Abstract
Introduction
The events in the cellular and molecular signaling triggered during inflammation mitigate tissue healing. The metabolic check-point control mediated by 5′-adenosine monophosphate-activated protein kinase (AMPK) is crucial for switching the cells into an activated state capable of mediating inflammatory events. The cell metabolism involved in the inflammatory response represents a potential therapeutic target for the pharmacologic management of inflammation.
Areas covered
In this article, a critical review is presented on triggering receptor expressed on myeloid cell (TREM) receptors and their role in the inflammatory responses, as well as homeostasis between different TREM molecules and their regulation. Additionally, we discussed the relationship between TREM and AMPK to identify novel targets to limit the inflammatory response. Literature search was carried out from the National Library of Medicine’s Medline database (using PubMed as the search engine) and Google Scholar and identified relevant studies up to March 30, 2016 using inflammation, TREM, AMPK, as the key words.
Expert commentary
The prevention of phenotype switching of immune cells during inflammation by targeting AMPK and TREM-1 could be beneficial for developing novel management strategies for inflammation and associated complications.
Keywords: Triggering Receptor Expressed on Myeloid Cells, Immune cells, Inflammation, 5′-adenosine monophosphate-activated protein kinase, Cell metabolism
1 Introduction
Inflammation is a vital response elicited by the immune system against infections, injury, and pathogenesis meant to restore tissue structure and physiological function. Inflammation is orchestrated by a cascade of molecular events mediated through the biomolecular signals between immune cells and target tissues. The key cellular events in inflammation are initiated by the influx of leukocytes into the affected area. The first mediator to enter the site is granulocytes such as neutrophils, which are quickly followed by monocytes. Monocyte migration from the circulation to the target tissue, and subsequent differentiation and proliferation allow for maturation into macrophages, inevitably progressing into the inflammatory response. These events cause the principal symptoms of inflammation; erythema, hyperemia, and edema. Organ dysfunction is frequently considered the fifth feature of inflammation [1]. Once the initiating stimulus subsides after a vigorous phagocytic phase, the inflammatory events are resolved with the aid of molecules like resolvins [2]. During the resolving phase, neutrophil withdrawal is prominent and granulocytes revert back to their pre-inflammatory phenotype, allowing for the restoration of physiological function. Any dysfunction in these events allow for scar tissue formation, organ dysfunction, and disease progression [3].
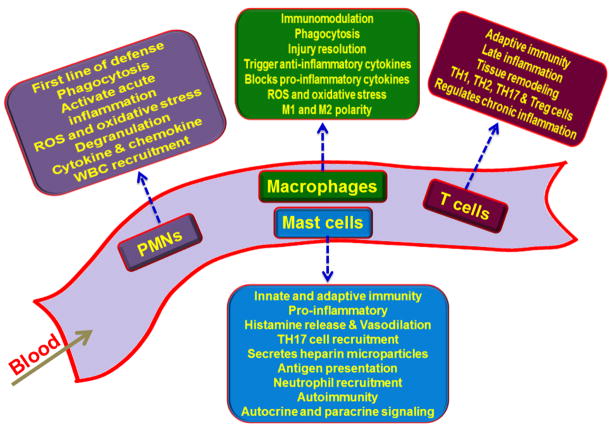
Inflammation can be either acute or chronic and is highly regulated. The alterations of the regulatory signals associated with inflammation are linked to the development and progression of chronic diseases like coronary artery disease, diabetes, obesity, and oncologic processes [4]. The extent of inflammation depends on the severity of injury [5]. Acute inflammation can lead to chronic inflammation depending on affected tissue, genetic makeup of the subject, and severity of the injury [5]. The interplay between the inflammatory cells, host cells, and biomolecules/signaling molecules is key in the regulation of inflammation and specific inflammatory pathways [6]. The delicate balance between these cells and their mediators is essential for the regulation of inflammatory responses. The major cells associated with inflammation and their functions are shown in Figure 1.
Figure 1. Specific role of immune cells in the progression and regulation of inflammatory responses.
Polymorphonuclear leukocytes (PMNs) are phagocytic cells associated with acute inflammation; macrophages have immunomodulatory effect owing to their phagocytic activity, cytokine release and repair responses; mast cells enhance inflammation due to histamine release and induction of vasodilation, and the T cells play role in tissue remodeling after injury.
Inflammatory responses begin when the stimuli signals bind to surface receptors on immune cells. These receptors include G-protein coupled transmembrane receptors, Fc receptors, cytokine receptors, cell adhesion molecules and pattern recognition receptors like TLRs and C-type lectins [7],. Activation of these receptors results in inflammatory events like phagocytosis, degranulation, reactive oxygen species production, chemotaxis and cytokine release [8, 9]. Triggering Receptor Expressed on Myeloid cells (TREM) are a recently discovered cell surface receptor of immunoglobulin superfamily which has implications on inflammation associated with pathologies. Among characterized TREMs, TREM-1 and TREM-2 are of greater importance owing to their antagonistic effects. TREM-1 amplifies proinflammatory mediators where TREM-2 is an anti-inflammatory modulator [10, 11]. The following sections deals with current understanding about TREM biology with respect to metabolic aspects of inflammation.
2 Triggering Receptor Expressed on Myeloid cells (TREM)
The inflammatory cells present a wide array of receptors that constantly evaluate the immune status (either innate or adaptive) of the body [12]. For example, G-protein coupled transmembrane receptors provoke inflammatory responses upon encountering a specific signal (pathogens, FMLP [N-formyl methionyl leucyl phenylalanine], lipid mediators, complement factors proinflammatory chemokines, endogenous molecules, among others) [13]. Toll-like receptors (TLRs) located on macrophages and dendritic cells are able to perceive general molecular patterns of microbes, like the structure of lipopolysaccharide (LPS) [14]. Similarly, mannose and scavenger receptors bind through mannose or mannose binding lectin and sets up the cell for phagocytosis [15]. In short, inflammatory reactions begin with interactions between receptors collectively called pattern recognition receptors (PRRs). Triggering receptor expressed on myeloid cells (TREM) are another class of cell-surface receptors that can magnify or lessen the inflammatory status depending on the type of PRR signal [16]. The relationship between these two receptors is still a topic of debate.
As the name implies, TREM has been expressed predominantly in myeloid cells, especially neutrophils and monocytes [17]. Granulocytes, dendritic cells and natural killer cells (NK) cells have also been found to have higher TREM expression. T cells and most B cell subsets express TREM molecules in low levels [18]. Apart from cells of the immune system, recent research indicates that TREM expression is not limited to immune cells, but in fact are found on non-immune cells also: fibroblasts, epithelial cells, lymph nodes, spinal cord, lungs, heart, placenta, kidney, and bones all have been found to express TREM [19]. Unfortunately, the exact signaling mechanism and function of these molecules in non-immune cells is still unknown.
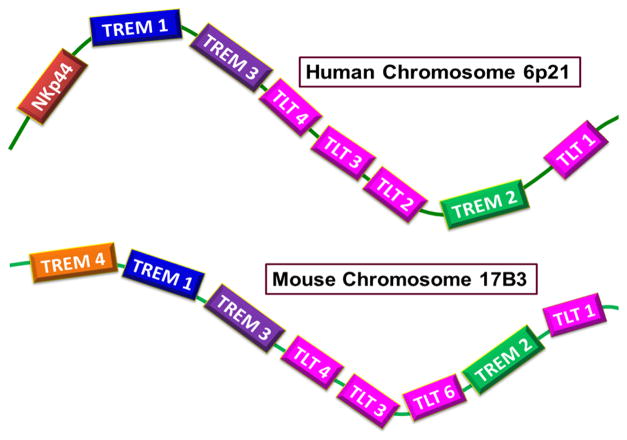
Human and mouse are both extensively studied systems for TREM biology. The individual genes of TREM (TREM-1 to TREM-4) belong to the immunoglobulin variable (IgV) domain receptor family. TREM, a cell surface activating transmembrane receptor, is comprised of a positive charged trans membrane domain (due to a Lys residue) and a cytoplasmic tail [20]. The genes for human TREM molecules (TREM-1 and TREM-2) are located on 6p21 (Figure 2). The DNA sequences encoding the TREM are contained within a gene cluster of structurally related receptors that trigger immune responses. TREM-like transcripts (TLT1 to TLT5) and NKp44 are homologous to TREM and they coexist within the structurally similar cluster. TLT genes code for IgV and NKp44 proteins that comprise a NK cell receptor [21, 22]. TLT-l is expressed in megakaryocytes α-granules and neonate thrombocytes, which offers protection against inflammation associated with hemorrhage.
Figure 2. Loci and arrangement of human and mouse TREM gene clusters.
Human TREM (triggering receptor expressed on myeloid cells) gene cluster is located on chromosome 6p21 carrying sequences for TREMs and TLTs. Mouse counterpart is located at chromosome 17B3 which bears an additional TREM gene (TREM-4) on comparing with that of human.
TLT-2 is found in B cells and peripheral lymphoid tissues. Upregulation of TLT-2 is seen in macrophages and neutrophils that are responding to inflammation but expression is minimal in naïve monocytes. Red pulp and marginal metallophilic macrophages in the spleen are the major sites for TLT-4 expression. Dendritic cells also express TLT-4 and are resistant to cavitation by TLR ligands [23]. The first discovery of the sequence homology of TREM-1 open reading frame (ORF) threw light to the elucidation of TREM-2 in murine macrophage cell line and then in humans [16]. Unlike TREMs, TLTs possess a tyrosine receptor inhibitory domain at the cytoplasmic tail domain [21]. Murine gene clusters for TREM has been mapped on chromosome 17C3 (Figure 2) [24]. TREM-3 [25] and TREM-4 [26] are exclusively found in mouse. TREM-3 shares around 40% sequence similarities with TREM-1 and is functionally similar [25, 27]. Plasmacytoid dendritic cells (pDCs) of mouse express TREM-4 (also called pDCTREM) which has around 20% amino acid homology with TREM-1 and TREM-2 [26].
TREM molecules require DAP12 (DNAX activation protein 12) for activation. DAP12 is an immunoreceptor tyrosine-based adaptor that transmits signals generated through surface receptors like TREM [28]. Human DAP12 gene is located on chromosome 19q13.1 and is translated into a 113 amino acid residue, homodimeric protein. Two Cys residues at positions 33 and 35 cross link to form a disulfide bond which constitutes the DAP12 homodimer.
Immunoreceptor tyrosine-based activation motif (ITAM) regulates cellular responses like adhesion, proliferation, migration, differentiation, gene expression, and is necessary for the action of DAP12. ITAMs (first identified in immune system) regulate cellular responses like adhesion, proliferation, migration, differentiation and gene expression. The ITAM motif on the cytoplasm is subjected to phosphorylation by casein kinase II and protein kinase C, an action that is necessary for the regulation of DAP12 [29]. On the other hand, TLTs have an intra-cellular immunoreceptor tyrosine-based inhibitory motif (ITIM) in their cytoplasmic tail to regulate downstream signaling [30]. ITAM bearing DAP12 adaptors bears an acidic amino acid (especially Asp) in transmembrane domain which interacts non-covalently to the transmembrane Lys residue of the receptors including TREMs [31, 32].
More than 20 DAP12 associated receptors have been characterized in humans and mice that act as either activators or inhibitors of the immune responses [33, 34]. These receptors belong to two families: the former to C-type lectin family, which includes MDL-1 (myeloid DAP12-associating lectin-1) and mouse NKG2D, and the latter to the Ig domain superfamily (Ig-SF), which includes TREM, NKp44, SIRP-β (signal-regulatory protein beta), and MAIR-II (myeloid-associated immunoglobulin-like receptor) [35, 36]. The binding between DAP12 and corresponding receptors cannot initiate the intra cellular signaling on their own [35]. Also, the phosphorylated ITAM activates Syk (Spleen tyrosine kinase) and NTAL/LAB (Non-T cell activation linker /linker for activation of B cells), which activates specific downstream signaling pathways including Akt, Ca2+, and MAPK (mitogen-activated protein kinase) [37, 38]. ITAMs are responsible for signal transduction of TREM pathways [39].
2.1 TREM-1
TREM-1 modulates inflammation through cytokine, chemokine and receptor upregulation [40]. TREM-1 is also reported to have the potential to magnify inflammatory responses initiated by TLRs and LPS [41]. The ligands of TREM-1 have been identified in the surface of platelets [42]. The existence of soluble TREM-1 ligands has also been discovered [43]. It has also been found that some damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) are also ligands for TREM-1. These molecules can act as PRR and function with a similar mechanism to TLRs [44]. Among the DAMPs, high-mobility group box 1 protein (HMGB1) and heat shock protein 70 (HSP70) are thought to be ligands for TREM1 [45]. All this data suggests that TREM1 functions by accepting a diverse array of ligands. The nature and binding kinetics, the mechanism and energetics of their interactions are still unknown.
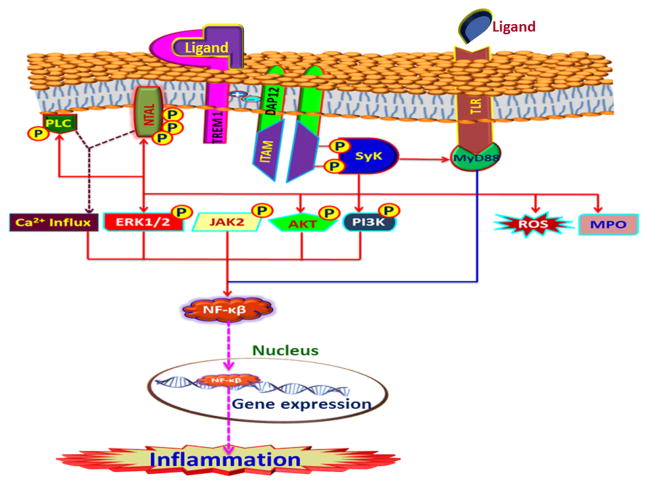
TREM-1 can elicit diverse responses through a multitude of mediators [46]. TREM-1 signaling mediated inflammatory responses are observed in both infectious and non-infectious models [47]. Upon encountering a ligand, TREM1 phosphorylates ITAM along with DAP12 and the downstream pathway is triggered. Src kinases phosphorylate specific proteins that recruit non-receptor tyrosine kinase Syk, which activates downstream signaling molecules, which, in turn, regulate inflammatory genes as well as NF-κB. These signaling molecules include PI3K, PLCγ, ERK1/2, and MAP kinases. Likewise, TREM-1 signaling modulates Ca2+ influx via Syk phosphorylation in an unknown mechanism [48]. In addition to cytokine upregulation, TREM-1 mediated degranulation and production of reactive oxygen species (ROS) aggravate inflammatory responses [42]. Attenuation of inflammation in TREM-1 deficient animal models confirms the proinflammatory signaling of TREM-1 [49].
Even though the exact interaction between TREM-1 and TLRs is unknown, it is believed that cross talk between them exists. TLR ligands have the capacity to upregulate TREM-1 mRNA expression and magnify TLR induced inflammatory responses. The expression of TREM-1 mRNA in LPS challenged macrophages was proven to be mediated by TLR-4/NF-κ B pathway [50]. TLR signaling does not elicit the expected similar result, [48]. Activation studies of TREM-1 by TLR ligands on monocytes also revealed the cumulative amplification of inflammatory cytokines [20]. Ornatowska et al. reported that silencing of TREM-1 genes had no direct effect on TLR-4 expression but down regulated Myd88 (myeloid differentiation primary-response gene 88) (adaptor protein) and IL-1b and IL-10, as seen in Figure 3, which suggests a possible mechanism of TREM-1/TLR cross talk [51, 52].
Figure 3. TREM-1 signaling mediated through DAP12 and downstream kinases leading to the expression of pro-inflammatory genes.
Upon ligand binding the phosphorylation of ITAM (immunoreceptor tyrosine-based activation motif) associated with the adaptor protein DAP12 (DNAX activation protein of 12 kDa) occurs resulting in the recruitment and activation of Syk (spleen tyrosine kinase). Syk phosphorylates a battery of downstream kinases that activates NF-κβ and facilitates its nuclear translocation where it functions as a transcription factor for a panel of pro-inflammatory genes. TLR (toll-like receptor) signaling also integrates with TREM-1 pathway via NF-κβ signaling. All these events result in inflammation.
The adaptor protein non-T cell activation linker (NTAL) is phosphorylated following TREM-1 activation [53]. NTAL has been found to be expressed in B cells, NK cells, monocytes, and mast cells. NTAL occupies a downstream position after DAP12 and Syk and binds with growth factor receptor-bound protein-22 (Grb-2), casitas B-lineage lymphoma-1 (c-Cbl) (ubiquitin ligase), and son of sevenless-1(Sos-1) (guanine nucleotide exchange factor) [54, 55], [46]. A study using siRNA knockout NTAL in myelomonocytic cell line revealed negative regulation of TREM-1 responses by way of ERK phosphorylation, TNF-α and IL-8 production, and Ca2+ flux alteration. Thus NTAL acts as a checkpoint for regulating the DAP12 mediated activation signals [46].
Caspase-recruitment domain-9 (CARD9) is another mediator of TREM-1 induced NF-κβ activation and production of cytokines. CARD9 joins with Bcl-10 after TREM-1 activation, allowing for IL-2 production. Similarly, CARD11 joins with mucosa associated lymphoid tissue translocation protein 1 (MALT1) and Bcl-10 for NF-κβ activation in lymphocytes [56, 57]. TREM-1 was reported to work with several other PRRs like NAIP, CIITA, HET-E and TP-1-leucine-rich repeat and activates inflammation [58].
2.2 TREM-2
TREM-2 (first identified in human monocyte derived dendritic cells) is an immune regulator that is expressed mainly in myeloid cells and acts by ITAM mediated DAP12 adaptor. Apart from the immunological function TREM-2 is also involved in osteoclastogenesis, brain homeostasis and phagocytosis of bacteria [59]. TREM-2 elicits a protective role from autoimmune diseases [60]. The non-myeloid epithelial cells of the genitourinary tract and fallopian tubes constitutively express TREM-2. TREM-2 signaling also activates downstream effector kinases like ZAP70, SYK and PI3K and PLC-γ. TREM-2 deficient macrophages exhibit an increased susceptibility to inflammation, a finding that illustrates the role of TREM-2 as an anti-inflammatory agent [61].
Like TREM-1, the TREM-2 receptor possesses several ligands on myeloid and non-myeloid cells [37] but many ligands are still unknown. TREM-2 activation results in the upregulation of receptors like CD86, CD40 and MHC class II in dendritic cells. Stimulation of TREM-2 protects the dendritic cells from apoptosis that allows for cytoprotective actions [62]. TREM-2 binds to its corresponding ligands along with the downstream mediators like DAP12, plexin-A1, and semaphorin 6D to form a multimeric complex, the functions of which remain unknown [28].
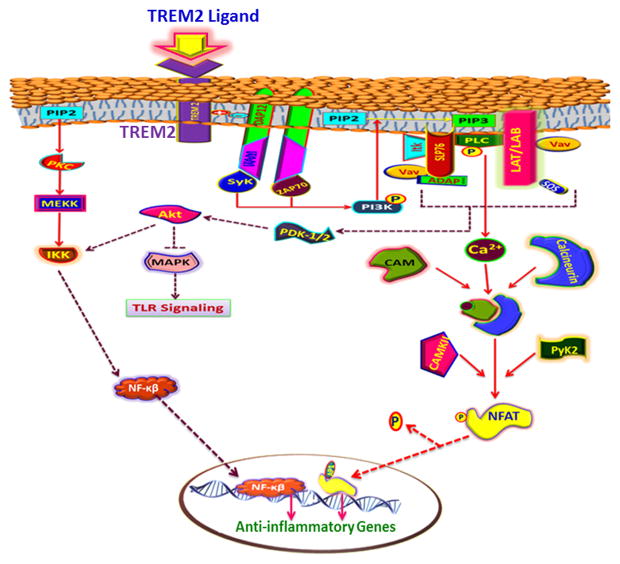
TREM-2 signaling can also proceed through DAP12, an action that shuts down inflammatory pathways. The TREM-2 pathway runs without NF-κβ but proceeds with Ca2+ influx following ERK and PI3K activation. The down-regulation of inflammatory cytokines, especially TNF-α, reveals the antagonistic effects of TREM-2 over TREM-1 [19]. TREM-2 is secreted in Golgi complex and is transported to cell membrane upon stimulation [63]. TREM-2 pathway is mainly involved in cell survival, cell activation, cell differentiation and cytoskeleton orientation [64]. Upon ligand binding with the TREM-2, ITAM associates with the DAP12 and are phosphorylated by SRC kinase, which in turn recruits Syk and ZAP70 kinases. In mouse Syk alone is prominent while in human both Syk and ZAP70 couple to the ITAMs via the SH2 domain. These kinases phosphorylate PI3K, activating it. Then, PI3K phosphorylates membrane phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-triphosphate (PIP3) and recruits PLC-γ, TEC-family kinases, and Vav to cell surface. The complex formation of these mediator proteins activates the Akt pathway. Akt/PI3K regulates the translocation of NF-κβ and the expression of inflammatory genes and inhibits the TLR by inhibiting the MAPK signaling at RAF [65].
ZAP70 has also been found to be a target for LAT (T cells), LAB (B cells), and SLP-76 (SH-2 domain-containing leukocyte protein of 76 kDa). Activation of these cells can trigger multiple signaling cascades [66]. LAT/LAB phosphorylation results in the recruitment of mediators like PLC-γ, IL-2-induced tyrosine kinase (Itk), growth factor-receptor-bound protein 2 (GRB2), son of sevenless homologue (SOS), and GRB2-related adaptor downstream of She protein (Gads). Unlike LAT, LAB lacks the PLC-γ binding domain and acts mainly in coordination with GRB2 [67, 68]. LAT recruits SLP-76 and the N-terminal domain of SLP-76 binds Vav, NCK (adaptor protein), and Itk. Gads and PLC-γ interacts with the PRP domain while HPK1 (hematopoietic progenitor kinase 1) and ADAP (degranulation-promoting adapter protein) bind to the C-terminal domain of SLP-76 [66, 69]. In short, both LAT and SLP-76 are major factors in assembly and stabilization of the signaling complex for TREM-2.
Itk (IL-2 inducible Tcell kinase) presents Vav to the antigen presenting cell (APC) surface in a kinase-independent fashion. Active Itk stimulates PLC-γ by phosphorylating and hydrolyzing PIP2 into diacylglycerol (DAG) and inositol (1,4,5)-triphosphate (IP3) within the membrane. DAG and IP3 are second messengers for T-cell differentiation, proliferation and response [24]. DAG activation opens NF-κβ signaling through protein kinase Ch (PKCh) and MAPK pathway via Ras guanyl nucleotide-releasing proteins (RasGRPs) [70, 71]. IP3 acts as a ligand for the Ca2+ channel in the endoplasmic reticulum (ER) membrane [66, 72]. IP3 activation initiates Ca2+ influx which activates the phosphatase activity of calcineurin. Calcineurin is responsible for the activation of kinases, including Ca2+/calmodulin-dependent protein kinase (CaMK) and protein tyrosine kinase 2 (Pyk2), as well as calcineurin-mediated dephosphorylation of nuclear factor activated T-cell (NFAT) results its nuclear translocation. In addition calcineurin mediates dephosphorylation of NFAT results its nuclear translocation. Inside the nucleus, NFAT regulates a wide range of transcriptional factor for various biological responses [73].
As in TREM-1, ITIM controls ITAM activation. The binding of SH2 domain-containing inositol phosphatase-1 (SHIP1) to DAP12 inhibits Syk recruitment, thereby inhibiting PI3K. The regulation of TREM-2 pathway is crucial to preventing uncontrolled cell activation and proliferation [64].
TREM-2 can act in coordination with RANK and its ligands. Both can activate DAP12 by recruiting Syk to activate downstream signaling and, ultimately, result in nuclear translocation of NFATc1 [73]. Sema6D (a family of secreted and membrane associated proteins) was also reported to activate TREM-2/DAP12 signaling by phosphorylating DAP12 which demonstrates a link between TREM-2, cell adhesion, and motility functions [74]. Similarly, the TREM-2/DAP12 complex cross talk with cytokine pathways, integrin signaling and TLRs, which are mediated through Ca2+ signaling and Syk activation. At the same time, TLR signaling complex formation is inhibited by DAP12 through PLC-γ activation. PLC-γ acts by limiting the PIP2 availability, which is necessary for the recruitment of the TIRAP/Mal (toll-interleukin 1 receptor domain-containing adaptor protein/MyD88 adapter-like) and MyD88. TIRAP/Mal and MyD88 plays a crucial role in initiating TLR signaling [74, 75]. The key events of TREM-2 signaling are summarized in Figure 4.
Figure 4. TREM-2 signaling mediated through DAP12 and downstream kinases leading to the expression of anti- inflammatory genes.
TREM-2 ligand binding activates DAP12 and downstream Syk and ZAP-70 (Zeta-chain-associated protein kinase 70) which in turn activate PI3K (phosphatidylinositol-4,5-bisphosphate 3-kinase) pathway resulting in Ca2+ influx. Ca2+ activates NFAT (nuclear factor of activated T cells) by phosphorylation through calmodulin kinase. NFAT translocates to nucleus and triggers the expression of anti-inflammatory genes. PI3K inhibits TLR (toll-like receptor) signaling via Akt pathway which in turn inhibits inflammation.
2.3 sTREM
Certain clinical conditions, like infections, induce shedding of a soluble form of TREM receptors from the membrane surface into the body fluids. Soluble TREM (sTREM) can be a diagnostic tool for clinically [76]. The plasma level of sTREM-1 is found to be increased after sepsis, systemic inflammatory response syndrome, cardiac arrest, cancer, arthritis, and lung disorders [77]. sTREM-1 is a glycoprotein of 27kDa which may be cleaved from its extra cellular domain by matrix metalloproteinases (MMPs). This discovery was substantiated by the finding that TREM-1 from CD14+ monocyte cells was decreased after six hours of LPS challenge [78]. TREM-1 mRNA can undergo alternate splicing that may results in sTREM1, the inciting factors of which are not known [79]. sTREM-1 has also been found in cerebral spinal fluid (CSF), in addition to the plasma, of bacterial meningitis patients and can be a marker to distinguish bacterial meningitis from non-bacterial forms [80]. Compared to soluble receptors like ICAM-1 and VCAM-1, it is believed that sTREM-1 and sTREM-2 negatively regulate the TREM receptors by eliciting a neutralizing effect [19].
A myriad of reports are being published in TREM signaling every year. Still the actual mechanism of action of these molecules and its implication to cell metabolism, apoptosis, and cell differentiation as well as a correlation of these biological responses with inflammatory status is limited. To the best of our knowledge the literature correlating the metabolism of immune cells and the target cells with inflammation is lacking.
3 Inflammation versus Metabolism – a gap to be filled
Recent findings suggest that cells that upregulate inflammation, such as Th17 lymphocytes and M1 macrophages, rely mostly on aerobic glycolysis for energy production when challenged with LPS. While the cells that mitigate inflammation, Treg cells, M2 macrophages, and memory T cells, prefer oxidative metabolism with limited glycolysis [81]. The energy demand of pro-inflammatory cells is higher than that of unchallenged cells. For instance, upon inflammation, macrophages switch to an activated form that promotes secretion and release of host response signals, facilitating phagocytosis and presenting antigens. [81, 82].
The increase in glycolysis allows for a rapid increase of intercellular ATP that is sufficient to maintain mitochondrial integrity after LPS challenge [83]. This increase in ATP maintains the viability and function of macrophages and other cells associated with inflammation until they perform their designated functions [84]. Similarly the activation of the pentose phosphate pathway provides intermediates for nucleotide biosynthesis and metabolic energy [85]. TH17 cells shift to Treg upon inhibition of glycolysis with 2-deoxyglucose. This shift shows that the plasticity of these cells is a function of their metabolic status [86, 87]. Also, TH1 cells sequester glyceraldehyde phosphate dehydrogenase (GAPDH) from IFN-γ mRNA and this prevents the IFN-γ mRNA from the inhibition by GAPDH to increase the glycolysis [88]. Similarly, the oxidative metabolism of the memory T cells switches to glycolysis upon shifting to effector T cells [89]. This cellular physiology demonstrates a link between inflammation and metabolism [81].
During the resting state, dendritic cells rely on oxidative metabolism and activation with PAMPs, like TLRs, that switch to glycolysis as a main route of metabolism. The activated dendritic cells are characterized by increased surface expression of the glucose transporter GLUT1, enhanced lactate accumulation, limited mitochondrial oxygen consumption and increased flux of PI3K and Akt signaling [83]. The anti-inflammatory cytokine IL-10 blocks TLR activation, prevents the switch to glycolysis, and favors oxidative metabolism in dendritic cells. TLR4 impedes the mitochondrial metabolism by upregulating the iNOS enzyme [90]. NO (nitric oxide) competes with oxygen and inhibits the cytochrome c oxidase reaction of the electron transport chain. In this way, NO alters the mitochondrial integrity, causing the release of cytochrome c into the cytosol, leading to the activation of BAX and thereby apoptosis [85]. These facts are the evidences of anti-inflammatory status of the mitochondrial metabolism [83].
M1 macrophages express glycolytic regulator enzyme 6-phosphofructo-2-kinase isoform PFKFB3. This enzyme increases the glycolytic flux by accumulating the intermediate fructose-2,6-bisphosphate [91]. However, on M2 macrophages where the oxidative metabolism is predominant, PFKFB1 is expressed [91]. In M2 macrophages, outstanding oxidative lipid metabolism is evident and mediated through STAT6 induction of peroxisome-proliferator-activated receptor-γ co-activator-1β (PGC-1β) [92]. These changes depend on the activation of M2 macrophages by IL-4. In vivo studies showed that the PGC-1β prefers M2 phenotype to reduce macrophage mediated inflammation. This was confirmed by the limited oxidative metabolism in PGC-1β knockdown cells [93].
The expression of pro-inflammatory receptors like TREM-1 on immune cells during the process of inflammation alters the metabolic status of these cells. The demand for high energy biomolecules and the overall energy status of cells increase during the inflammatory phase. Hence, the control of energy yielding metabolic pathways in such cells limits them from conversion to an activated state which, in turn, minimize inflammation.
4 Inflammation versus metabolic checkpoint – a new target to be explored
Cellular energy homeostasis is regulated at the metabolic check point (5′adenosine monophosphate activated protein kinase) based on intercellular nutrient/ATP levels. This process is mediated by 5′adenosine monophosphate activated protein kinase (AMPK). AMPK is a protein complex that senses the cellular energy status of the cell with respect to the AMP:ATP ratio. AMPK is especially active during hypoxia, starvation and other physiological stress [94]. The phosphorylation by AMPK inhibits several key regulatory proteins of lipid and carbohydrate biosynthesis and metabolism. At the same time, AMPK activates energy yielding pathways like fatty acid oxidation, glucose and caloric influx, and mitochondrial biogenesis and activation. AMPK regulates the homeostasis between anabolic and catabolic pathways of energy metabolism [95].
Recent research on energetics of inflammation has focused on alterations and regulation of metabolic pathways in inflammatory cells and the target tissues. AMPK is a Ser/Thr protein kinase allosterically activated by AMP (and also by ADP) [96]. A heterotrimeric protein, AMPK possesses a catalytic α-subunit and two regulatory β and γ subunits. The highly conserved Thr 172 of the α-subunit is the site of phosphorylation for the activation of the enzyme by the protein kinase LKB1 (Liver kinase B1). LKB1 is a constitutively expressed protein, thereby, activating the AMPK continuously [97]. Binding of ATP to the γ-subunit reduces Thr 172 phosphorylation while ADP/AMP enhances AMPK activation [98]. If the ratio of AMP:ATP remains unaltered, AMPK can be activated by intracellular Ca2+. Under such conditions, the Ca2+/calmodulin-dependent protein kinase kinase b (CaMKKb) phosphorylates Thr172 for AMPK activation [99]. H2O2 has also been reported to activate AMPK by oxidative modification of Cysteine residues in the α-subunit [100]. Being a key regulator of metabolism AMPK can be a central target for inflammatory diseases.
The activation of AMPK has been found to inhibit the severity of inflammation. The aggravation of inflammatory reactions in AMPK inhibited cells has provided some proof as to its anti-inflammatory effects [101]. AMPK suppresses iNOS in the presence of AICAR (5′-aminoimidazole-4-carboxamide ribonucleoside, an AMPK activator) activated in L6 myocytes and microglial cells which shows its anti-inflammatory role [102, 103]. In palmitate-challenged HUVECs (human umbilical cord endothelial cells), the NF-κβ reporter gene expression was effectively down regulated by AMPK [104]. AMPK mediated NF-κβ inhibition has also been reported in astrocytes, hepatic stellate cells, chondrocytes, neutrophils, and macrophages [101]. The decreased deterioration of IκBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha) and subsequent binding of p65 to IL-6 promoter in LPS challenged macrophages by AMPK is another evidence for anti-inflammatory effects [105]. There are two possible mechanisms by which AMPK inhibits NF-κβ signaling. The first involves the phosphorylation of a transcriptional co-activator, p300, and masking of acetylation at Lys22 of p65 by constitutively expressed AMPK (endothelial cells). This expression in turn blocks the TNF-α binding of NF-κβ to the inflammatory genes [106]. The second involves AMPK phosphorylating IKKb at Ser 177 and Ser 181 to inhibit subsequent phosphorylation of IκBα and p65 (COS-7 cells). This inhibition also blocks the NF-κβ via TNF-α [107].
As with the AMP:ATP ratio, the NAD+ levels in the cells also represents the metabolic status. NAD+ regulates inflammation by a link with the NLRP3 inflammasome via sirtuin deacetylases. This enzyme mediates the proinflammatory IL-1β function and also activates AMPK [108]. The link between alterations in metabolism and NLRP3 expression has been well established in macrophages. The depletion of NAD+ in activated macrophages due to mitochondrial dysfunction reduces the activity of NAD+-dependent deacetylase SIRT. SIRT hypofunction causes the cellular building up of acetylated tubulin, the SIRT substrate, and leads to SIRT polymerization. Mitochondrial ROS activates NLRP3 and NLRP3 cause the localization of tubulin in mitochondria [109, 110]. SIRT1 and SIRT6 switches glycolysis to fatty acid oxidation by way of nicotinamide phosphoribosyl transferase (NAMPT) enzyme [111]. Deacetylation of p65 by SIRT1 inactivates NF-κβ pathway [112] and deacetylation of PGC-1β (also by SIRT1) promotes fatty acid oxidation [113, 114]. Inhibition of PPARγ (a transcription factor that modulate genes for lipid storage) by SRIT1 shifts energy production from glycolysis to fatty acid oxidation [115].
A positive feedback relationship between AMPK and SIRT1 exists [116]. SIRT1 mediated deacetylation of LKB1facilitates its cytosolic translocation and AMPK activation The anti-inflammatory effects of SIRT1 is further confirmed by the enhanced proinflammatory cytokine response in SIRT1 knockdown macrophages [117].
Molecular signaling pathways like JNK, p38, ERK1/2, and MAPK aggravate inflammatory responses [118]. AMPK suppresses the proinflammatory cytokine mediated activation of MAPK and JNK [119]. AMPK suppresses JAK-STAT (Janus kinase-signal transducer and activates transcription) signaling by induction of the orphan nuclear receptor protein SHP (small heterodimer partner). This prevents STAT3 from accessing DNA and prevents the recruitment of downstream mediator SOCS3 (suppressor of cytokine signaling 3) to its promoter following the IL-6 challenge [120, 121]. AMPK can also decrease leukocyte mobility to the inflammatory site by down-regulating the adhesion molecules like VCAM-1 (vascular cell adhesion protein 1), ICAM-1 (Intercellular Adhesion Molecule 1), selectins, and MCP-1 [122]. AMPKs can also decrease inflammation through the regulation of lipid metabolism. The infiltration of M1 macrophages in adipose tissue and accumulation of lipids in their cytoplasm is considered one of the hallmarks of inflammation [123]. AMPK regulates lipid metabolism by balancing lipid synthesis and oxidation. AMPK mediates phosphorylation of the lipid biosynthetic enzyme acetyl-CoA carboxylase (ACC), leading to its inactivation and subsequent lipogenesis. On the other hand, AMPK enhances lipid oxidation via mitochondrial activation through PGC-1α (peroxisome proliferator-activated receptor gamma coactivator) [124, 125], a very crucial step since fatty acids like palmitate can activate NF-κβ and JNK pathways. Inhibition of AMPK triggers the both NF-κβ and JNK pathways [126]. The anti-inflammatory role of AMPK is given in Figure 5.
Figure 5. Integration of various cellular signaling pathways of metabolism by AMPK leading to inhibition of inflammatory responses.
AMPK (5′ AMP-activated protein kinase) is activated by LKB1 (liver kinase B1) through SITR1 (sirtuin 1 - silent mating type information regulation 2 homolog 1) and Ca2+ through CAMKKb (calmodulin-dependent protein kinase kinase 2). On activation, AMPK inhibits a battery of enzymes and pathways to enhance inflammation. At the same time, AMPK activates lipid oxidation through PGC-1α (Peroxisome proliferator-activated receptor-γ coactivator-1α) and inhibits fatty acid synthesis which limits the availability of lipid moieties for the synthesis of pro-inflammatory mediators like prostaglandins.
AMPK has been considered as a cellular metabolic check point as it regulates all the energy metabolism of the body. Activation of AMPK suppresses the pathways triggering NF-κβ activation and subsequent expression of pro-inflammatory genes. AMPK also inhibit lipid accumulation in the inflammatory and target cells that alleviate inflammation. Strategies that enhance the activity of AMPK in the cells associated with inflammation could result in newer opportunities in the better management of inflammation.
5 Future perspectives
Inflammation is an essential immunological response of the body. It is a delicate balance between leukocyte subsets, secreted biomolecule signals from these cells, and signals from target cells. Chronic inflammation slows the inherent repair process of the body and can become pathologic. The balance between cellular and molecular components is necessary for the regulation of inflammation. PMNLs, macrophages/monocytes, mast cells and T cells are necessary for the initiation and execution of inflammatory responses. The secreted chemokines and cytokines, especially interleukins TNFα and TGFβ, are also significant for inflammation. Targeting inflammation therapeutically is a common approach for disease management.
TREMs play and important role in the inflammatory process. Among the characterized TREM molecules, TREM-1 is considered pro-inflammatory while TREM-2 mitigates the inflammatory response. Even though certain mechanisms have been proposed for the TREM action, the actual mechanism is still unknown. DAP12 mediated signaling for TERM is widely accepted model that involves other mediators such as ERK, MAPK, JNK, PI3K, and calcium. The actual ligands for both TREM-1 and TREM-2 have to yet be defined. The existence of a link between TLR pathway and TREM is also a mystery. Variants of TREM other than TREM-1 and TREM-2 and their soluble forms are yet to be unveiled. Targeting TREM-1 for alleviating the inflammation may be an effective target for developing future anti-inflammatory therapies.
The metabolism and energetics of inflammatory leukocytes and their switching to active forms is the key to study life and longevity of these cells. The energy to deal with the harsh environments like ischemia, ROS and hypoxia during acute inflammation allows these cells to execute their desired function promptly. It is known that activated cells prefer glycolysis rather than oxidative metabolism. Such a shift in metabolism in inflammatory cells is mediated by metabolic checkpoint executed by AMPK. Apart from these, AMPK plays a central role in the inflammatory response by regulating several pro-inflammatory pathways. AMPK limits the availability of lipid metabolites for the synthesis of inflammatory mediators like leukotrienes by channeling them to mitochondria for oxidation. The anti-inflammatory role of AMPK provides a link between metabolism and inflammation.
AMPK may have implications in TREM physiology and their interplay may regulate inflammation. There is no reported literature addressing this concept. Since AMPK may mitigate the inflammatory response, it is thought to exhibit an inhibitory effect on TREM-1 and promote TREM-2 activation. These pathways both share NF-κβ signaling. The accumulation of lipids in inflamed and inflammatory cells after chronic inflammation signifies the down regulation of AMPK. In such cells the TREM-1 expression is greater than TREM-2 expression, which conveys the severity of inflammation. The possibility of alleviating inflammation by AMPK activation, and subsequent TREM-1 inhibition, may provide an opportunity for new regimes for inflammation management.
6 Expert commentary
Mechanism of activation of immune cells to trigger an inflammatory response is the central focus of research aiming to ameliorate the ill effects of inflammation. The prevention of phenotype switching of various immune cell types prior and post inflammation can open new opportunities for the management of inflammation and associated complications. Metabolism and energetics of activated immune cells and the correlation between AMPK and TREM-1 are yet to be unveiled. Antagonistic effects of TREM-1 and TREM-2 in regarding to inflammation is interesting but the underlying mechanism is largely unknown. Simultaneous targeting of AMPK and TREM-1 will be beneficial for the therapeutic management of inflammation.
7 Five-Year view
Even though TREM-1 and TREM-2 takes part in inflammation, their underlying mechanism of activation and action, the potential signals involved, functional ligands, detailed studies on the thermodynamics of ligand binding are warranted. Role of TREM-1 and TREM-2 in non-immune cells in regulating inflammation needs to be explored. The metabolism and energetics of activated inflammatory cells is also gaining significance in the current scenario. The potential role of AMPK in regulating inflammation by altering metabolic pathways has to be fully elucidated. The link between AMPK and TREM expression and signaling is poorly known. Novel approaches to specifically target the activated immune cells can also contribute for developing promising strategies for the treatment of inflammation.
8 Key issues.
Inflammation is associated with cellular and molecular components.
Persistence of inflammation causes delay in tissue healing responses after injury/pathology.
PMNLs, mast cells, monocytes/macrophages, and T cell population are key cellular players of inflammation.
Present understanding about the mechanism of regulation of inflammation by TREMs is reviewed.
Signaling pathways and triggers of TREM-1 and TREM-2 can form excellent targets for management of inflammation.
Metabolism of activated immune cells varies significantly from their pre-activated state.
Role of AMPK in metabolism of inflammatory cells’ and their subsequent activation is well known.
The possibilities of AMPK mediated TREM activation or vice versa are still a dogma.
Strategies for targeting AMPK and TREMs simultaneously are beneficial for the management of inflammation
Footnotes
Declaration of Interests
This work was supported by research grants R01 HL116042, R01 HL112597 and R01 HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA, and Haddix grant to MF Dilisio. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Libby P. Inflammatory Mechanisms: The Molecular Basis of Inflammation and Disease. Nutr Rev. 2007 Dec;65:140–146. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 2.Cash JL, Norling LV, Perretti M. Resolution of inflammation: targeting GPCRs that interact with lipids and peptides. Drug Discov. Today. 2014 Aug;19:1186–1192. doi: 10.1016/j.drudis.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol. 2011 May;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Shea JJ, Murray PJ. Cytokine Signaling Modules in Inflammatory Responses. Immunity. 2008 Apr;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart DA. Curbing Inflammation in Multiple Sclerosis and Endometriosis: Should Mast Cells Be Targeted? Int J Inflamm. 2015;2015:1–10. doi: 10.1155/2015/452095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punchard NA, Whelan CJ, Adcock I. The Journal of Inflammation. J Inflamm Lond Engl. 2004 Sep;1:1. doi: 10.1186/1476-9255-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Futosi K, Fodor S, Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013 Nov;17:638–650. doi: 10.1016/j.intimp.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi O, Akira S. Pattern Recognition Receptors and Inflammation. Cell. 2010 Mar;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Hall SC, Agrawal DK. Toll-like receptors, triggering receptor expressed on myeloid cells family members and receptor for advanced glycation end-products in allergic airway inflammation. Expert Rev Respir Med. 2016 Feb;10:171–184. doi: 10.1586/17476348.2016.1133303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen AH, Koenck C, Quirk SK, Lim VM, Mitkov MV, Trowbridge RM, Hunter WJ, Agrawal DK. Triggering Receptor Expressed on Myeloid Cells in Cutaneous Melanoma: TREMs in Melanoma. Clin Transl Sci. 2015 Oct;8:441–444. doi: 10.1111/cts.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelham CJ, Agrawal DK. Emerging roles for triggering receptor expressed on myeloid cells receptor family signaling in inflammatory diseases. Expert Rev Clin Immunol. 2014 Feb;10:243–256. doi: 10.1586/1744666X.2014.866519. [DOI] [PubMed] [Google Scholar]
- 12***.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006 Dec;7:1266–1273. doi: 10.1038/ni1411. This article reviews TREM expression and function in various cell types, the TREM ligands and challenges in TREM biology. [DOI] [PubMed] [Google Scholar]
- 13.Funk CD. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science. 2001 Nov;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 14.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000 Aug;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 15.Linehan SA, Martinez-Pomares L, Gordon S. Mannose receptor and scavenger receptor: two macrophage pattern recognition receptors with diverse functions in tissue homeostasis and host defense. Adv Exp Med Biol. 2000;479:1–14. doi: 10.1007/0-306-46831-X_1. [DOI] [PubMed] [Google Scholar]
- 16.Genua M, Rutella S, Correale C, Danese S. The triggering receptor expressed on myeloid cells (TREM) in inflammatory bowel disease pathogenesis. J Transl Med [Internet] 2014 Dec; doi: 10.1186/s12967-014-0293-z. [cited 2015 Dec 14];12. Available from: http://www.translational-medicine.com/content/12/1/293. [DOI] [PMC free article] [PubMed]
- 17.Yuan Z, Syed MA, Panchal D, Joo M, Colonna M, Brantly M, Sadikot RT. Triggering Receptor Expressed on Myeloid Cells 1 (TREM-1)-mediated Bcl-2 Induction Prolongs Macrophage Survival. J Biol Chem. 2014 May;289:15118–15129. doi: 10.1074/jbc.M113.536490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rigo I, McMahon L, Dhawan P, Christakos S, Yim S, Ryan LK, Diamond G. Induction of triggering receptor expressed on myeloid cells (TREM-1) in airway epithelial cells by 1,25(OH)2 vitamin D3. Innate Immun. 2012 Apr;18:250–257. doi: 10.1177/1753425911399796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roe K, Gibot Sã©, Verma S. Triggering receptor expressed on myeloid cells-1 (TREM-1): a new player in antiviral immunity? Front Microbiol [Internet] 2014 Nov; doi: 10.3389/fmicb.2014.00627. [cited 2015 Dec 16];5. Available from: http://journal.frontiersin.org/article/10.3389/fmicb.2014.00627/abstract. [DOI] [PMC free article] [PubMed]
- 20.Bouchon A, Dietrich J, Colonna M. Cutting Edge: Inflammatory Responses Can Be Triggered by TREM-1, a Novel Receptor Expressed on Neutrophils and Monocytes. J Immunol. 2000 May;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 21.Allcock RJN, Barrow AD, Forbes S, Beck S, Trowsdale J. The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur J Immunol. 2003 Feb;33:567–577. doi: 10.1002/immu.200310033. [DOI] [PubMed] [Google Scholar]
- 22.Cantoni C, Bottino C, Vitale M, Pessino A, Augugliaro R, Malaspina A, Parolini S, Moretta L, Moretta A, Biassoni R. NKp44, A Triggering Receptor Involved in Tumor Cell Lysis by Activated Human Natural Killer Cells, Is a Novel Member of the Immunoglobulin Superfamily. J Exp Med. 1999 Mar;189:787–796. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Won K-J, Park S-W, Lee S, Kong I-K, Chae J-I, Kim B, Lee E-J, Kim D-K. A New Triggering Receptor Expressed on Myeloid Cells (TREM) Family Member, TLT-6, is Involved in Activation and Proliferation of Macrophages. Immune Netw. 2015;15:232. doi: 10.4110/in.2015.15.5.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paradowska-Gorycka A, Jurkowska M. Structure, expression pattern and biological activity of molecular complex TREM-2/DAP12. Hum Immunol. 2013 Jun;74:730–737. doi: 10.1016/j.humimm.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Chung D-H, Seaman WE, Daws MR. Characterization of TREM-3, an activating receptor on mouse macrophages: definition of a family of single Ig domain receptors on mouse chromosome 17. Eur J Immunol. 2002 Jan;32:59–66. doi: 10.1002/1521-4141(200201)32:1<59::AID-IMMU59>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 26.Watarai H, Sekine E, Inoue S, Nakagawa R, Kaisho T, Taniguchi M. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc Natl Acad Sci. 2008 Feb;105:2993–2998. doi: 10.1073/pnas.0710351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radaev S, Kattah M, Rostro B, Colonna M, Sun PD. Crystal Structure of the Human Myeloid Cell Activating Receptor TREM-1. Structure. 2003 Dec;11:1527–1535. doi: 10.1016/j.str.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Klesney-Tait J, Colonna M. Uncovering the TREM-1-TLR connection. Am J Physiol - Lung Cell Mol Physiol. 2007 Dec;293:L1374–L1376. doi: 10.1152/ajplung.00415.2007. [DOI] [PubMed] [Google Scholar]
- 29.Call ME, Wucherpfennig KW, Chou JJ. The structural basis for intramembrane assembly of an activating immunoreceptor complex. Nat Immunol. 2010 Nov;11:1023–1029. doi: 10.1038/ni.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King RG, Herrin BR, Justement LB. Trem-Like Transcript 2 Is Expressed on Cells of the Myeloid/Granuloid and B Lymphoid Lineage and Is Up-Regulated in Response to Inflammation. J Immunol. 2006 May;176:6012–6021. doi: 10.4049/jimmunol.176.10.6012. [DOI] [PubMed] [Google Scholar]
- 31.Underhill DM, Goodridge HS. The many faces of ITAMs. Trends Immunol. 2007 Feb;28:66–73. doi: 10.1016/j.it.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol Baltim Md 1950. 2006 Aug;177:2051–2055. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 33.Van Bergen J, Koning F. The tortoise and the hare: slowly evolving T-cell responses take hastily evolving KIR. Immunology. 2010 Nov;131:301–309. doi: 10.1111/j.1365-2567.2010.03337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguilar H, Álvarez-Errico D, García-Montero AC, Orfao A, Sayós J, López-Botet M. Molecular Characterization of a Novel Immune Receptor Restricted to the Monocytic Lineage. J Immunol. 2004 Dec;173:6703–6711. doi: 10.4049/jimmunol.173.11.6703. [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Barriocanal A, Sayós J. Molecular and functional characterization of CD300b, a new activating immunoglobulin receptor able to transduce signals through two different pathways. J Immunol Baltim Md 1950. 2006 Sep;177:2819–2830. doi: 10.4049/jimmunol.177.5.2819. [DOI] [PubMed] [Google Scholar]
- 36.Cao H, Lakner U, de Bono B, Traherne JA, Trowsdale J, Barrow AD. SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur J Immunol. 2008 Aug;38:2303–2315. doi: 10.1002/eji.200738078. [DOI] [PubMed] [Google Scholar]
- 37.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009 Feb;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharif O, Knapp S. From expression to signaling: Roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008 Nov;213:701–713. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Hamerman JA, Ni M, Killebrew JR, Chu C-L, Lowell CA. The expanding roles of ITAM adapters FcRγ and DAP12 in myeloid cells. Immunol Rev. 2009 Nov;232:42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molloy EJ. Triggering Receptor Expressed on Myeloid Cells (TREM) family and the application of its antagonists. Recent Patents Anti-Infect Drug Disc. 2009 Jan;4:51–56. doi: 10.2174/157489109787236292. [DOI] [PubMed] [Google Scholar]
- 41.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001 Apr;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 42.Haselmayer P, Grosse-Hovest L, von Landenberg P, Schild H, Radsak MP. TREM-1 ligand expression on platelets enhances neutrophil activation. Blood. 2007 Apr;110:1029–1035. doi: 10.1182/blood-2007-01-069195. [DOI] [PubMed] [Google Scholar]
- 43.Wong-Baeza I, Gonzalez-Roldan N, Ferat-Osorio E, Esquivel-Callejas N, Aduna-Vicente R, Arriaga-Pizano L, Astudillo-de la Vega H, Villasis-Keever MA, Torres-Gonzalez R, Estrada-Garcia I, Lopez-Macias C, Isibasi A. Triggering receptor expressed on myeloid cells (TREM-1) is regulated post-transcriptionally and its ligand is present in the sera of some septic patients. Clin Exp Immunol. 2006 Sep;145:448–455. doi: 10.1111/j.1365-2249.2006.03158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohamadzadeh M, Coberley SS, Olinger GG, Kalina WV, Ruthel G, Fuller CL, Swenson DL, Pratt WD, Kuhns DB, Schmaljohn AL. Activation of Triggering Receptor Expressed on Myeloid Cells-1 on Human Neutrophils by Marburg and Ebola Viruses. J Virol. 2006 Jul;80:7235–7244. doi: 10.1128/JVI.00543-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Mezayen R, El Gazzar M, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett. 2007 Jul;111:36–44. doi: 10.1016/j.imlet.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tessarz AS, Cerwenka A. The TREM-1/DAP12 pathway. Immunol Lett. 2008 Mar;116:111–116. doi: 10.1016/j.imlet.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 47.Hommes TJ, Hoogendijk AJ, Dessing MC, van’t Veer C, Florquin S, Colonna M, de Vos AF, van der Poll T. Triggering receptor expressed on myeloid cells-1 (TREM-1) improves host defence in pneumococcal pneumonia: Role of TREM-1 in pneumococcal pneumonia. J Pathol. 2014 Aug;233:357–367. doi: 10.1002/path.4361. [DOI] [PubMed] [Google Scholar]
- 48.Arts RJW, Joosten LAB, van der Meer JWM, Netea MG. TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. J Leukoc Biol. 2013 Feb;93:209–215. doi: 10.1189/jlb.0312145. [DOI] [PubMed] [Google Scholar]
- 49.Weber B, Schuster S, Zysset D, Rihs S, Dickgreber N, Schürch C, Riether C, Siegrist M, Schneider C, Pawelski H, Gurzeler U, Ziltener P, Genitsch V, Tacchini-Cottier F, Ochsenbein A, Hofstetter W, Kopf M, Kaufmann T, Oxenius A, Reith W, Saurer L, Mueller C. TREM-1 Deficiency Can Attenuate Disease Severity without Affecting Pathogen Clearance. In: Modlin R, editor. PLoS Pathog. Vol. 10. 2014. Jan, p. e1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S-Y, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku C-L, Casrouge A, Zhang X-X, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova J-L. TLR3 Deficiency in Patients with Herpes Simplex Encephalitis. Science. 2007 Sep;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 51.Ornatowska M, Azim AC, Wang X, Christman JW, Xiao L, Joo M, Sadikot RT. Functional genomics of silencing TREM-1 on TLR4 signaling in macrophages. AJP Lung Cell Mol Physiol. 2007 Sep;293:L1377–L1384. doi: 10.1152/ajplung.00140.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu L, Du Z, Zhao G, Jiang N, Lin J, Wang Q, Xu Q, Cong L, Qiu S. Role of TREM-1 in response to Aspergillus fumigatus infection in corneal epithelial cells. Int Immunopharmacol. 2014 Nov;23:288–293. doi: 10.1016/j.intimp.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Tessarz AS, Weiler S, Zanzinger K, Angelisová P, Horejsí V, Cerwenka A. Non-T Cell Activation Linker (NTAL) Negatively Regulates TREM-1/DAP12-Induced Inflammatory Cytokine Production in Myeloid Cells. J Immunol. 2007 Feb;178:1991–1999. doi: 10.4049/jimmunol.178.4.1991. [DOI] [PubMed] [Google Scholar]
- 54.Janssen E, Zhu M, Zhang W, Koonpaew S, Zhang W. LAB: A new membrane-associated adaptor molecule in B cell activation. Nat Immunol. 2003 Feb;4:117–123. doi: 10.1038/ni882. [DOI] [PubMed] [Google Scholar]
- 55.Brdicka T, Imrich M, Angelisová P, Brdicková N, Horváth O, Spicka J, Hilgert I, Lusková P, Dráber P, Novák P, Engels N, Wienands J, Simeoni L, Osterreicher J, Aguado E, Malissen M, Schraven B, Horejsí V. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J Exp Med. 2002 Dec;196:1617–1626. doi: 10.1084/jem.20021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hara H, Ishihara C, Takeuchi A, Imanishi T, Xue L, Morris SW, Inui M, Takai T, Shibuya A, Saijo S, Iwakura Y, Ohno N, Koseki H, Yoshida H, Penninger JM, Saito T. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat Immunol. 2007 Jun;8:619–629. doi: 10.1038/ni1466. [DOI] [PubMed] [Google Scholar]
- 57.Thome M, Weil R. Post-translational modifications regulate distinct functions of CARMA1 and BCL10. Trends Immunol. 2007 Jun;28:281–288. doi: 10.1016/j.it.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Netea MG, Azam T, Ferwerda G, Girardin SE, Kim SH, Dinarello CA. Triggering receptor expressed on myeloid cells-1 (TREM-1) amplifies the signals induced by the NACHT-LRR (NLR) pattern recognition receptors. 2006:1461. doi: 10.1189/jlb.1205758. [Internet] [cited 2015 Dec 28]; Available from: http://repository.ubn.ru.nl/handle/2066/51102. [DOI] [PubMed]
- 59.Tomasello E, Vivier E. KARAP/DAP12/TYROBP: three names and a multiplicity of biological functions. Eur J Immunol. 2005 Jun;35:1670–1677. doi: 10.1002/eji.200425932. [DOI] [PubMed] [Google Scholar]
- 60.Helming L, Tomasello E, Kyriakides TR, Martinez FO, Takai T, Gordon S, Vivier E. Essential Role of DAP12 Signaling in Macrophage Programming into a Fusion-Competent State. Sci Signal. 2008 Oct;1:ra11–ra11. doi: 10.1126/scisignal.1159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang W, Huang S, Huang X, Li J, Ye P, Xu J, Zheng P, Shen H, Huang J. Regulation of human mesenchymal stem cell differentiation by TREM-2. Hum Immunol [Internet] 2015 Jun; doi: 10.1016/j.humimm.2015.06.005. [cited 2015 Dec 29]; Available from: http://linkinghub.elsevier.com/retrieve/pii/S0198885915001718. [DOI] [PubMed]
- 62.Omar Sharif SK, Sharif O, Knapp S. From expression to signaling: roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008;213:701–713. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Sessa G, Podini P, Mariani M, Meroni A, Spreafico R, Sinigaglia F, Colonna M, Panina P, Meldolesi J. Distribution and signaling of TREM2/DAP12, the receptor system mutated in human polycystic lipomembraneous osteodysplasia with sclerosing leukoencephalopathy dementia. Eur J Neurosci. 2004 Nov;20:2617–2628. doi: 10.1111/j.1460-9568.2004.03729.x. [DOI] [PubMed] [Google Scholar]
- 64.Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal. 2010;3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nat Rev Immunol. 2007 Feb;7:155–161. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 66.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fuller DM, Zhu M, Ou-Yang C-W, Sullivan SA, Zhang W. A tale of two TRAPs: LAT and LAB in the regulation of lymphocyte development, activation, and autoimmunity. Immunol Res. 2010 Dec;49:97–108. doi: 10.1007/s12026-010-8197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orr SJ, McVicar DW. LAB/NTAL/Lat2: a force to be reckoned with in all leukocytes? J Leukoc Biol. 2011 Jan;89:11–19. doi: 10.1189/jlb.0410221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwartzberg PL, Finkelstein LD, Readinger JA. TEC-family kinases: regulators of T-helper-cell differentiation. Nat Rev Immunol. 2005 Apr;5:284–295. doi: 10.1038/nri1591. [DOI] [PubMed] [Google Scholar]
- 70.Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004 Apr;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 71.Okkenhaug K, Ali K, Vanhaesebroeck B. Antigen receptor signalling: a distinctive role for the p110δ isoform of PI3K. Trends Immunol. 2007 Feb;28:80–87. doi: 10.1016/j.it.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volná P, Lebduska P, Dráberová L, Símová S, Heneberg P, Boubelík M, Bugajev V, Malissen B, Wilson BS, Horejsí V, Malissen M, Dráber P. Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J Exp Med. 2004 Oct;200:1001–1013. doi: 10.1084/jem.20041213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat Immunol. 2009 Apr;10:340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005 Jun;6:579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kagan JC, Medzhitov R. Phosphoinositide-Mediated Adaptor Recruitment Controls Toll-like Receptor Signaling. Cell. 2006 Feb;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 76.Li Z, Wang H, Liu J, Chen B, Li G. Serum Soluble Triggering Receptor Expressed on Myeloid Cells-1 and Procalcitonin Can Reflect Sepsis Severity and Predict Prognosis: A Prospective Cohort Study. Mediators Inflamm. 2014;2014:1–7. doi: 10.1155/2014/641039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barraud D, Gibot S. Triggering Receptor Expressed on Myeloid Cell 1. Crit Care Clin. 2011 Apr;27:265–279. doi: 10.1016/j.ccc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Gomez-Pina V, Soares-Schanoski A, Rodriguez-Rojas A, del Fresno C, Garcia F, Vallejo-Cremades MT, Fernandez-Ruiz I, Arnalich F, Fuentes-Prior P, Lopez-Collazo E. Metalloproteinases Shed TREM-1 Ectodomain from Lipopolysaccharide-Stimulated Human Monocytes. J Immunol. 2007 Sep;179:4065–4073. doi: 10.4049/jimmunol.179.6.4065. [DOI] [PubMed] [Google Scholar]
- 79.Gingras M-C, Lapillonne H, Margolin JF. TREM-1, MDL-1, and DAP12 expression is associated with a mature stage of myeloid development. Mol Immunol. 2002 Mar;38:817–824. doi: 10.1016/s0161-5890(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 80.Bishara J, Hadari N, Shalita-Chesner M, Samra Z, Ofir O, Paul M, Peled N, Pitlik S, Molad Y. Soluble triggering receptor expressed on myeloid cells-1 for distinguishing bacterial from aseptic meningitis in adults. Eur J Clin Microbiol Infect Dis. 2007 Aug;26:647–650. doi: 10.1007/s10096-007-0343-z. [DOI] [PubMed] [Google Scholar]
- 81.O’Neill LAJ, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013 Jan;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 82.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011 Dec;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010 Jun;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vazquez A, Liu J, Zhou Y, Oltvai ZN. Catabolic efficiency of aerobic glycolysis: The Warburg effect revisited. BMC Syst Biol. 2010;4:58. doi: 10.1186/1752-0509-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garedew A, Henderson SO, Moncada S. Activated macrophages utilize glycolytic ATP to maintain mitochondrial membrane potential and prevent apoptotic cell death. Cell Death Differ. 2010 Oct;17:1540–1550. doi: 10.1038/cdd.2010.27. [DOI] [PubMed] [Google Scholar]
- 86.O’Connor W, Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nat Immunol. 2010 Jun;11:471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 87.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011 Jul;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang C-H, Curtis JD, Maggi LB, Faubert B, Villarino AV, O’Sullivan D, Huang SC-C, van der Windt GJW, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013 Jun;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Windt GJW, Everts B, Chang C-H, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial Respiratory Capacity Is a Critical Regulator of CD8+ T Cell Memory Development. Immunity. 2012 Jan;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Everts B, Amiel E, van der Windt GJW, Freitas TC, Chott R, Yarasheski KE, Pearce EL, Pearce EJ. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012 Aug;120:1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodríguez-Prados J-C, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, Cascante M, Boscá L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol Baltim Md 1950. 2010 Jul;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 92.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006 Jul;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rőszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:1–16. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Viollet B, Horman S, Leclerc J, Lantier L, Foretz M, Billaud M, Giri S, Andreelli F. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010 Aug;45:276–295. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011 Sep;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011 Apr;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 98.Oakhill JS, Steel R, Chen Z-P, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011 Jun;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 99.Woods A, Dickerson K, Heath R, Hong S-P, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005 Jul;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to Hydrogen Peroxide Induces Oxidation and Activation of AMP-activated Protein Kinase. J Biol Chem. 2010 Oct;285:33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salt IP, Palmer TM. Exploiting the anti-inflammatory effects of AMP-activated protein kinase activation. Expert Opin Investig Drugs. 2012 Aug;21:1155–1167. doi: 10.1517/13543784.2012.696609. [DOI] [PubMed] [Google Scholar]
- 102.Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem. 2004 May;279:20767–20774. doi: 10.1074/jbc.M401390200. [DOI] [PubMed] [Google Scholar]
- 103.Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci Off J Soc Neurosci. 2004 Jan;24:479–487. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cacicedo JM, Yagihashi N, Keaney JF, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004 Nov;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 105.Yang Z, Kahn BB, Shi H, Xue B-Z. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010 Jun;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y, Qiu J, Wang X, Zhang Y, Xia M. AMP-Activated Protein Kinase Suppresses Endothelial Cell Inflammation Through Phosphorylation of Transcriptional Coactivator p300. Arterioscler Thromb Vasc Biol. 2011 Dec;31:2897–2908. doi: 10.1161/ATVBAHA.111.237453. [DOI] [PubMed] [Google Scholar]
- 107.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou M-H. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010 Apr;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006 Mar;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 109.Joosten LAB, Netea MG, Mylona E, Koenders MI, Malireddi RKS, Oosting M, Stienstra R, van de Veerdonk FL, Stalenhoef AF, Giamarellos-Bourboulis EJ, Kanneganti T-D, van der Meer JWM. Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1β production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum. 2010 Nov;62:3237–3248. doi: 10.1002/art.27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, Akira S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013 May;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 111.Liu TF, Vachharajani VT, Yoza BK, McCall CE. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem. 2012 Jul;287:25758–25769. doi: 10.1074/jbc.M112.362343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004 Jun;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kelly TJ, Lerin C, Haas W, Gygi SP, Puigserver P. GCN5-mediated Transcriptional Control of the Metabolic Coactivator PGC-1β through Lysine Acetylation. J Biol Chem. 2009 Jul;284:19945–19952. doi: 10.1074/jbc.M109.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005 Jun;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 115.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 Modulation of the Acetylation Status, Cytosolic Localization, and Activity of LKB1 POSSIBLE ROLE IN AMP-ACTIVATED PROTEIN KINASE ACTIVATION. J Biol Chem. 2008 Oct;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009 Apr;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu J-C, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009 Mar;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatol Oxf Engl. 2008 Apr;47:409–414. doi: 10.1093/rheumatology/kem297. [DOI] [PubMed] [Google Scholar]
- 119.Jeong HW, Hsu KC, Lee J-W, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009 Apr;296:E955–E964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 120.Nerstedt A, Johansson A, Andersson CX, Cansby E, Smith U, Mahlapuu M. AMP-activated protein kinase inhibits IL-6-stimulated inflammatory response in human liver cells by suppressing phosphorylation of signal transducer and activator of transcription 3 (STAT3) Diabetologia. 2010 Nov;53:2406–2416. doi: 10.1007/s00125-010-1856-z. [DOI] [PubMed] [Google Scholar]
- 121.Kim YD, Kim YH, Cho YM, Kim DK, Ahn SW, Lee JM, Chanda D, Shong M, Lee CH, Choi HS. Metformin ameliorates IL-6-induced hepatic insulin resistance via induction of orphan nuclear receptor small heterodimer partner (SHP) in mouse models. Diabetologia. 2012 May;55:1482–1494. doi: 10.1007/s00125-012-2494-4. [DOI] [PubMed] [Google Scholar]
- 122.Wang Q, Zhang M, Liang B, Shirwany N, Zhu Y, Zou M-H. Activation of AMP-Activated Protein Kinase Is Required for Berberine-Induced Reduction of Atherosclerosis in Mice: The Role of Uncoupling Protein 2. PLoS ONE [Internet] 2011 Sep; doi: 10.1371/journal.pone.0025436. [cited 2016 Jan 5];6. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3181327/ [DOI] [PMC free article] [PubMed]
- 123.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008 Dec;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davies SP, Sim ATR, Hardie DG. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem. 1990 Jan;187:183–190. doi: 10.1111/j.1432-1033.1990.tb15293.x. [DOI] [PubMed] [Google Scholar]
- 125.Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006 Jan;55:120–127. [PubMed] [Google Scholar]
- 126.Galic S, Fullerton MD, Schertzer JD, Sikkema S, Marcinko K, Walkley CR, Izon D, Honeyman J, Chen Z-P, van Denderen BJ, Kemp BE, Steinberg GR. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011 Dec;121:4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]