Abstract
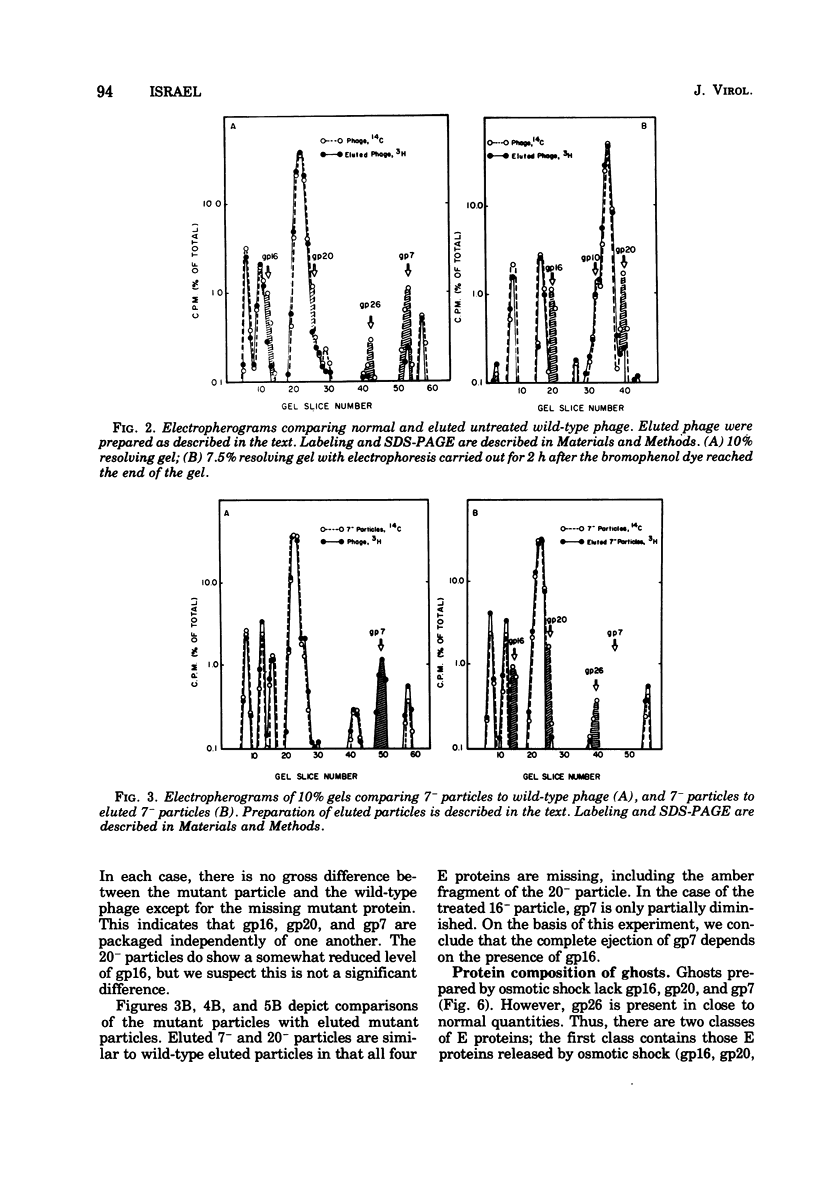
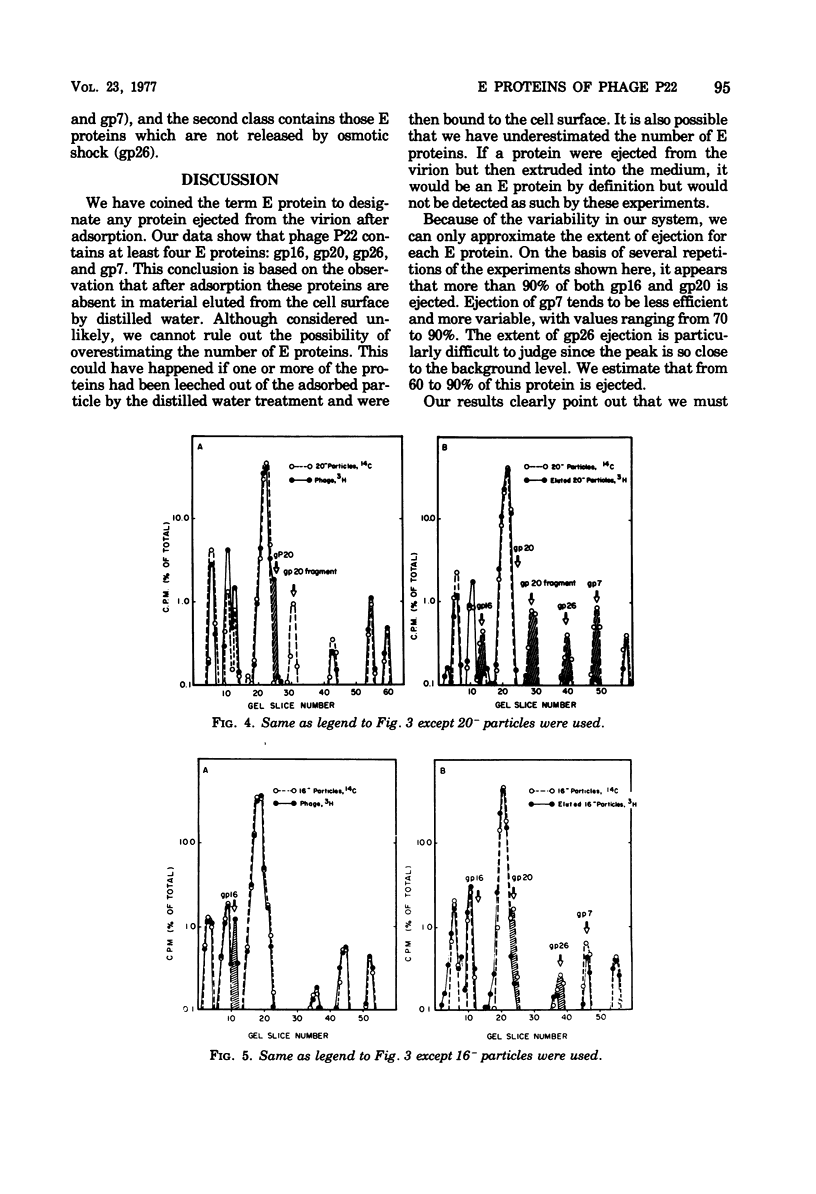
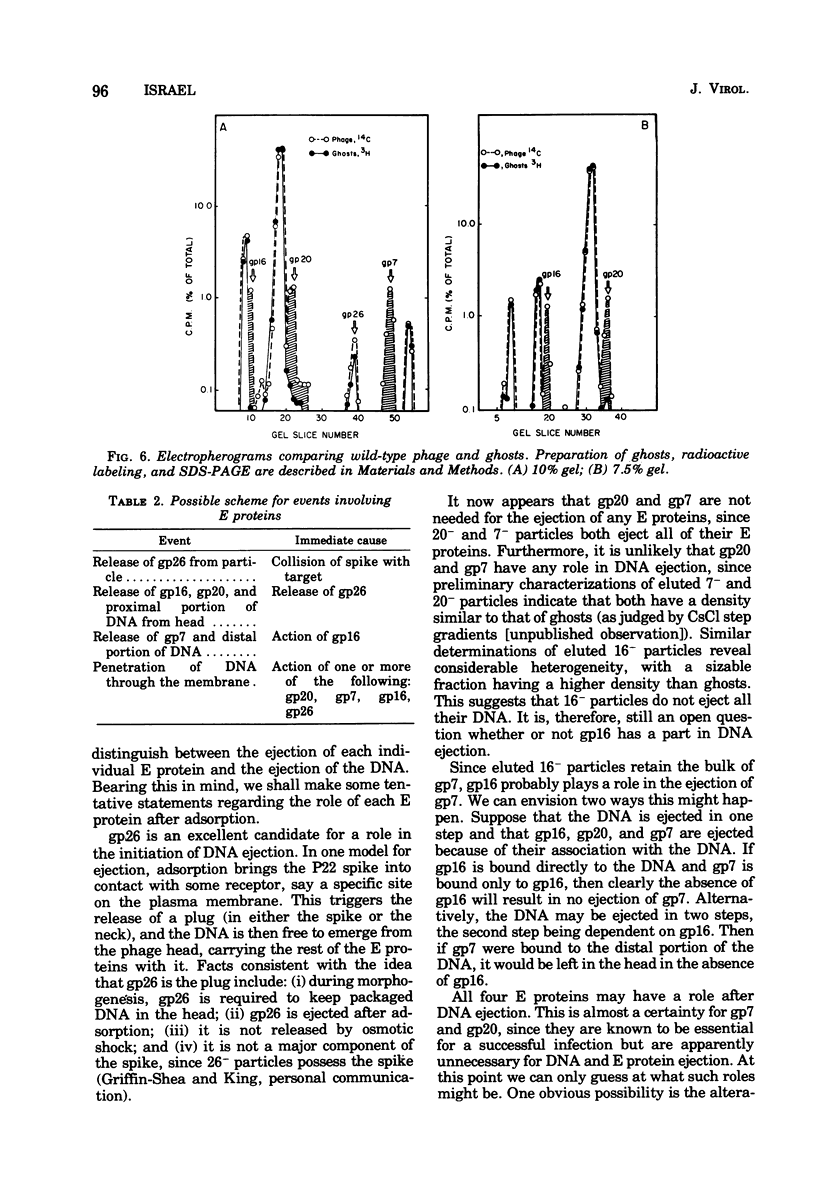
Of the nine proteins found in the virion of phage P22, four are ejected into the cell after adsorption. The four ejected proteins, termed E proteins, are gp16, gp20, gp26, and gp7. This was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of radioactively labeled phage that had been adsorbed to cells and then eluted off the surface with distilled water. Phage particles that lack gp7 (7- particles) or gp20 (20- particles) successfully eject all their E proteins. The 16- particles do not eject gp7. Analysis of phage ghosts showed that they lack gp16, gp20, and gp7, but they have gp26 in close to normal quantities. Our results suggest roles for gp16 and gp26 in DNA and E protein ejection. All four E proteins are possible candidates for roles in helping the phage DNA cross the plasma membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnberg A. C., Arwert F. DNA-protein complex in circular DNA from Bacillus bacteriophage GA-1. J Virol. 1976 May;18(2):783–784. doi: 10.1128/jvi.18.2.783-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert F., Venema G. Protease-sensitive transfection of Bacillus subtilis with bacteriophage GA-1 DNA: a probable case of heterologous transfection. J Virol. 1974 Mar;13(3):584–589. doi: 10.1128/jvi.13.3.584-589.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Chan R. K., Waddell C. H. Genetics of bacteriophage P22. II. Gene order and gene function. Virology. 1972 Jul;49(1):268–282. doi: 10.1016/s0042-6822(72)80028-x. [DOI] [PubMed] [Google Scholar]
- Botstein D. Synthesis and maturation of phage P22 DNA. I. Identification of intermediates. J Mol Biol. 1968 Jun 28;34(3):621–641. doi: 10.1016/0022-2836(68)90185-x. [DOI] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. P22 morphogenesis. I: Catalytic scaffolding protein in capsid assembly. J Supramol Struct. 1974;2(2-4):202–224. doi: 10.1002/jss.400020215. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D. Some minor components of bacteriophage T2 particles. Virology. 1957 Oct;4(2):237–264. doi: 10.1016/0042-6822(57)90061-2. [DOI] [PubMed] [Google Scholar]
- Hirokawa H. Transfecting deoxyribonucleic acid of Bacillus bacteriophage phi 29 that is protease sensitive. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1555–1559. doi: 10.1073/pnas.69.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B., Levine M. Bacteriophage P22 virion protein which performs an essential early function. I. Analysis of 16-ts mutants. J Virol. 1975 Dec;16(6):1536–1546. doi: 10.1128/jvi.16.6.1536-1546.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B., Levine M. Bacteriophage P22 virion protein which performs an essential early function. II. Characterization of the gene 16 function. J Virol. 1975 Dec;16(6):1547–1559. doi: 10.1128/jvi.16.6.1547-1559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel J. V., Anderson T. F., Levine M. in vitro MORPHOGENESIS OF PHAGE P22 FROM HEADS AND BASE-PLATE PARTS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):284–291. doi: 10.1073/pnas.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V., Rosen H., Levine M. Binding of bacteriophage P22 tail parts to cells. J Virol. 1972 Dec;10(6):1152–1158. doi: 10.1128/jvi.10.6.1152-1158.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., Lindberg A. A., Kornberg A. The gene H spike protein of bacteriophages phiX174 and S13. I. Functions in phage-receptor recognition and in transfection. Virology. 1975 Jul;66(1):283–293. doi: 10.1016/0042-6822(75)90198-1. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., Marco R., Kornberg A. A coat protein of the bacteriophage M13 virion participates in membrane-oriented synthesis of DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):205–209. doi: 10.1073/pnas.70.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M., Nathans D. Fate of maturation protein during infection by coliphage MS2. Nat New Biol. 1971 Sep 15;234(50):209–211. doi: 10.1038/newbio234209a0. [DOI] [PubMed] [Google Scholar]
- Krahn P. M., O'Callaghan R. J., Paranchych W. Stages in phage R17 infection. VI. Injection of A protein and RNA into the host cell. Virology. 1972 Mar;47(3):628–637. doi: 10.1016/0042-6822(72)90552-1. [DOI] [PubMed] [Google Scholar]
- LEVINE M., CURTISS R. Genetic fine structure of the C region and the linkage map of phage P22. Genetics. 1961 Dec;46:1573–1580. doi: 10.1093/genetics/46.12.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Chakravorty M., Bronson M. J. Control of the replication complex of bacteriophage P22. J Virol. 1970 Oct;6(4):400–405. doi: 10.1128/jvi.6.4.400-405.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H. O., LEVINE M. TWO SEQUENTIAL REPRESSIONS OF DNA SYNTHESIS IN THE ESTABLISHMENT OF LYSOGENY BY PHAGE P22 AND ITS MUTANTS. Proc Natl Acad Sci U S A. 1964 Aug;52:356–363. doi: 10.1073/pnas.52.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames R. B., Lorkiewicz Z. K., Kozinski A. W. Injection of ultraviolet-damage-specific enzyme by T4 bacteriophage. J Virol. 1973 Jul;12(1):1–8. doi: 10.1128/jvi.12.1.1-8.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., Black L. W. Assembly core of bacteriophage T4: an intermediate in head formation. Nat New Biol. 1973 Mar 21;242(116):70–75. doi: 10.1038/newbio242070a0. [DOI] [PubMed] [Google Scholar]
- Silverstein J. L., Goldberg E. B. T4 DNA injection. II. Protection of entering DNA from host exonuclease V. Virology. 1976 Jul 1;72(1):212–223. doi: 10.1016/0042-6822(76)90324-x. [DOI] [PubMed] [Google Scholar]
- Whitfield H. J., Jr, Martin R. G., Ames B. N. Classification of aminotransferase (C gene) mutants in the histidine operon. J Mol Biol. 1966 Nov 14;21(2):335–355. doi: 10.1016/0022-2836(66)90103-3. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO N., ANDERSON T. F. Genomic masking and recombination between serologically unrelated phages P22 and P221. Virology. 1961 Aug;14:430–439. doi: 10.1016/0042-6822(61)90334-8. [DOI] [PubMed] [Google Scholar]
- Yanofsky S., Kawamura F., Ito J. Thermolabile transfecting DNA from temperature-sensitive mutant of phage phi29. Nature. 1976 Jan 1;259(5538):60–63. doi: 10.1038/259060a0. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D. Lysogenization and superinfection immunity in Salmonella. Virology. 1958 Apr;5(2):291–326. doi: 10.1016/0042-6822(58)90025-4. [DOI] [PubMed] [Google Scholar]



