC) by a designated investigator who ensured that all other investigators were blinded to the treatment group. The cecum was exposed through a ventral midline incision. A single ligature of 4-0 silk was used to ligate approximately 50% of the cecum distal to the ileocecal junction. The antimesenteric border of the ligated cecum was perforated twice (through-and-through procedure) by using a 21-gauge needle. Cecal contents were gently expressed to ensure patency of the perforations. The abdominal musculature was closed with sutures, followed by closure of the skin by using wound clips. Sterile saline (0.5 mL) was administered intraperitoneally immediately after the procedure. Mice were identified by numbering the tails with nontoxic markers for future recordkeeping and were returned to their home cages once they had recovered completely from anesthesia. The procedure time for each mouse was approximately 5 to 10 min, with a total anesthesia time of less than 15 min. Mice did not display any evidence of anesthetic complication. The procedures were divided over 2 d, and all surgeries were performed by the same investigator, who was blinded to treatment group, and began at the same time each day.
Animal health monitoring postoperatively.
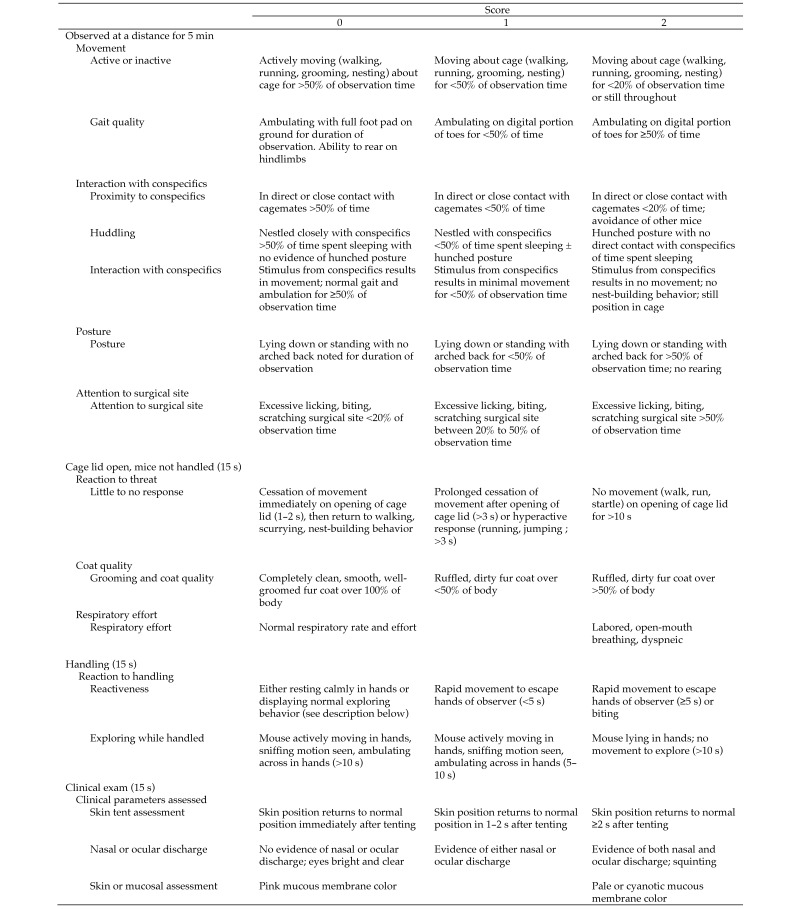
Individual mice were monitored immediately after the CLP procedure and at 6, 12, 24, 30, and 36 h thereafter, concurrent with Bup HCl dosing. Animal mortality was documented at each time point. All observations were performed by the same investigator, who was female to avoid potential stress-related analgesia bias and who was blinded to treatment group.44 Several clinical signs associated with activity and response to stimulus were used to create a scoring system. Observations with clinical assessment scoring were performed at 12, 24, and 36 h after the CLP procedure; the monitoring at the 6- and 30-h time points included a general health check, with no clinical assessment scoring, and euthanasia as warranted. Beginning 48 h after the CLP procedure, all mice were observed every 12 h for general health, with no clinical scoring and euthanasia if warranted. All observations occurred prior to the administration of the analgesic. An abbreviated version of a validated behavioral scoring system14,48 was used to create the current scoring system, integrating physiologic and behavioral parameters to evaluate clinical condition of postoperative mice. Duration of all behaviors was recorded by using a stopwatch in real time. Individual mice initially were observed for 5 min from a distance while they were in their home cage. Mice were observed for movement, interaction with conspecifics, posture, and amount of time spent focused at the surgical site. After the initial observation, mice were observed for 15 s immediately after the home cage lid was opened, to evaluate responsiveness to cage opening, grooming, and respiratory effort. Finally, mice were evaluated for reactiveness and exploration for 15 s during handling. During handling, a clinical exam was performed, and skin tenting for hydration status, nasal or ocular discharge, and color of mucous membranes were noted. Scores of 0 (normal), 1 (mild presentation), or 2 (severe presentation) were assigned for each feature in every mouse. The clinical condition scale with a complete description of all clinical signs can be found in Figure 1; the maximal total condition score was 30. Mice that scored greater than 12 or that were unable to right themselves were euthanized by CO2 asphyxiation followed by cervical dislocation.
Figure 1.
Behavioral ethogram. Mice were observed for the listed clinical parameters during the time period indicated. Mice were scored on a scale of 0 to 2; the maximal total clinical condition score was 30. Mice that scored higher than 12 or that were unable to right themselves were euthanized by CO2 asphyxiation followed by cervical dislocation.
All cages contained approximately 3 g of nesting material, and the mice's nesting behaviors scored at the same time each evening after the animals had experienced at least 10 h of the light cycle. Nest scores were graded prior to the start of this experiment, and all cages had nests that scored as 4 or higher. Because of the randomization of mice in groups, nest scores could not be accurately compared between before and after CLP.
Time-to-integrate-to-nest test (TINT) scoring was attempted at the same time each morning, within 3 h of initiation of the light cycle. TINT scoring began the day after the CLP procedure and was continued for the duration of the experiment. As described in previous reports,40,41 a single square (1 in. × 1 in.) of cotton was placed opposite the established nest and left for a total of 10 min. When the nesting square was integrated into the established home nest, the cage was scored as positive. When the cotton square was untouched, the cage was scored as negative.
Analgesic dosing.
All analgesics were provided subcutaneously after induction of anesthesia. The mice assigned to the Bup HCl group (n = 16) were given a single subcutaneous dose of Bup HCl at time of the CLP procedure and 0.5 mL saline intraperitoneally at the completion of the procedure. Additional doses of Bup HCl (0.1 mg/kg) were given at 6, 12, 24, 30, and 36 h postoperatively for a total of 6 doses of Bup HCl. The Bup SR group (n = 16) received a single subcutaneous dose of Bup SR (1 mg/kg) at the time of the CLP procedure and 0.5 mL saline intraperitoneally. To maintain investigator blinding and to reduce the potential confounding effect of increased disturbance to the Bup HCl group, after the first Bup SR dose, mice in the Bup SR treatment group received an equal volume of saline without analgesics at the same time points as the Bup HCl groups were treated. In total, 1 dose of analgesic and 5 doses of saline without analgesics were given in the Bup SR group. The initial analgesic injection was given by one investigator, who recorded the drug given and thus the analgesic groups. All additional injections were given by a second investigator, who was blinded to the identity of the analgesic groups.
Blood collection and processing.
Approximately 25 μL blood was collected from the saphenous vein at 24 h postoperatively. Previous studies have shown that the highest single-day mortality and highest expression of circulating cytokines typically occur at 24 h after the CLP procedure.35 The same experienced investigator collected blood from all animals. The proinflammatory cytokine IL6 and chemotactic cytokine MCP1 were evaluated in all collected samples by using the Cytometric Bead Array Mouse Flex Kit (BD Biosciences, Franklin Lakes, NJ) according to the manufacturer's instructions.
Statistical analysis.
Kaplan–Meier survival curves were calculated for each treatment group, and differences between curves were analyzed by using log-rank statistics. Observational scores and MCP1 and IL6 levels were compared between groups by using nonparametric 2-tailed Mann–Whitney U testing. A P value of less than 0.05 was considered significant. All statistical analyses were performed by using Prism 5 (GraphPad Software; La Jolla, CA).
Results
Survival rate.
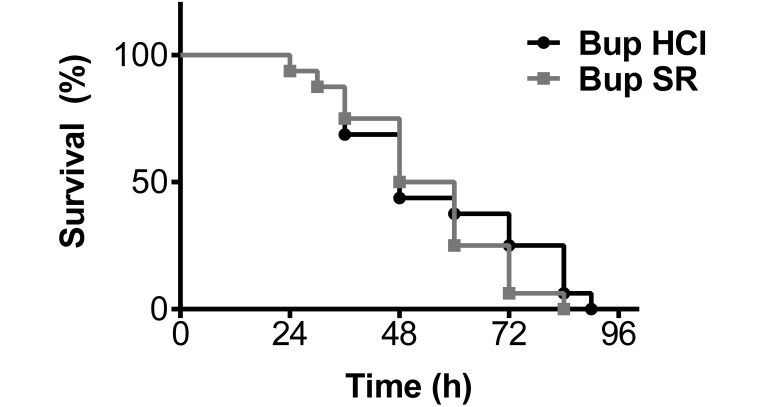
In both analgesic groups, the survival rate was approximately 50% at 48 h after CLP and reached 100% by 84 h postoperatively. Overall, no significant difference in mortality rate was detected between the analgesic groups during the 5-d course of this study (Figure 2).
Figure 2.
Kaplan–Meier survival curve show a 50% survival rate for both Bup HCl (black) and Bup SR (gray) treatment groups at 48 h postoperatively. Both treatment groups reached 100% mortality at 84 h after undergoing CLP, and mortality rate did not differ significantly between the analgesic treatment groups during this study.
Clinical condition analysis.
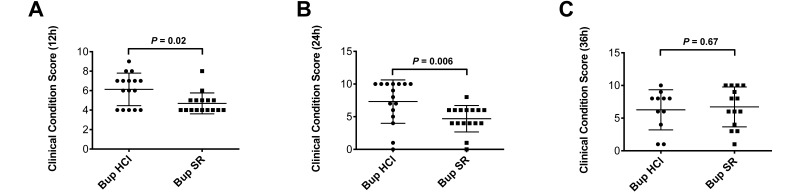
Clinical condition scores (mean ± 1 SD) in the Bup SR mice at 12 h (4.69 ± 1.08) and 24 h (4.69 ± 2.02) postoperatively were significantly better (that is, lower) than those in mice given Bup HCl (12 h, 6.13 ± 1.67, P = 0.02; 24 h, 6.93 ± 3.38, P = 0.006; Figure 3). However, clinical condition scores at 36 h did not differ between the 2 groups (Figure 3). Because of study attrition bias due to dropout from euthanasia, clinical condition scores were not compared between the 2 groups after 48 h.
Figure 3.
Clinical condition analysis scores of Bup HCl (circle) and Bup SR (square) treatment groups at 3 time points after CLP. (A) At 12 h after the CLP procedure, mice dosed with Bup SR had significantly (P = 0.02) better (that is, lower) condition scores (mean ± 1 SD) than did mice given Bup HCl (4.69 ± 1.08 and 6.13 ± 1.67, respectively). (B) At the 24-h time point, Bup SR mice again had significantly (P = 0.006) better condition scores than did Bup HCl mice (4.69 ± 2.02 compared with 7.313 ± 3.32). (C) At 36 h after CLP, the condition score did not differ significantly (P = 0.67) between the mice treated with Bup SR (6.71 ± 3.05) and those given Bup HCl (6.27 ± 3.07).
During the current experiment, few cotton squares were manipulated throughout the study. The results regarding nest scores were not statistically significant and therefore are not shown. In addition, all mice were negative according to TINT throughout this experiment.
Immunologic parameters.
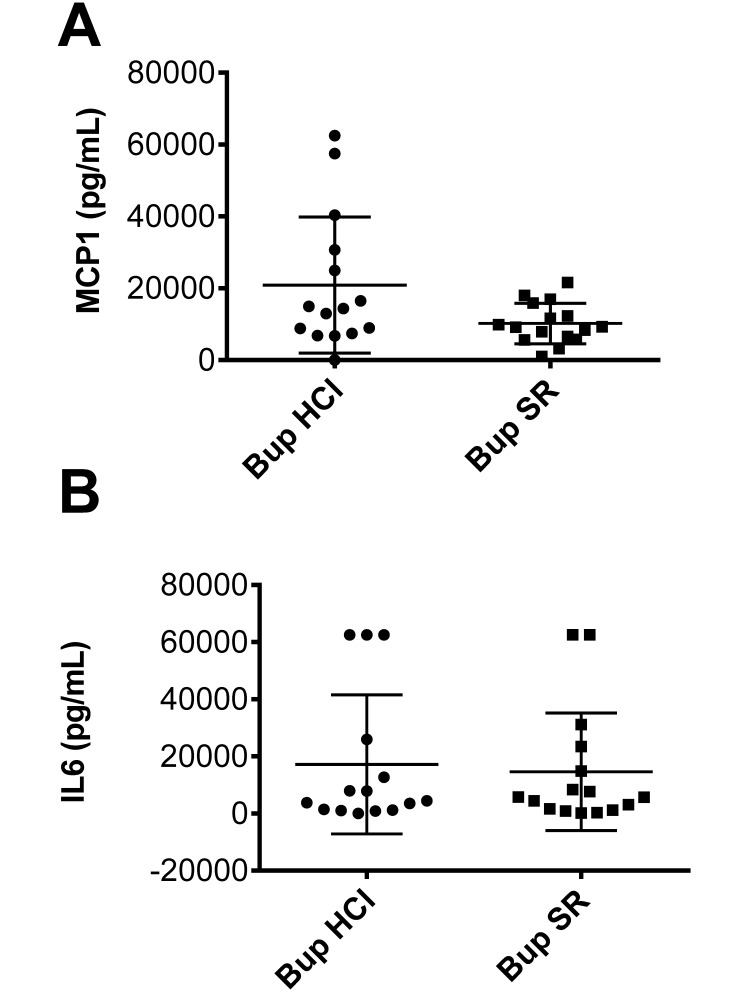
One mouse in the Bup HCl group was moribund at 24 h postoperatively and therefore did not undergo blood collection at this point. Proinflammatory IL6 and MCP1 cytokine–chemokine levels were evaluated on all other samples. In the Bup SR group, the MCP1 concentration was 10,212 ± 5615 pg/mL and the IL6 level was 14,612 ± 20,607 pg/mL, compared with 20,918 ± 18,941 pg/mL and 17,221 ± 24,320 pg/mL, respectively, in the Bup HCl mice. These cytokine levels did not differ significantly between the treatment groups (Figure 4).
Figure 4.
Circulating MCP1 and IL6 levels at 24 h after CLP in Bup HCl- (circles) and Bup SR- (squares) treated mice. (A) MCP1 levels (mean ± 1 SD) were 10,212 ± 5,615 pg/mL in the Bup SR group and 20,918 ± 18,941 in the Bup HCl group. These values did not differ significantly between the 2 groups. (B) IL6 levels (mean ± 1 SD) were 22,657 ± 41,783 pg/mL in Bup SR mice and 43,010 ± 82,853 pg/mL in Bup HCl mice. Circulating levels of IL6 did not differ significantly between the analgesic treatment groups.
Discussion
Sepsis (Greek, ‘to make putrid’) is characterized by oxidative stress and a systemic inflammatory response to infection.4,26,38,43 This inflammatory reaction is mediated by the release of cytokines from neutrophils and macrophages. Cytokines then activate the extrinsic coagulation cascade and inhibit fibrinolysis, leading to disruptions of the coagulation pathway, the formation of microvascular thrombi, the consumption of endogenous anticoagulant, and ultimately organ dysfunction. Outcomes include aberrant mediator production and cellular dysfunction, both of which are being investigated as potential causes of widespread tissue injury.26,38
Sepsis is a leading cause of mortality in human and veterinary medicine.39,42,43 In 2012, bacterial sepsis was the 8th leading cause of death in newborns, and septicemia ranked as the 10th leading cause of death in females. Septicemia ranks within the top 10 causes of death in various other populations.1,18,31 The mortality rate from sepsis among human and veterinary populations has remained stable over the past few years and appears to be a result of a host's inability to respond to the pathogenesis of sepsis.31,39,42,43
The CLP procedure is frequently considered the gold standard of murine sepsis models because it replicates the clinical progression of sepsis in humans.10,21,39 In the murine CLP model, sepsis originates from a polymicrobial infectious focus within the abdominal cavity, followed by bacterial translocation into the blood compartment, triggering a systemic inflammatory response.39 This model often presents challenges to IACUC due to the invasive abdominal surgery, induction of peritonitis, and severe postoperative outcomes. Perioperative analgesia is considered standard of care in veterinary medicine, and federally funded research must be in compliance with the Public Health Service policy. However, investigators may be hesitant to provide analgesics in animal models of sepsis because of the risk of potential immunomodulatory effects. Currently, investigators are tasked with providing appropriate analgesia or providing adequate justification for the withholding of analgesics.21 This dilemma has been a topic of discussion in recent laboratory animal veterinary forums, with consensus in favor of providing analgesics in mice undergoing the CLP procedure.
In the current study, we evaluated the effects of 2 formulations of buprenorphine on mortality, MCP1 and IL6 cytokine response, behavior, and perceived pain in C57BL/6 male mice after a CLP procedure. We chose Bup HCl as the control analgesic in this study because previous reports concluded that the use of Bup HCl in mice undergoing the CLP procedure had limited adverse effects in this model.10 In addition, we modified an IACUC protocol in which the use of Bup HCl was already approved to evaluate the 2 analgesic formulations in a murine model of polymicrobial sepsis. The doses of buprenorphine that we used reflect current recommendations in the literature and in commonly used veterinary formularies.7,15 The dosages chosen for Bup HCl and Bup SR were at the higher end of the recommended dose range, as was protocol-approved standard dosing. The recommended dosing frequency for Bup HCl ranges from every 6 to every 12 h.7,15 Our study used the maximal time between doses of Bup HCl, because this schedule mimics the common practice at many institutions of implementing the least invasive treatment regimen. In addition, this schedule followed the dosing regimen of a preapproved IACUC protocol, further demonstrating the common use of this dosing frequency. Bup SR is reported to be efficacious for as long as 72 h,6,8,9 thus exceeding the 48-h analgesic coverage required by the IACUC-approved protocol.
We administered Bup HCl (0.1 mg/kg) at the time of the CLP procedure and at 6, 12, 24, 30, and 36 h postoperatively, for a total of 6 doses. Bup SR was given once at the time of the procedure, and saline was injected at the remaining time points to control for handling effects and fluid volume between groups. No significant difference in mortality or levels of IL6 and MCP1 were observed. IL6 is a proinflammatory mediator that is significantly elevated in the plasma of septic patients; it can also serve as a biomarker of the systemic inflammatory response.37,43 MCP1 levels are significantly elevated in animal models of sepsis and in septic human patients.5,36,49,50 Both MCP1 and IL6 have been used as predictive markers of disease severity and survival in murine CLP models of sepsis.25,37,36,45 Previous reports have shown increased levels of MCP1 in the peripheral blood and peritoneal fluid at 24 h after CLP procedures.45,46 Another report25 concluded that measuring IL6 at 24 h after CLP can be used as a predictive method of determining survival in murine CLP models of sepsis. The highest single-day mortality and highest expression of circulating cytokines has been reported to occur at 24 h after CLP.35 Because of these reasons, we chose the 24-h time point for measuring circulating cytokines. Although chemokines are essential for the host immune response, overproduction of these mediators plays an important role in the pathogenesis of sepsis.36 Mice that received Bup HCl or Bup SR had a survival rate of 50% at 48 h, and both treatment groups reached 100% mortality by 84 h postoperatively. Taken together, these results suggest that Bup HCl and Bup SR have similar effects on the MCP1 and IL6 cytokine levels at 24 h after the procedure and mortality. One limitation of this study is that only 2 cytokines were measured to assess the systemic inflammatory reaction. However, both of these cytokines have been documented as markers of mortality in animal models of sepsis.25,35,36,38,45,46
We modified an abbreviated version of a validated behavioral scoring system14,48 to evaluate the clinical condition of mice after CLP (Figure 1). The scoring system integrated physiologic and behavioral parameters that are recognized easily by the observer, including activity, posture, breathing pattern, coat condition, interaction with conspecifics, and response to external stimulation.2,17,23,24,33,48 Using this adapted clinical condition scoring system, we observed a significant decrease in the number of clinical signs and behaviors indicative of pain at both 12 h and 24 h after CLP in the Bup SR mice compared with those given Bup HCl. At 6 h, Bup HCl mice were given their second dose, and Bup SR mice received their first dose of saline. At the 12-h time point, Bup SR group had a clinical condition score of 4.69 ± 1.08, whereas the Bup HCl group scored 6.13 ± 1.67. At this time, all mice had fully recovered from anesthesia and, after their assessment scoring, mice in the Bup HCl group received another dose of the analgesic, and Bup SR mice were given saline. At 24 h, the Bup SR group scored 4.69 ± 2.02, and the Bup HCl group had a clinical condition score of 6.93 ± 3.38. At 12 h after the CLP procedure, Bup HCl mice had reached 6 h between doses, and at the 24-h time point, the Bup HCl group was reaching the maximal time between doses (12 h). This pattern suggests that the dosing of Bup HCl should occur more frequently than every 6 to 12 h for the initial 24 h after the CLP procedure.
Studies exploring nesting behaviors have been used to determine postoperative murine pain and animal clinical condition. Nest scoring is a behavioral parameter that has been previously measured by researchers to study this effect.3,16,19,27 Prior to the current study, all cages had nest scores of 4 or higher. Due to the randomization of animals, the nest scores could not be accurately compared before and after the CLP procedure. The nesting behaviors of each cage were scored at the same time each evening according to a previously described process,16,19,27 but few of the cotton squares were manipulated throughout the experiment. Similarly, TINT scoring was measured beginning on postoperative day 1 for the duration of the experiment, and all cages of mice received negative TINT results throughout the study. Previous reports have documented the importance of performing the TINT prior to a study to have a baseline measurement with which to compare manipulated animals. However, due to time constraints, we did not obtain a baseline TINT, which could have been a reason for the lack of TINT-positive cages.41 As previously reported, the use of buprenorphine may produce a motor depression that inhibits mice from forming complete nests and integrating nesting material into preestablished nests.41 A complete analysis of the effects of Bup SR and Bup HCl on TINT and nest scoring is beyond the scope of the current study.
Clinical observations and assessments of complex behaviors have been used in studies to evaluate pain and animal condition. The Mouse Grimace Score is a parameter validated for use to measure pain in mice. For this scoring, mice are placed in a chamber suitable for obtaining photographs or videorecordings the face of a mouse; these images then are scored by a trained observer.14,28,29,32 Due to its complex set-up, we did not use the Mouse Grimace Score for this project, and this type of scoring is beyond the scope of this study.
For mice undergoing surgical procedures, the analgesics selected for postoperative care should be considered carefully on the basis of efficacy, ease of application, and effects on research. Arguments have been raised regarding the use of analgesics for murine sepsis models. The current study concludes that, compared with Bup HCl, Bup SR does not alter mortality or plasma levels of the inflammatory cytokines MCP1 and IL6 and is superior in ameliorating signs of pain, as evidenced by the significantly decreased clinical condition scores in mice at 12 and 24 h following the CLP procedure. The recommended time between doses of Bup HCl varies, leading to inconsistent dosing schedules between studies. Compared with Bup HCl, Bup SR provides a more consistent dosing regimen and thus eliminates a potential confounding variable. The less frequent dosing schedule, consistent analgesia, and lack of effect on MCP1 and IL6 cytokine levels and mortality support the use of Bup SR in the CLP procedure.
References
- 1.1992. American College of Chest Physicians–Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874. [PubMed] [Google Scholar]
- 2.Applied Research Ethics National Association 2002. Institutional Animal Care and Use Committee guidebook, 2nd ed Bethesda (MD): NIH. [Google Scholar]
- 3.Arras M, Rettich A, Cinelli P, Kasermann HP, Burki K. 2007. Assessment of postlaparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res 3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP–SCCM Consensus Conference Committee. American College of Chest Physicians–Society of Critical Care Medicine. Chest 101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 5.Bossink AW, Paemen L, Jansen PM, Hack CE, Thijs LG, Van Damme J. 1995. Plasma levels of the chemokines monocyte chemotactic proteins 1 and 2 are elevated in human sepsis. Blood 86:3841–3847. [PubMed] [Google Scholar]
- 6.Carbone ET, Lindstrom KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51:815–819. [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter JW. 2013. Exotic animal formulary. St Louis (MO): Elsevier Saunders. [Google Scholar]
- 8.Chawarski MC, Schottenfeld RS, O'Connor PG, Pakes J. 1999. Plasma concentrations of buprenorphine 24 to 72 h after dosing. Drug Alcohol Depend 55:157–163. [DOI] [PubMed] [Google Scholar]
- 9.Clark TS, Clark DD, Hoyt RF., Jr 2014. Pharmacokinetic comparison of sustained-release and standard buprenorphine in mice. J Am Assoc Lab Anim Sci 53:387–391. [PMC free article] [PubMed] [Google Scholar]
- 10.Cotroneo TM, Hugunin KMS, Shuster KA, Hwang HJ, Kakaraparthi BN, Nemzek-Hamlin JA. 2012. Effects of buprenorphine on a cecal ligation and puncture model in C57BL/6 mice. J Am Assoc Lab Anim Sci 51:357–365. [PMC free article] [PubMed] [Google Scholar]
- 11.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moldawer LL, Efron PA. 2010. Cecal ligation and puncture, chapter 19, unit 13. In: Coico R. Current protocols in immunology, suppl 90 Hobeken (NJ): Wiley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deitch EA. 2005. Rodent models of intraabdominal infection. Shock 24:19–23. [DOI] [PubMed] [Google Scholar]
- 13.Dejager L, Pinheiro I, Dejonchkheere E, Libert C. 2011. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19:198–208. [DOI] [PubMed] [Google Scholar]
- 14.Faller KM, McAndrew DJ, Schneider JE, Lygate CA. 2015. Refinement of analgesia following thoracotomy and experimental myocardial infarction using the mouse grimace scale. Exp Physiol 100:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flecknell P. 2009. Laboratory animal anesthesia, 3rd ed New York (NY): Elsevier Science [Google Scholar]
- 16.Gaskill BN, Karas AZ, Garner JP, Pritchett-Corning KR. 2013. Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp 82:51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins P. 2002. Recognizing and assessing pain, suffering and distress in laboratory animals: a survey of current practice in the UK with recommendations. Lab Anim 36:378–395. [DOI] [PubMed] [Google Scholar]
- 18.Heron M. 2015. Deaths: leading causes for 2012. Natl Vital Stat Rep 64:1–93. [PubMed] [Google Scholar]
- 19.Hess SE, Rohr S, Dufour BD, Gaskill BN, Pajor EA, Garner JP. 2008. Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. J Am Assoc Lab Anim Sci 47:25–31. [PMC free article] [PubMed] [Google Scholar]
- 20.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, Bland KI, Chaudry IH. 2005. Cecal ligation and puncture. Shock 24:52–57. [DOI] [PubMed] [Google Scholar]
- 21.Hugunin KMS, Fry C, Shuster K, Nemzek JA. 2010. Effects of tramadol and buprenorphine on select immunologic factors in a cecal ligation and puncture model. Shock 34:250–260. [DOI] [PubMed] [Google Scholar]
- 22.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 23.Institute for Laboratory Animal Research 2008. Recognition and alleviation of distress in laboratory animals, 1st ed Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 24.Institute for Laboratory Animal Research 2009. Recognition and alleviation of pain in laboratory animals, 1st ed Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 25.Iskander KN, Craciun FL, Stepien DM, Duffy ER, Kim J, Moitra R, Vaickus LJ, Osuchowski MF, Remick DG. 2013. Cecal ligation and puncture induced murine sepsis does not cause lung injury. Crit Care Med 41:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobi J. 2002. Pathophysiology of sepsis. Am J Health Syst Pharm 59 Suppl 1:S3–S8. [DOI] [PubMed] [Google Scholar]
- 27.Jirkof P, Fleischmann T, Cesarovic N, Rettich A, Vogel J, Arras M. 2013. Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Lab Anim 47:153–161. [DOI] [PubMed] [Google Scholar]
- 28.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. [DOI] [PubMed] [Google Scholar]
- 29.Leach MC, Klaus K, Miller AL, Scotto di Perrotolo M, Sotocinal SG, Flecknell PA. 2012. The assessment of postvasectomy pain in mice using behaviour and the mouse grimace scale. PLoS One 7: e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowes DA, Webster NR, Murphy MP, Galley HF. 2013. Antioxidants that protect mitochondria reduce interleukin 6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth 110:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin GS. 2012. Sepsis, severe sepsis, and septic shock: changes in incidence, pathogens, and outcomes. Expert Rev Anti Infect Ther 10:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, King OD, Mogil JS. 2012. Using the mouse grimace scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci 51:42–49. [PMC free article] [PubMed] [Google Scholar]
- 33.Morton DB, Griffiths PH. 1985. Guidelines on the recognition of pain, distress, and discomfort in experimental animals and an hypothesis for assessment. Vet Rec 116:431–436. [DOI] [PubMed] [Google Scholar]
- 34.Office of Laboratory Animal Welfare 2015. Public Health Service policy on humane care and use of laboratory animals. Bethesda (MD): Department of Health and Human Services. [Google Scholar]
- 35.Osuchowski MF, Connett J, Welch K, Granger J, Remick DG. 2009. Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit Care Med 37:1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramnath RD, Ng SW, Guglielmotti A, Bhatia M. 2008. Role of MCP1 in endotoxemia and sepsis. Int Immunopharmacol 8:810–818. [DOI] [PubMed] [Google Scholar]
- 37.Rau S, Kohn B, Richter C, Fenske N, Kuchenhoff H, Hartmann K, Hartle S, Kaspers B, Hirschberger J. 2007. Plasma interleukin 6 response is predictive for severity and mortality in canine systemic inflammatory response syndrome and sepsis. Vet Clin Pathol 36:253–260. [DOI] [PubMed] [Google Scholar]
- 38.Remick DG. 2007. Pathophysiology of sepsis. Am J Pathol 170:1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. 2008. Immunodesign of experimental sepsis by ceca ligation and puncture. Nat Protoc 4:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rock ML, Karas AZ, Gallo MS, Pritchett-Corning K, Gaskill BN. 2014. Housing condition and nesting experience do not affect the time to integrate to nest test (TINT). Anim Welf 23: 381–385. [Google Scholar]
- 41.Rock ML, Karas AZ, Rodriguez KB, Gallo MS, Pritchett-Corning K, Karas RH, Aronovitz M, Gaskill BN. 2014. The time-to-integrate-to-nest test as an indicator of wellbeing in laboratory mice. J Am Assoc Lab Anim Sci 53:24–28. [PMC free article] [PubMed] [Google Scholar]
- 42.Siempos II, Lam HC, Ding Y, Choi ME, Choi AM, Ryter SW. 2014. Cecal ligation and puncture-induced sepsis as a model to study autophagy in mice. J Vis Exp 84:e51066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song R, Kim J, Yu D, Park C, Park J. 2012. Kinetics of IL6 and TNFα changes in a canine model of sepsis induced by endotoxin. Vet Immunol Immunopathol 146:143–149. [DOI] [PubMed] [Google Scholar]
- 44.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. 2014. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 11:629–632. [DOI] [PubMed] [Google Scholar]
- 45.Speyer CL, Gao H, Rancilio NJ, Neff TA, Huffnagle GB, Sarma JV, Ward PA. 2004. Novel chemokine responsiveness and mobilization of neutrophils during sepsis. Am J Pathol 165:2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinhauser ML, Hogaboam CM, Matsukawa A, Lukacs NW, Strieter RM, Kunkel SL. 2000. Chemokine C10 promotes disease resolution and survival in an experimental model of bacterial sepsis. Infect Immun 68:6108–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umbro I, Gentile G, Tinti F, Muiesan P, Mitterhofer AP. 2016. Recent advances in pathophysiology and biomarkers of sepsis-induced acute kidney injury. J Infect 72:131–142. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe AM, Kennedy LH, Na JJ, Nemzek-Hamlin JA. 2015. Efficacy of tramadol as a sole analgesic for postoperative pain in male and female mice. J Am Assoc Lab Anim Sci 54:411–419. [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Zhi L, Moochhala S, Moore PK, Bhatia M. 2007. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture induced sepsis in mice by upregulating the production of cytokines and chemokines via NFκB. Am J Physiol Lung Cell Mol Physiol 292:L960–L971. [DOI] [PubMed] [Google Scholar]
- 50.Zisman DA, Kunkel SL, Strieter RM, Tsai WC, Bucknell K, Wilkowski J, Standiford TJ. 1997. MCP1 protects mice in lethal endotoxemia. J Clin Invest 99:2832–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]