Abstract
Recent studies have identified the apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3B (APOBEC3B) as a source of mutations in various malignancies. APOBEC3B is overexpressed in several human cancer types, including breast cancer. In this study, we analyzed APOBEC3B mRNA expression in 305 primary breast cancers of Japanese women using quantitative reverse transcription-PCR, and investigated the relationships between the APOBEC3B mRNA expression and clinicopathological characteristics, prognosis, and TP53 mutations. The expression of APOBEC3B mRNA was detected in 277 tumors and not detected in 28 tumors. High APOBEC3B mRNA expression was significantly correlated with ER- and PR-negativity, high grade and high Ki67 index. The APOBEC3B mRNA expression was highest in the triple-negative and lowest in the hormone receptor-positive/HER2-negative subtypes. The TP53 gene was more frequently mutated in the tumors with high APOBEC3B mRNA expression. High APOBEC3B mRNA expression was significantly associated with poor recurrence-free survival in all cases and the ER-positive cases. These findings were almost consistent with the previous reports from the Western countries. In conclusion, high APOBEC3B mRNA expression was related to the aggressive phenotypes of breast cancer, high frequency of TP53 mutation and poor prognosis, especially in ER-positive tumors.
Introduction
Breast cancer is a heterogeneous disease both pathologically and genetically [1, 2]. Apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3B (APOBEC3B) is a member of a APOBEC3 family of DNA cytosine deaminases [3]. It normally functions in the innate immune responses that protect against retrovirus and retrotransposon propagation. However, this enzyme can also deaminate cytosines in the host genome and generate C-to-T mutations. Recent studies have revealed that APOBEC3B is a significant contributor to the somatic mutation burden among several cancer types [4–7]. APOBEC3B expression is up-regulated in various malignant tumors, including breast cancer, and high APOBEC3B expression is correlated with the presence of certain somatic mutations, particularly in TP53 [4, 5, 8–10]. High APOBEC3B expression has been associated with aggressive clinicopathological characteristics and was significantly associated with a poor prognosis in untreated ER-positive (ER+) patients as well as in ER+ patients receiving adjuvant endocrine therapy [11]. In ER+/lymph-node-negative cases, APOBEC3B expression was an independent poor prognostic factor for disease-free survival (DFS) [11]. Another recent study using two large datasets also revealed that APOBEC3B expression is associated with aggressive and proliferative features. Elevated APOBEC3B expression was associated with recurrence in the luminal tumor subtype [10]. Thus, APOBEC3B is a marker of poor prognosis for ER+ breast cancer, which strongly suggests that genetic aberrations induced by APOBEC3B contribute to breast cancer progression or resistance to treatment [10, 11].
On the other hand, a germ-line deletion polymorphism in APOBEC3B, which leads to no expression of APOBEC3B, has been associated with an increased risk of breast cancer [12, 13]. Homozygosity for the APOBEC3B null allele is associated with a higher incidence of breast cancer [14]. The frequencies of this deletion differ by ethnicity: 1% in Africans, 6% in Europeans, 37% in Asians and 93% in Oceanians. [15]. Homozygous deletion of APOBEC3B did not affect the tumor aggressiveness or the prognosis [10].
In the present study, we evaluated the expression of APOBEC3B mRNA and investigated the relationships between the APOBEC3B mRNA expression and the clinicopathological features, the frequency of the mutation in the TP53 gene, and the prognosis in Japanese women with breast cancer.
Materials and Methods
Patient population and tumor specimens
The institutional review board of Kyushu University approved this study. A total of 894 primary breast cancer patients underwent surgery without neoadjuvant systemic therapy in the Department of Surgery and Science, Kyushu University, between 1994 and 2012. Of these, 305 cases for which archival tissue samples for an immunohistochemical analysis and/or extraction of the genomic DNA and total RNA were available were included in this study. Immediately after surgery, the specimens for extraction of the genomic DNA and total RNA were placed in liquid nitrogen and stored at –80°C. Written informed consent was obtained from all of the patients in this study. The clinical data were obtained from the patients’ medical records. The AJCC/UICC TNM classification and stage groupings were used. The estrogen receptor (ER), progesterone receptor (PR), HER2 status, and Ki67 labeling index were evaluated as described previously [16].
APOBEC3B mRNA expression
APOBEC3B expression was analyzed using quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Total RNA was extracted from the frozen specimens with the TRIZOL reagent (Invitrogen Japan K.K., Tokyo, Japan) in accordance with the manufacturer’s recommendations. The extracted RNA was pretreated with RNase-free DNase, and the cDNA was then synthesized with random hexamer primers and Superscript III reverse transcriptase (Invitrogen Japan K.K.) in accordance with the manufacturer’s instructions. The APOBEC3B mRNA expression was normalized to that of the constitutive housekeeping gene TATA binding protein (TBP) [17]. The primers and probe were Hs00358981-m1 for APOBEC3B and Hs00427620-m1 for TBP (Applied Biosystems, Waltham, MA, USA).
PCR direct sequencing of the TP53 gene and single-nucleotide polymorphism- comparative genomic hybridization
Genomic DNA was extracted as described previously [18, 19]. PCR direct sequencing was performed for exon 5–9 of the TP53 gene as previously described [18]. The presence of genetic alterations was investigated by single-nucleotide polymorphism- comparative genomic hybridization (SNP-CGH, HumanOmni1-Quad BeadChip; Illumina Inc.), and the copy number variation was analyzed with the software program GenomeStudio V2009.1 (Illumina Inc.) as described previously [18, 20].
Statistical analyses
The statistical analyses were performed using the JMP software package, version 9.0.2 (SAS Institute Inc., Cary, NC, USA). The associations between the APOBEC3B mRNA expression and clinicopathological characteristics were assessed using χ2 tests. The relapse-free survival (RFS) was defined as the time from surgery to the first breast cancer event, including loco-regional recurrence, distant metastasis, or a new cancer in the contralateral breast. Survival curves were plotted using the Kaplan–Meier method and the association between survival and each variable was determined by the log-rank test. For multivariate analysis of the survival data, Cox proportional hazards model was used. Differences were considered to be significant at p < 0.05.
Results
APOBEC3B mRNA expression
Of the 305 cases evaluated, expression of APOBEC3B mRNA was not detected in 28 tumors, which was defined as “no” APOBEC3B mRNA expression. Among 277 tumors, in which APOBEC3B mRNA expression was detected, APOBEC3B mRNA expression was dichotomized based on the median value as either “low” or “high” expression. The associations between the APOBEC3B mRNA expression and the clinicopathological characteristics were compared among three groups: no, low, or high APOBEC3B mRNA expression (Table 1).
Table 1. The association between APOBEC3B mRNA expression and clinicopathological characteristcs.
| Factors | none (n = 28) | APOBEC3B mRNA expression | P-value | |
|---|---|---|---|---|
| low (n = 139) | high (n = 138) | |||
| Age (yo) (mean ± SE) | 53.2 ± 2.5 | 56.4 ± 1.1 | 55.5 ± 1.1 | 0.4863 |
| Nodal status | ||||
| Negative | 21 (75.0) | 89 (64.0) | 85 (61.6) | 0.3869 |
| Positive | 7 (25.0) | 50 (36.0) | 53 (38.4) | |
| pathological tumor size (pT) | ||||
| 0 | 0 (0) | 5 (2.9) | 5 (2.9) | 0.8032 |
| 1 | 10 (42.9) | 58 (38.4) | 51 (36.0) | |
| 2 | 15 (50.0) | 65 (46.4) | 71 (45.6) | |
| 3 | 3 (7.1) | 11 (10.1) | 11 (14.0) | |
| Histology | ||||
| DCIS | 0(0) | 5 (3.6) | 5 (3.6) | 0.2574 |
| Invasive ductal ca | 25 (89.3) | 125 (89.9) | 123 (89.1) | |
| Invasive lobular ca | 1 (3.6) | 4 (2.9) | 4 (2.9) | |
| Metaplastic ca | 0 (0) | 1 (0.7) | 5 (3.6) | |
| Others | 2 (7.1) | 4 (2.9) | 1 (0.7) | |
| Nuclear grade | ||||
| 1 | 7 (26.9) | 75 (55.6) | 24 (18.6) | <0.001 |
| 2 | 6 (23.1) | 24 (17.8) | 32 (24.8) | |
| 3 | 13 (50.0) | 36 (26.7) | 73 (56.6) | |
| Ki67 labeling index (mean ± SE) | ||||
| 21.9 ± 4.7 | 20.2 ± 2.3 | 33.1 ± 2.3 | 0.0002 | |
| ER | ||||
| Negative | 5 (17.9) | 20 (14.4) | 60 (43.5) | <0.001 |
| Positive | 23 (82.1) | 119 (85.6) | 78 (56.5) | |
| PR | ||||
| Negative | 8 (28.6) | 31 (22.3) | 70 (51.1) | <0.001 |
| Positive | 20 (71.4) | 108 (77.7) | 67 (48.9) | |
| HER2 | ||||
| Negative | 17 (60.7) | 116 (86.6) | 95 (72.0) | 0.002 |
| Positive | 10 (35.7) | 18 (13.4) | 37 (28.0) | |
| N.D. | 1 (3.6) | 0 (0) | 0 (0) | |
| Subtype | ||||
| HR+/HER2- | 14 (50.0) | 104 (77.6) | 63 (47.7) | <0.001 |
| HR+/HER2+ | 8 (28.6) | 11 (8.2) | 15 (11.4) | |
| HR-/HER+ | 2 (7.1) | 7 (5.2) | 22 (16.7) | |
| Triple negative | 3 (10.7) | 12 (9.0) | 32 (24.2) | |
| N.D. | 1 (3.6) | 0 (0) | 0 (0) | |
| (%) | ||||
pT; pathological tumor size, HR; horomne receptor, ND; not determined
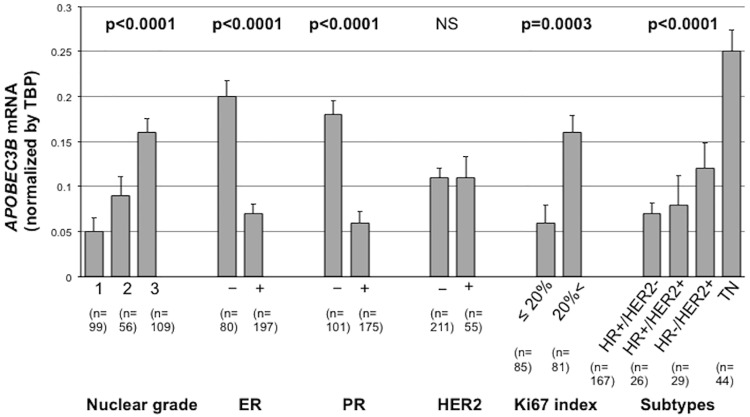
High APOBEC3B mRNA expression was significantly correlated with higher nuclear grade (p<0.0001), higher Ki67 labeling index (p = 0.0002) and ER and PR negativity (p<0.0001 and p<0.0001). There were no significant associations between the APOBEC3B mRNA expression and the age, lymph node metastasis, pathological stage and histology. HER2 positivity was highest in the tumors with no APOBEC3B mRNA expression, and lowest in the tumors with low APOBEC3B mRNA expression (p = 0.0023). In terms of breast cancer subtype, high APOBEC3B mRNA expression was significantly associated with the triple negative (TN) subtype. Low APOBEC3B mRNA expression was associated with HR+/HER2- subtype, and no expression was most frequent in HR+/HER2+ subtype (p<0.0001) (Table 1). Intriguingly, the tumors lacking APOBEC3B mRNA expression seemed to be more aggressive than those with low APOBEC3B mRNA expression, due to higher nuclear grade and higher HER2-positivity. Fig 1 shows the APOBEC3B mRNA expression according to the clinicpoathplogical factors. In Fig 1, the relative APOBEC3B mRNA expression level normalized by TBP mRNA level was shown. The data from tumors without APOBEC3B mRNA expression were excluded. Relative APOBEC3B mRNA expression were higher in high-nuclear-grade (p<0.0001), ER–(p<0.0001), PR–(p<0.0001) tumors. APOBEC3B mRNA expression was also higher in tumors with high Ki67 index (p = 0.0003) and TN tumors (p<0.0001) (Fig 1).
Fig 1. APOBEC3B mRNA expression according to the clinicopathological factors.
APOBEC3B mRNA expression was significantly higher in higher grade, ER−, PR− and high-Ki67 tumors. APOBEC3B mRNA expression was highest in TN tumors. HR; hormone receptor.
Association between APOBEC3B mRNA expression and the patient prognosis
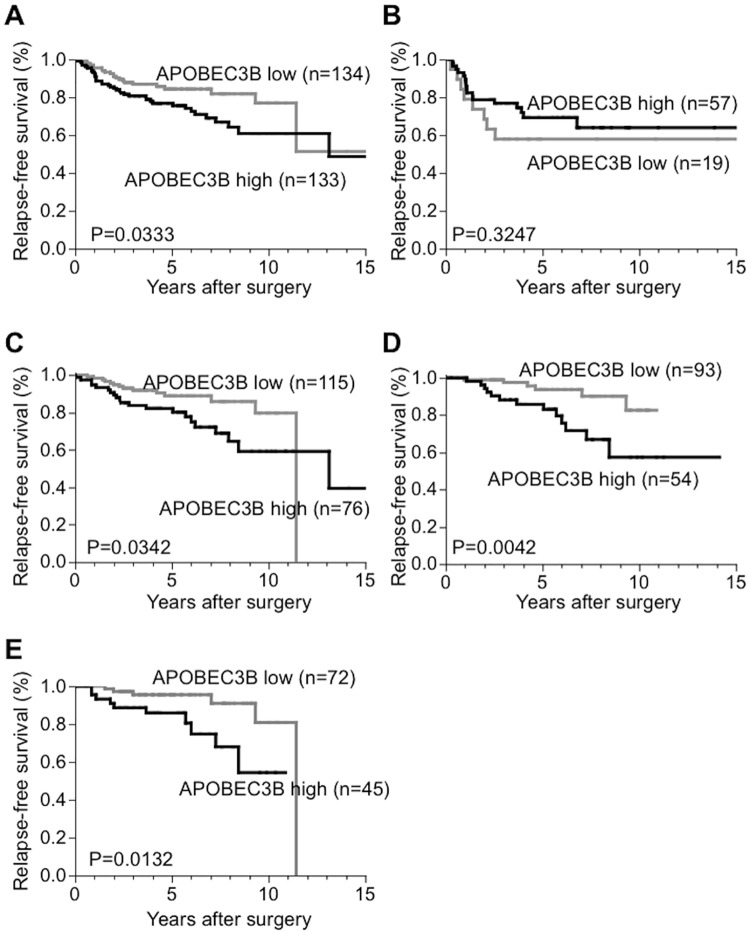
The association between APOBEC3B mRNA expression and the patient prognosis was examined in 295 invasive breast cancer patients.The median follow-up period was 4.93years (range, 0.5–19.9 years). Fig 2 shows the prognosis data of the patients with high and low APOBEC3B mRNA expression. As shown in Fig 2, high APOBEC3B mRNA expression was associated with shorter RFS compared with than low expression (p = 0.0333, Fig 2A). While APOBEC3B mRNA expression was not associated with RFS in ER–tumors (Fig 2B), high APOBEC3B mRNA expression was significantly associated with shorter RFS in ER+ tumors (p = 0.0342, Fig 2C), especially in the patients who had received adjuvant endocrine therapy (p = 0.0042, Fig 2D) or the node-negative patients (p = 0.0132, Fig 2E). There were no statistically significant differences in the prognosis between high and no APOBEC3B mRNA expression, and no marked differences were recognized in the prognosis between low and no APOBEC3B mRNA expression (data not shown).
Fig 2. The relationship between APOBEC3B mRNA and the prognosis (relapse-free survival).
A. All cases, B. ER− cases, C. ER+ cases, D. ER+ and adjuvant endocrine+ cases, E. ER+ and node-negative cases. High APOBEC3B expression was associated with shorter relapse-free survival in all cases, ER+ cases, ER+ with adjuvant endocrine therapy cases and ER+ and node-negative cases (A, C, D, E).
Univariate and multivariate analyses were performed to assess the effect of the clinicopathological factors on the RFS (Table 2). The “no expression cases” were not included in “low expression group”. According to the univariate analysis, high APOBEC3B mRNA expression was significantly associated with poor RFS in all cases and ER+ cases. However, a multivariate Cox hazard analyses analysis showed that high APOBEC3B mRNA was not an independent prognostic factor. When analyzing the ER+ with adjuvant endocrine therapy or LN-negative ER+ cases, high APOBEC3B mRNA expression was significantly associated with poor RFS on both univariate and multivariate analyses (Table 2).
Table 2. Univariate and multivariate analysis for recurrence-free survival.
| Factors | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| All cases (n = 267) | |||||||
| Age (y.o.) | ≤40 vs. 41≤ | 1.73 | 0.32–1.14 | 0.1074 | |||
| Tumor size | T2, T3 vs. T1 | 2.70 | 1.56–4.93 | 0.0003 | 2.00 | 1.06–3.96 | |
| LN meta. | positive vs. negative | 2.59 | 1.55–4.41 | 0.0003 | 1.94 | 1.07–3.54 | |
| Nuclear grade | 3 vs. 1, 2 | 2.10 | 1.22–3.65 | 0.0076 | 1.49 | 0.77–2.89 | |
| ER | positive vs. negative | 0.50 | 0.30–0.84 | 0.0099 | 0.97 | 0.42–2.21 | |
| PR | positive vs. negative | 0.40 | 0.23–0.67 | 0.0005 | 0.44 | 0.20–0.97 | |
| HER2 | positive vs. negative | 1.98 | 1.09–3.45 | 0.0253 | 1.00 | 0.50–1.96 | |
| APOBEC3B mRNA | high vs. low | 1.76 | 1.05–3.02 | 0.0324 | 1.26 | 0.71–2.31 | |
| ER-negative cases (n = 76) | |||||||
| Age (y.o.) | ≤40 vs. 41≤ | 1.13 | 0.27–3.26 | 0.8480 | |||
| Tumor size | T2, T3 vs. T1 | 3.49 | 1.47–9.60 | 0.0037 | 2.49 | 1.01–7.03 | 0.0463 |
| LN meta. | positive vs. negative | 4.50 | 1.97–11.1 | 0.0003 | 3.63 | 1.56–9.19 | 0.0027 |
| Nuclear grade | 3 vs. 1, 2 | 0.74 | 0.30–2.06 | 0.5350 | |||
| PR | positive vs. negative | 0.55 | 0.03–2.61 | 0.5229 | |||
| HER2 | positive vs. negative | 1.27 | 0.56–2.80 | 0.5521 | |||
| APOBEC3B mRNA | high vs. low | 0.66 | 0.29–1.61 | 0.3420 | |||
| ER-positive cases (n = 191) | |||||||
| Age (y.o.) | ≤40 vs. 41≤ | 2.47 | 1.08–5.14 | 0.0329 | 3.44 | 1.37–7.94 | |
| Tumor size | T2, T3 vs. T1 | 2.29 | 1.13–5.00 | 0.0207 | 2.43 | 1.13–5.69 | |
| LN meta. | positive vs. negative | 1.72 | 0.87–3.44 | 0.1151 | |||
| Nuclear grade | 3 vs. 1, 2 | 2.63 | 1.24–5.49 | 0.0127 | 2.07 | 0.92–4.30 | |
| PR | positive vs. negative | 0.39 | 0.19–0.90 | 0.0282 | 0.31 | 0.14–0.74 | |
| HER2 | positive vs. negative | 2.22 | 0.82–5.12 | 0.1102 | |||
| APOBEC3B mRNA | high vs. low | 2.07 | 1.05–4.19 | 0.0364 | 1.41 | 0.67–3.01 | |
| ER-positive with adjuvant endocrine therapy cases (n = 147) | |||||||
| Age (y.o.) | ≤40 vs. 41≤ | 1.89 | 0.53–5.27 | 0.2938 | |||
| Tumor size | T2, T3 vs. T1 | 1.70 | 0.67–4.86 | 0.2679 | |||
| LN meta. | positive vs. negative | 1.13 | 0.44–2.81 | 0.7853 | |||
| Nuclear grade | 3 vs. 1, 2 | 1.93 | 0.67–5.04 | 0.2103 | |||
| PR | positive vs. negative | 0.23 | 0.07–0.74 | 0.0160 | 0.30 | 0.11–0.97 | 0.0440 |
| HER2 | positive vs. negative | 1.47 | 0.24–4.47 | 0.5592 | |||
| APOBEC3B mRNA | high vs. low | 3.73 | 1.47–10.6 | 0.0052 | 3.28 | 1.27–9.45 | 0.0138 |
| ER-positive and LN-negative cases (n = 117) | |||||||
| Age (y.o.) | ≤40 vs. 41≤ | 1.97 | 0.55–5.81 | 0.2727 | |||
| Tumor size | T2, T3 vs. T1 | 3.81 | 1.33–12.4 | 0.0121 | 3.40 | 1.00–13.4 | 0.0493 |
| Nuclear grade | 3 vs. 1, 2 | 3.32 | 1.06–10.1 | 0.0394 | 1.54 | 0.44–5.31 | 0.4875 |
| PR | positive vs. negative | 0.69 | 0.19–4.45 | 0.6460 | |||
| HER2 | positive vs. negative | 4.08 | 1.11–12.3 | 0.0356 | 2.73 | 0.63–10.7 | 0.1672 |
| APOBEC3B mRNA | high vs. low | 3.57 | 1.27–11.5 | 0.0158 | 4.51 | 1.31–18.3 | 0.0169 |
LN meta.; lymph node metastasis, ER; estrogen receptor, PR; progesterone receptor
Associations between APOBEC3B mRNA expression and TP53 mutation
We next examined the relationship between APOBEC3B mRNA expression and a mutation in the TP53 gene, exon 5–9. We evaluated the TP53 sequence in 28 tumors lacking APOBEC3B as well as in 60 tumors with low and 60 tumors with high APOBEC3B mRNA expression. Any mutations in TP53 gene were evaluated. Confirmation whether it is non-germline SNPs or not was not performed. Mutations in the TP53 gene were detected in 15 (25%), 7 (11.9%) and 2 (7.1%) tumors with high, low and no APOBEC3B mRNA expression, respectively. High APOBEC3B mRNA expression was significantly associated with high frequencies of the TP53 mutation compared with no or low APOBEC3B mRNA (p = 0.0312, Table 3).
Table 3. The association between APOBEC3B mRNA expression and TP53 gene mutation.
| TP 53 | APOBEC3B mRNA | P-value | |
|---|---|---|---|
| exon 5–9 | none (n = 28) or low (n = 60) | high (n = 60) | |
| wild-type | 78 (88.6) | 45 (75.0) | 0.0312 |
| mutant | 10 (11.4) | 15 (25.0) | |
| (%) | |||
Deletion at the APOBEC3B gene locus in the tumor lacking APOBEC3B mRNA expression
SNP-CGH data were available for 26 tumors. Of these, apparent deletion of APOBEC3B gene locus was recognized in only one case (Fig 3). In this case, no APOBEC3B mRNA expression was detected. The lack of APOBEC3B mRNA may have been associated with the deletion of the APOBEC3B gene locus in this case.
Fig 3. Deletion of APOBEC3B gene locus at chromosome 22 analyzed by SNP-CGH.
An apparent deletion was recognized at the APOBEC3B gene locus (arrow). In this case, APOBEC3B mRNA expression was not detected.
Discussion
In this study, we demonstrated that high expression of APOBEC3B mRNA was significantly associated with aggressive phenotypes of breast cancer and poor RFS. The poor prognosis of the patients with high-expression tumors are more significant in ER+ tumors, especially in patients who had received adjuvant endocrine therapy and node-negative patinets. High expression of APOBEC3B mRNA was also associated with TP53 gene mutation. We also found that there are some individuals in which APOBEC3B mRNA could not be detected, and these tumors are more often HER2-positive, relatively high grade, and have low frequencies of TP53 mutation, with a deletion of the APOBEC3B gene locus observed in one particular case. This is the first study to evaluate the relationship between APOBEC3B mRNA expression and the clinicopathological factors, prognosis, and TP53 mutation status in a large cohort of Japanese breast cancer patients including tumors lacking APOBEC3B mRNA expression.
In a previous study conducted in a large cohort of breast cancer cases, the APOBEC3B mRNA levels were significantly higher in subjects with a high tumor grade and ER—and PR—tumors [11]. These results are consistent with the data obtained in our study. Sieuwerts et al. showed that high APOBEC3B mRNA expression was associated with poor prognosis in ER+ breast cancer, and that high APOBEC3B mRNA expression was an independent prognostic factor in ER+, lymph-node-negative patients [11]. In our study, high APOBEC3B mRNA was expression significantly associated with poor RFS in all cases as well as ER+ cases, although not to a statistically significant degree in multivariate analyses. However, the analysis for ER+, lymph-node-negative patients revealed that high APOBEC3B mRNA expression was an independent poor prognostic factor. There was substantial heterogeneity in our data, probably due to the small sample size. However, the fact that similar results were obtained is very meaningful.
Previous studies have shown the association of APOBEC3B for the elevated levels of DNA damage and mutation in several breast cancer cell lines [4, 5, 21]. APOBEC3B knockdown cells had fewer C-to-T mutations in the TP53 and c-Myc genes than controls [4, 5]. Moreover, in tumors with TP53 mutations, APOBEC3B expression was elevated [10]. TP53 mutations are common in breast cancer, and approximately 30% of breast cancers harbor a somatic mutation in the TP53 gene [9, 22, 23]. Thus, high expression of APOBEC3B might cause TP53 mutation in breast cancer. In our study, the frequency of the TP53 gene mutation was also higher in tumors with high APOBEC3B mRNA expression than in those with low or lacking APOBEC3B mRNA expression. Although the mechanism of the upregulation of APOBEC3B mRNA expression has not been been fully elucidated, a recent study showed the induction of APOBEC3B in vitro by environmental factors such as DNA damage and viral infection [24].
It has been shown that APOBEC3B is lacking in some people due to a deletion polymorphism near the APOBEC3B gene [14, 25]. There is a large ethnic difference in the frequency of the deletion. The incidence of APOBEC3B deletion polymorphism is higher in East-Asian, American and Oceanian populations than in African and European populations [25]. In our study, the tumor in which APOBEC3B gene locus was deleted did not express APOBEC3B mRNA. Thus, the deletion of APOBEC3B gene locus might be a mechanism of lacking APOBEC3B mRNA in this tumor.
Tsuboi et al. recently reported on the APOBEC3B mRNA expression in Japanese breast cancer patients [26]. In their report, APOBEC3B mRNA expression was not related to the frequency of TP53 mutation or survival time, although the high APOBEC3B mRNA expression were associated with subtype, lymph node metastasis and nuclear grade. However, they analyzed only 93 cases, a smaller sample size than ours. Therefore, their results were not conclusive.
Several limitations associated with the present study warrant mention. This study is retrospective, and the sample size is smaller than those of previous reports from European populations. There was also substantial heterogeneity in the background of the patients. However, we were able to demonstrate that high APOBEC3B mRNA expression is associated with the aggressiveness of breast cancer and a poor prognosis in ER+ breast cancer, regardless of ethnic difference.
Conclusions
Our findings here suggest that high APOBEC3B mRNA expression is associated with aggressive phenotypes, high frequency of TP53 mutation, and poor prognosis of breast cancer, especially for ER+ breast cancer.
Acknowledgments
We are grateful to Ms. Y. Kubota, Ms. T. Shishino and Ms. N. Makikusa for their valuable technical assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by JSPS KAKENHI Grant Number 23591896.
References
- 1.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486(7403):400–4. 10.1038/nature11017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Refsland EW, Harris RS. The APOBEC3 family of retroelement restriction factors. Curr Top Microbiol Immunol. 2013;371:1–27. 10.1007/978-3-642-37765-5_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns MB, Lackey L, Carpenter MA, Rathore A, Land AM, Leonard B, et al. APOBEC3B is an enzymatic source of mutation in breast cancer. Nature. 2013;494(7437):366–70. 10.1038/nature11881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns MB, Temiz NA, Harris RS. Evidence for APOBEC3B mutagenesis in multiple human cancers. Nature genetics. 2013;45(9):977–83. 10.1038/ng.2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor BJ, Nik-Zainal S, Wu YL, Stebbings LA, Raine K, Campbell PJ, et al. DNA deaminases induce break-associated mutation showers with implication of APOBEC3B and 3A in breast cancer kataegis. eLife. 2013;2:e00534 10.7554/eLife.00534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8. 10.1038/nature12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuong KJ, Loeb LA. APOBEC3B mutagenesis in cancer. Nature genetics. 2013;45(9):964–5. 10.1038/ng.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silwal-Pandit L, Vollan HK, Chin SF, Rueda OM, McKinney S, Osako T, et al. TP53 mutation spectrum in breast cancer is subtype specific and has distinct prognostic relevance. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(13):3569–80. [DOI] [PubMed] [Google Scholar]
- 10.Cescon DW, Haibe-Kains B, Mak TW. APOBEC3B expression in breast cancer reflects cellular proliferation, while a deletion polymorphism is associated with immune activation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(9):2841–6. 10.1073/pnas.1424869112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sieuwerts AM, Willis S, Burns MB, Look MP, Meijer-Van Gelder ME, Schlicker A, et al. Elevated APOBEC3B correlates with poor outcomes for estrogen-receptor-positive breast cancers. Hormones & cancer. 2014;5(6):405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long J, Delahanty RJ, Li G, Gao YT, Lu W, Cai Q, et al. A common deletion in the APOBEC3 genes and breast cancer risk. Journal of the National Cancer Institute. 2013;105(8):573–9. 10.1093/jnci/djt018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xuan D, Li G, Cai Q, Deming-Halverson S, Shrubsole MJ, Shu XO, et al. APOBEC3 deletion polymorphism is associated with breast cancer risk among women of European ancestry. Carcinogenesis. 2013;34(10):2240–3. 10.1093/carcin/bgt185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nik-Zainal S, Wedge DC, Alexandrov LB, Petljak M, Butler AP, Bolli N, et al. Association of a germline copy number polymorphism of APOBEC3A and APOBEC3B with burden of putative APOBEC-dependent mutations in breast cancer. Nature genetics. 2014;46(5):487–91. 10.1038/ng.2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson S, Fenton T. APOBEC3 genes: retroviral restriction factors to cancer drivers. Trends Mol Med. 2015;21(5):274–84. 10.1016/j.molmed.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 16.Hisamatsu Y, Tokunaga E, Yamashita N, Akiyoshi S, Okada S, Nakashima Y, et al. Impact of FOXA1 expression on the prognosis of patients with hormone receptor-positive breast cancer. Annals of surgical oncology. 2012;19(4):1145–52. 10.1245/s10434-011-2094-4 [DOI] [PubMed] [Google Scholar]
- 17.Leonard B, Hart SN, Burns MB, Carpenter MA, Temiz NA, Rathore A, et al. APOBEC3B upregulation and genomic mutation patterns in serous ovarian carcinoma. Cancer research. 2013;73(24):7222–31. 10.1158/0008-5472.CAN-13-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeki H, Kitao H, Yoshinaga K, Nakanoko T, Kubo N, Kakeji Y, et al. Copy-neutral loss of heterozygosity at the p53 locus in carcinogenesis of esophageal squamous cell carcinomas associated with p53 mutations. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(7):1731–40. [DOI] [PubMed] [Google Scholar]
- 19.Okada S, Tokunaga E, Kitao H, Akiyoshi S, Yamashita N, Saeki H, et al. Loss of heterozygosity at BRCA1 locus is significantly associated with aggressiveness and poor prognosis in breast cancer. Annals of surgical oncology. 2012;19(5):1499–507. 10.1245/s10434-011-2166-5 [DOI] [PubMed] [Google Scholar]
- 20.Peiffer DA, Le JM, Steemers FJ, Chang W, Jenniges T, Garcia F, et al. High-resolution genomic profiling of chromosomal aberrations using Infinium whole-genome genotyping. Genome Res. 2006;16(9):1136–48. 10.1101/gr.5402306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RS. Molecular mechanism and clinical impact of APOBEC3B-catalyzed mutagenesis in breast cancer. Breast cancer research: BCR. 2015;17:8 10.1186/s13058-014-0498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borresen-Dale AL. TP53 and breast cancer. Human mutation. 2003;21(3):292–300. 10.1002/humu.10174 [DOI] [PubMed] [Google Scholar]
- 23.Olivier M, Langerod A, Carrieri P, Bergh J, Klaar S, Eyfjord J, et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(4):1157–67. [DOI] [PubMed] [Google Scholar]
- 24.Middlebrooks CD, Banday AR, Matsuda K, Udquim KI, Onabajo OO, Paquin A, et al. Association of germline variants in the APOBEC3 region with cancer risk and enrichment with APOBEC-signature mutations in tumors. Nature genetics. 2016;48(11):1330–8. 10.1038/ng.3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd JM, Newman TL, Tuzun E, Kaul R, Eichler EE. Population stratification of a common APOBEC gene deletion polymorphism. PLoS genetics. 2007;3(4):e63 10.1371/journal.pgen.0030063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuboi M, Yamane A, Horiguchi J, Yokobori T, Kawabata-Iwakawa R, Yoshiyama S, et al. APOBEC3B high expression status is associated with aggressive phenotype in Japanese breast cancers. Breast Cancer. 2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.