Supplemental Digital Content is available in the text.
Abstract
Background:
The authors present a novel mesh suture design aimed at minimizing the early laparotomy dehiscence that drives ventral hernia formation. The authors hypothesized that modulation of the suture-tissue interface through use of a macroporous structure and increased aspect ratio (width-to-height ratio) would decrease the suture pull-through that leads to laparotomy dehiscence.
Methods:
Incisional hernias were produced in 30 rats according to an established hernia model. The rat hernias were randomized to repair with either two 5-0 polypropylene sutures or two midweight polypropylene mesh sutures. Standardized photographs were taken before repair and 1 month after repair. Edge-detection software was used to define the border of the hernia defect and calculate the defect area. Histologic analysis was performed on all mesh suture specimens.
Results:
Seventeen hernias were repaired with mesh sutures and 13 were repaired with conventional sutures. The mean area of the recurrent defects following repair with mesh suture was 177.8 ± 27.1 mm2, compared with 267.3 ± 34.1 mm2 following conventional suture repair. This correlated to a 57.4 percent reduction in defect area after mesh suture repair, compared with a 10.1 percent increase in defect area following conventional suture repair (p < 0.0007). None (zero of 34) of the mesh sutures pulled through the surrounding tissue, whereas 65 percent (17 of 26) of the conventional sutures demonstrated complete pull-through. Excellent fibrocollagenous ingrowth was observed in 13 of 17 mesh suture specimens.
Conclusions:
Mesh sutures better resisted suture pull-through than conventional polypropylene sutures. The design elements of mesh sutures may prevent early laparotomy dehiscence by more evenly distributing distracting forces at the suture-tissue interface and permitting tissue incorporation of the suture itself.
Innovations by plastic surgeons in the field of abdominal wall reconstruction have served to limit the morbidity and increase the durability of ventral hernia repair procedures.1–5 However, the most significant innovation would be one that effectively prevents the laparotomy failure that drives incisional hernia formation.6 Over the past 3000 years, sutures used to oppose divided tissues have changed little from their initial flexible linear design.7 Also unchanged during that time is the problem of suture pull-through. Localized pressure at the suture-tissue interface acts like a cheese cutter to slice through intact tissue either acutely or chronically over time.8 The problem of suture pull-through is universal to all branches of surgery; however, the formation of hernias after closure of a midline laparotomy is an extreme and common example of sutures failing to fulfill their primary objective. Multiple strategies to improve surgical outcomes through new materials, suturing techniques, and meshes and efforts to modulate wound healing have not served to reduce the number of laparotomy closures that go on to form incisional hernias, with greater than 300,000 incisional hernias requiring repair annually in the United States.9
We hypothesized that it is possible to modulate the suture-tissue interface with a novel mesh suture design. This suture design aims to decrease suture pull-through by means of increased elasticity, larger suture-tissue interface area, and progressive tissue incorporation of the suture. The ability of this novel suture to resist tissue pull-through was evaluated in an established rat midline hernia model.
MATERIALS AND METHODS
We acclimated Sprague-Dawley rats (Harlan Laboratories, Indianapolis, Ind.) weighing 250 to 300 g and housed them under standard conditions. Animals were allowed ad libitum intake of standard rat chow and water throughout the study. We performed all animal care and operative procedures in accordance with the U.S. Public Health Service Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 86-23, revised 1985). The animal use protocol was approved by the Northwestern University Institutional Animal Care and Use Committee.
Mesh Suture Creation
Macroporous polypropylene sutures (mesh sutures) were created in the following manner. Strips of uncoated mid-weight macroporous polypropylene mesh (Prolene Soft Mesh; Ethicon, Inc., Somerville, N.J.) were cut at various widths, after which force extension curves were generated for each width using an Instron Tensiometer (model 5542; Instron Corp., Canton, Mass.) equipped with a 50-N static load cell set at a crosshead speed of 10 mm/minute. These force extension curves were compared to those generated for conventional 5-0 polypropylene suture (Prolene). Tensiometric testing demonstrated mesh sutures with a width of 4 mm to have comparable tensile strength (maximal load at failure: mesh suture, 4.6 N; 5-0 polypropylene suture, 6.7 N) and greater extensibility (extension at tensile strength: mesh suture, 25.1 mm; 5-0 polypropylene suture, 8.0 mm).
Incisional Hernia Formation
We produced incisional hernias in 30 rats according to the model described previously by DuBay et al.10 In an avascular prefascial plane, 6 × 3-cm rectangular full-thickness skin flaps based 2 cm lateral to the ventral midline were raised. This served to separate the skin incisions from the midline laparotomy incision. We then created a 5-cm midline laparotomy incision through the linea alba. This laparotomy incision was repaired with two interrupted, rapidly absorbed, 5-0 plain catgut sutures (Covidien, Mansfield, Mass.) placed approximately 3 mm from the cut fascial edges and equidistant from the incision ends and to each other. The skin flap was then secured in place with three deep 4-0 polyglycolic acid sutures (Polysorb; Covidien), after which the skin incision was closed in running fashion using a 4-0 poly glycolic acid suture. Neck collars were required to prevent autophagy of the incisions. The temporary laparotomy closures successfully produced fascial defects in all 30 animals. By the time the fascial closure failed, herniation of the abdominal viscera was contained by the intact skin flap closure. The incisional hernias were allowed to mature for 28 days before undergoing repair.
Incisional Hernia Repair
All 30 animals underwent repair of the established incisional hernia. The previously designed abdominal skin flap was reelevated along the existing incisions, and the borders of the hernia ring were dissected free under 2.5× loupe magnification. In all cases, the hernia ring was identified as a discrete margin surrounding the hernia defect. A 3 × 6-cm rectangle of waterproof 2-mm grid graph paper (Rite in the Rain; JL Darling LLC, Tacoma, Wash.) was placed intraabdominally against the posterior aspect of the abdominal wall for photographic assessment of the defect size. Once photographed, the rat was assigned randomly to hernia repair with either conventional 5-0 polypropylene or mesh suture. Randomization was achieved using a random number generator, with even numbers corresponding to conventional suture repair and odd numbers dictating mesh suture repair. Hernia repair using conventional suture was performed by means of two interrupted sutures placed 5 mm from the fascial edges and equidistant from each other and the rostral and caudal extent of the hernia defect. The suture was tightened until the hernia edges had been reapproximated and then secured in standard fashion using a surgeon’s knot. Mesh suture repairs were performed in similar fashion, with two differences in technique. First, the mesh suture was introduced through the abdominal wall tissues with the assistance of a flat 3-mm-wide needle that had been swedged to the mesh suture. Second, the ends of the mesh ribbon were temporarily secured in place with a medium hemoclip, and then sutured together with a single 5-0 polypropylene suture that did not pass into the tissues. After closure of the hernia defect, the skin flap was replaced and sutured in analogous fashion to the prior procedure. Each animal was then followed for an additional 28 days.
Necropsy Measurement of Hernia Area
To measure the area of the hernia defect, we completed necropsies on postoperative day 28 after the hernia repair (postoperative day 56 after initial laparotomy procedure). Within 30 minutes of killing the animal, we circumferentially dissected the skin free of the abdominal wall. We then excised the thin-wall hernia sac from the surrounding hernia ring, which at this point was well developed and clearly identified with 2.5× loupe magnification. Suture pull-through was recorded if the suture was no longer in contact with both sides of the abdominal wall. Two 4-cm transverse incisions were made 1 cm from the rostral and caudal ends of the hernia defect, through which a 4 × 8-cm sheet of waterproof 2-mm grid graph paper was passed. The graph paper was positioned in contact with the posterior aspect of the abdominal wall and the postrepair hernia defect was then photographed. With the 2-mm grid graph paper serving as a calibration reference for each sample, all photographs were recorded in Tagged Image File Format. To minimize the amount of investigator bias introduced in defining and measuring the hernia defect size, edge-detection software provided by the open-source image processing package, Fiji, was used to define the border of the hernia defect and calculate the defect area. All area calculations were calibrated based on the 2-mm grid present in each photograph (Fig. 1).
Fig. 1.
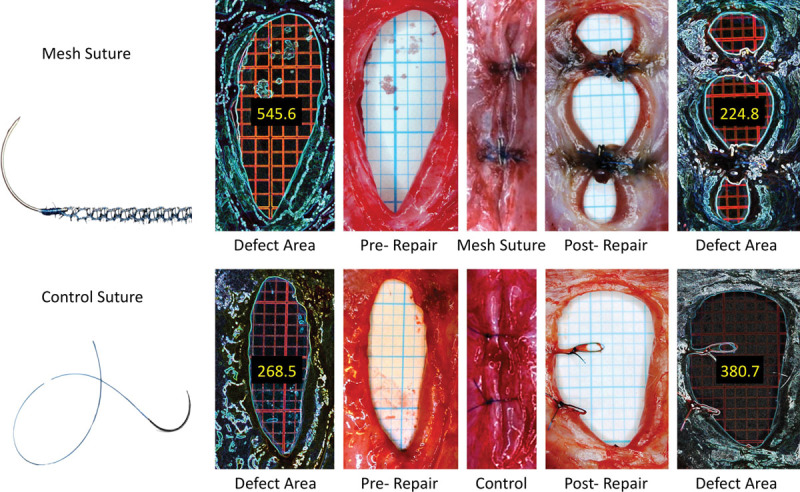
Original hernia defect, defect closure, and postrepair hernia defect following mesh suture (above) and conventional suture (below) repair. Defect area measurement by means of edge-detection technique is demonstrated.
Histology
Once the hernia defect had been photographed, biopsy specimens were taken of the rostral mesh suture and 1 cm of surrounding abdominal wall in all animals that had undergone mesh suture repair. These tissue samples were fixed in formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Five nonoverlapping fields per specimen were evaluated at 100× magnification and graded according to a four-point scale adapted from the American Society for Testing and Materials guidelines, as described previously by Melman et al.11 Higher scores correlate with a greater degree of tissue ingrowth.
Mechanical Testing
Tensiometric analysis had been intended, but a useful comparison between the suture and mesh repairs was precluded by the frequency with which the conventional suture repair pulled completely through the contralateral abdominal wall, thus failing to leave a spanning tissue segment on which to perform testing. Likewise, tensiometry of the strength of the mesh suture-tissue interface failed to yield interpretable data, as mechanical tissue disruption lateral to the suture-tissue interface occurred with each trial. This mechanical disruption resulted from tearing of the specimen along the pneumatic grip rather than the intended pull-through phenomenon and thus did not provide useful data with respect to the tissue-mesh interaction.
Statistical Analysis
Data were analyzed with the use of GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, Calif.). An unpaired t test with Welch’s correction was used to determine the difference in prerepair and postrepair defect area between the mesh ribbon and conventional suture groups. Values were reported as mean ± SEM. Values of p < 0.05 were considered significant.
RESULTS
Hernia Area Measurements
All 30 rats developed an incisional hernia by postoperative day 28. After randomization to repair type, 17 hernias were repaired with mesh suture and 13 hernias were repaired with conventional 5-0 polypropylene suture. The mean area of the hernia defects repaired with mesh suture was 391.9 ± 33.4 mm2, whereas that of the hernia defects repaired with conventional 5-0 polypropylene suture was 255.4 ± 23.3 mm2. Despite a rigid randomization protocol, the defects repaired with mesh ribbon were significantly larger than those undergoing conventional suture repair (p < 0.0025). The mean area of the recurrent defect following repair with the mesh ribbon suture was 177.8 ± 27.1 mm2, compared with 267.3 ± 34.1 mm2 following conventional suture repair (Fig. 2, left). When compared with the initial defect area, the mesh suture repairs produced recurrent defects with a mean area that was 42.6 percent that of the original defect, whereas the conventional suture repairs resulted in a recurrent defect area that averaged 110.1 percent that of the original defect (p < 0.0001). This correlated to a 57.4 percent reduction in defect area after mesh suture repair, whereas conventional suture repairs yielded a 10.1 percent increase in postrepair defect area (p < 0.0007) (Fig. 2, right). Notably, none (zero of 34) of the mesh sutures pulled through the surrounding abdominal wall tissue, whereas 65 percent (17 of 26) of the conventional sutures used for repair demonstrated complete pull-through.
Fig. 2.
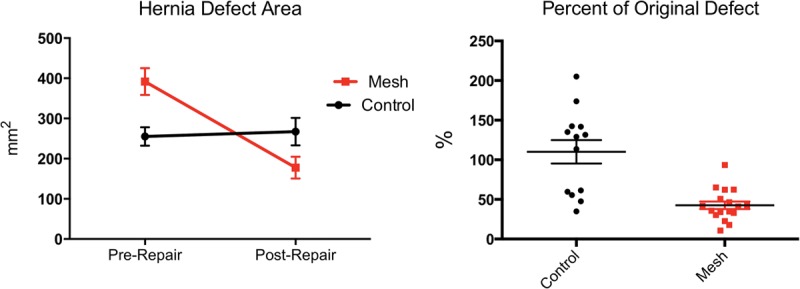
(Left) Comparison of prerepair and postrepair hernia defect area. (Right) Percentage change in hernia defect area following repair.
Histologic Analysis
Excellent (American Society for Testing and Materials 3) ingrowth was observed in 13 of 17 mesh ribbon specimens, whereas the remaining four specimens demonstrated good (American Society for Testing and Materials 2) ingrowth. A histologic specimen demonstrating excellent ingrowth is depicted in Figure 3.
Fig. 3.
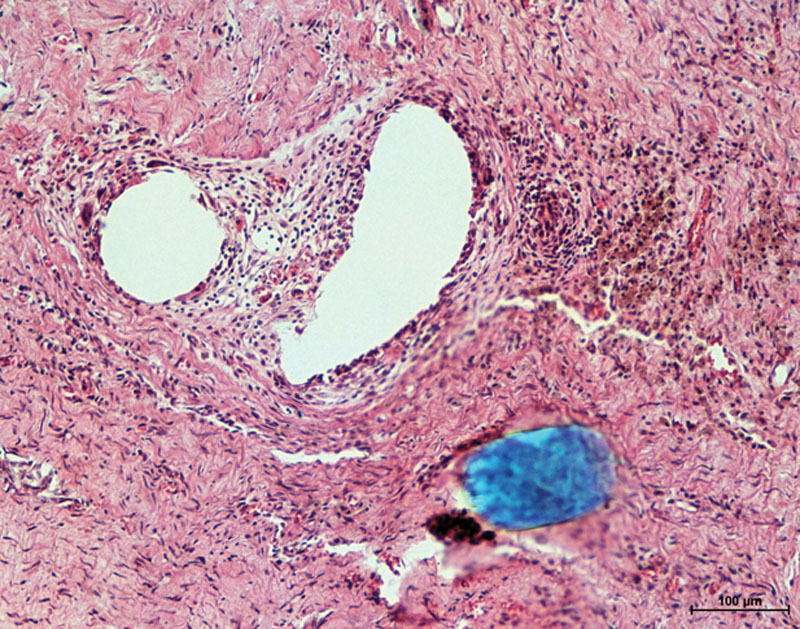
Sample hematoxylin and eosin photomicrograph of the mesh suture 1 month after implantation at 100× magnification. Vacuolated regions represent mesh fibers.
DISCUSSION
Plastic surgeons have become increasingly involved in the management of the ventral hernia patient. Combining an expertise in wound healing and soft-tissue management with mechanical concepts borrowed from experience with breast and hand surgery, plastic surgeons have been instrumental in the introduction and evaluation of new techniques and materials for abdominal wall reconstruction.3,4,12–18 However, although these advances have yielded improved surgical outcomes with regard to hernia repair, there has not been an equivalent improvement in the general surgeon’s ability to provide a reliable laparotomy closure. A recent review of outcomes from two large prospective trials reported an incisional hernia rate of 22.4 percent at 3 years, a rate comparable to that reported more than 30 years ago.9,19 Frustrated by the lack of progress, surgeons have been left to blame the patient, with multiple reports citing risk factors such as smoking and obesity as principal causes for an inability to provide durable closure of the linea alba after incision.20–22 However, recent clinical and preclinical studies suggest that this high rate of hernia formation is driven primarily by a process of early occult laparotomy dehiscence.6,23,24 Likewise, it is well established that acute laparotomy dehiscence occurs through a mechanical process of suture pull-through.8,25–32 Patient factors may contribute to the tendency for suture to tear through tissue, with obesity or chronic obstructive pulmonary disease increasing tension on the laparotomy closure; and steroids, malnutrition, or connective tissue disease weakening the abdominal wall tissues.33 However, suture pull-through remains the common pathway through which all hernias develop. Thus, techniques or materials that target the fundamental process of suture pull-through hold the greatest promise of actually preventing incisional hernia formation.
The forces experienced at each suture-tissue interface can be understood as the total force opposing abdominal closure divided by the number of suture fixation points. The total mechanical force required for closure is variable based on the volume of the intraabdominal contents and the compliance of the abdominal wall. The dynamic nature of the abdominal wall puts further stress on the abdominal closure, as muscular contraction associated with coughing, breathing, and movement transiently increase the intensity of the distracting force experienced at the suture-tissue interface.34 Cobb et al. estimated the tensile strength needed to resist abdominal wall contraction to be between 11 and 27 N/cm, depending on the intensity of the activity performed.35 Immediately after laparotomy closure, these forces are resisted solely by the suture, and are distributed across a suture-tissue interface area defined by the size of the suture and the number of fixation points. This is completely analogous to two-, four-, and six-strand flexor tendon repairs, where the early strength of the repair is dependent entirely on the strength of the suture and the ability of the repair technique to grasp the tendon fibers. The greater the force applied to the tendon, and the fewer locking components incorporated into the repair, the greater the likelihood for rupture.37 The same holds true for the acute laparotomy closure. The higher the tensile strength required to maintain midline closure, and the smaller the suture-tissue interface area, the greater the likelihood for acute laparotomy failure.26 If the fascial edges or cut tendon ends are maintained in apposition over time, biological healing occurs to add intrinsic support to the extrinsic strength provided by the suture. In essence, fascial healing serves to offload the mechanical construct by increasing the area across which this tension is distributed.
However, biological healing, and any therapy or intervention aimed to optimize it, is predicated on the ability of the repair to maintain apposition of the involved tissue edges. Just as gapping of the tendon ends is known to predispose a tendon repair to rupture, early laparotomy dehiscence predicts eventual hernia formation.23,24 Although splinting and positioning can be used to offload a flexor tendon repair to minimize the forces experienced at the suture-tissue interface, no such adjuncts exist to protect an abdominal wall closure. Viewed along the lines of an unprotected flexor tendon repair, it is easier to appreciate the mechanical challenge posed by laparotomy closure, and to understand why incisional hernia formation remains the most common operative complication following abdominal surgery.6
The importance of the suture-tissue interface has been the subject of multiple prior reports, but alterations in suture size, extensibility, and surgical closure technique have thus far been unable to sufficiently modulate this interface enough to prevent suture pull-through.8,25–28,36,38,39 Efforts to broaden the suture-tissue interface area by increasing the size of the suture simultaneously increase the volume of the implanted foreign material, and are thus prone to issues of palpability, discomfort, and the potential for infection or foreign body response.40 Increasing the aspect ratio (width-to-height ratio) of the suture serves as an alternative way of increasing the suture-tissue interface area without dramatically increasing the overall size of the suture. Conventional suture is cylindrical, with an aspect ratio of 1 (width-to-height ratio of 1:1), whereas the aspect ratio increases as the suture becomes progressively more elliptical and ultimately ribbon-like. Preliminary tensiometric testing with porcine linea alba has demonstrated increased resistance to suture pull-through with increasing suture aspect ratio (width-to-height ratio). This has also been demonstrated in a rat acute abdominal burst model. (See Video, Supplemental Digital Content 1, which shows a rat acute abdominal burst model demonstrating mesh suture’s increased resistance to pull-through over conventional 5-0 polypropylene suture. Balloon insufflation of a rat abdomen closed with two conventional sutures fails at the suture-tissue interface, whereas identical balloon insufflation of an abdomen closed with mesh suture fails by means of rupture of the abdominal wall itself, http://links.lww.com/PRS/B194.) The video qualitatively shows that suture shape, even without tissue integration, can influence suture pull-through. Balloon insufflation of a rat abdomen closed with two conventional sutures fails at the suture-tissue interface, whereas identical balloon insufflation of an abdomen closed with mesh suture failed by means of rupture of the abdominal wall itself. Clinically, this concept is already being applied to median sternotomy closure, where 4-mm-wide cable ties have been used in place of conventional wires to avoid sternal instability caused by wire pull-through.41
Video.
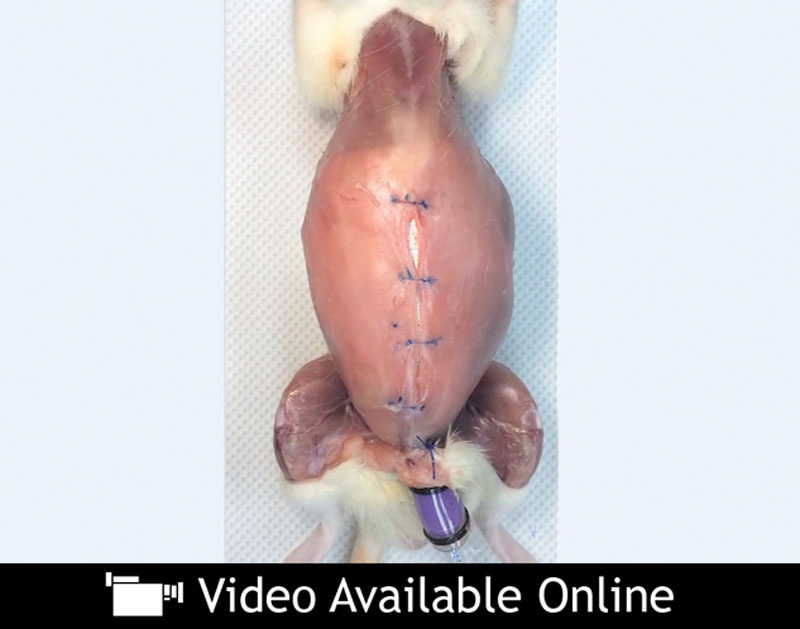
Supplemental Digital Content 1 shows a rat acute abdominal burst model demonstrating mesh suture’s increased resistance to pull-through over conventional 5-0 polypropylene suture. Balloon insufflation of a rat abdomen closed with two conventional sutures fails at the suture-tissue interface, whereas identical balloon insufflation of an abdomen closed with mesh suture fails by means of rupture of the abdominal wall itself, http://links.lww.com/PRS/B194.
In addition to an increased aspect ratio, the mesh suture also benefits from increased extensibility. Enhanced extensibility serves to better absorb episodic increases in force at the suture-tissue interface that occur with coughing and other functions associated with transient abdominal wall contraction. Rodeheaver et al. showed that a polybutester monofilament suture with increased elasticity better resisted suture pull-through than the more rigid nylon and polyglycolic sutures to which it was compared.36 Finally, the macroporous structure of the mesh suture design further decreases the amount of implanted foreign material and also fosters fibrocollagenous ingrowth. Suture materials with an impervious or microporous surface structure are encapsulated by scar, whereas materials with a macroporous structure have the potential to become incorporated into the surrounding tissue.42 Although cellular integration may not necessarily impart greater strength on the implanted suture itself, limiting the foreign body response should make it less prone to the chronic suture pull-through that occurs by means of protease-mediated breakdown of adjacent scar tissue.6,25 As anticipated based on clinical experience with the use of macroporous polypropylene mesh, the majority of the mesh suture specimens exhibited excellent ingrowth on histologic evaluation, with the few remaining specimens demonstrating good tissue integration. More in-depth histologic evaluation was not performed, as the biocompatibility of macroporous midweight polypropylene has been previously well defined.42
We hypothesized that a mesh suture design featuring increased elasticity and a larger suture-tissue interface area would better distribute force across the repair and thus be less prone to acute suture pull-through. Furthermore, we anticipated that the macroporous mesh construct would also better resist a chronic pull-through mechanism. Improved distribution of forces should minimize the extent to which pressure-induced necrosis occurs, and any tendency toward chronic cheese-wiring should be offset by fibrocollagenous ingrowth within the suture itself. Although the relative importance of each of these mechanisms is still unknown, the ability of the overall construct to resist suture pull-through was clearly demonstrated by the rat hernia model presented here.
Given the intent for mesh suture to be used for primary laparotomy closure rather than to be applied as a means of repairing an established hernia, one may question why a hernia model was used in place of a laparotomy model. Unfortunately, acute laparotomy failure is a uniquely human phenomenon.6 As a result, we elected to use an established ventral hernia model as a “challenged” laparotomy, one in which the fascial edges possess the healing characteristics of a chronic wound and thus contribute little tissue bridging to offset the forces experienced at the suture-tissue interface. This model is known to produce predictable hernia recurrence when repaired with conventional polypropylene suture.10 The suture pull-through mechanism by which this hernia recurrence occurs has been shown to be similar in nature to that which occurs clinically.10 Thus, this “stressed” model was ideally suited to test suture pull-through rather than the efficacy of the hernia repair. Given the limitations of the model, hernia recurrence was expected in all animals, with the size of the recurrent hernia defect intended to serve as a proxy for the extent of suture pull-through. To accentuate this, only two sutures were used for repair, rather than the five or six that would have been required to mimic a clinical laparotomy closure. Overall, the data clearly suggest that the mesh suture better resisted cheese-wiring, supporting the primary hypothesis that it is possible to modify the suture-tissue interface with use of an unconventional suture design.
We recognize that these findings are preliminary in nature. In addition to the limitations discussed previously, our rat model is further restrained by the issue of scale, which prohibits immediate translation of our findings to the clinical setting. In addition, planned tensiometric testing aimed at quantifying the tensile strength of the suture-tissue interface was limited by technical constraints. Because of the frequency with which the conventional sutures pulled completely through the contralateral abdominal wall, there were insufficient specimens for testing. In addition, evaluation of the tensile strength at the suture-tissue interface of the mesh suture specimens was prevented by tissue tearing elsewhere in the specimen. Beyond these scientific limitations, there exist practical challenges to incorporating these design characteristics into a clinically useful suture. The ribbon-like shape of the mesh suture used for investigation is hampered by poor handling characteristics, as it requires precise placement to ensure that the broad portion of the suture serves as the suture-tissue interface. Efforts are underway to harness these design concepts in the form of a hollow, macroporous, three-dimensional suture capable of flattening against the tissue when exposed to distracting forces. Such a design would eliminate the need to orient the suture and would dramatically improve its handling characteristics. Testing is currently underway of a mesh suture for human use in a large-animal model.
CONCLUSIONS
In conclusion, macroporous mesh suture demonstrated an improved ability to approximate tissues under tension over time. Although encouraged by these results, we recognize the limitations of the described model. Nevertheless, the concepts introduced represent an entirely novel and seemingly effective approach to the challenging problem of suture pull-through. Although this problem is perhaps most apparent in the form of laparotomy failure, it is certainly not limited to this clinical entity. From tendon repairs to rhytidectomies, all surgical procedures that aim to join tissues under tension are plagued by suture pull-through. In an era of molecular medicine and rational drug design, it is only reasonable to expect surgical suture to exhibit design characteristics better suited to its intended purpose.
Supplementary Material
Footnotes
Presented at the 59th Annual Meeting of the Plastic Surgery Research Council, in New York, New York, March 7 through 9, 2014; the 16th Annual Meeting of the Americas Hernia Society, in Las Vegas, Nevada, March 12 through 15, 2014, where it was awarded Best Resident Presentation; and presented in part at the Technology Innovation in Plastic Surgery meeting, in San Francisco, California, May 31 through June 2, 2013, where the suture prototype that has been developed based on this work won the Trends in Plastic Surgery Innovation Challenge.
Supplemental digital content is available for this article. Direct URL citations appear in the text; simply type the URL address into any Web browser to access this content. Clickable links to the material are provided in the HTML text of this article on the Journal’s Web site (www.PRSJournal.com).
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Saulis AS, Dumanian GA. Periumbilical rectus abdominis perforator preservation significantly reduces superficial wound complications in “separation of parts” hernia repairs. Plast Reconstr Surg. 2002;109:2275–2280; discussion 2281. doi: 10.1097/00006534-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Ghali S, Turza KC, Baumann DP, Butler CE. Minimally invasive component separation results in fewer wound-healing complications than open component separation for large ventral hernia repairs. J Am Coll Surg. 2012;214:981–989. doi: 10.1016/j.jamcollsurg.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel KM, Bhanot P, Franklin B, Albino F, Nahabedian MY. Use of intraoperative indocyanine-green angiography to minimize wound healing complications in abdominal wall reconstruction. J Plast Surg Hand Surg. 2013;47:476–480. doi: 10.3109/2000656X.2013.787085. [DOI] [PubMed] [Google Scholar]
- 4.Souza JM, Dumanian GA. Routine use of bioprosthetic mesh is not necessary: A retrospective review of 100 consecutive cases of intra-abdominal midweight polypropylene mesh for ventral hernia repair. Surgery. 2013;153:393–399. doi: 10.1016/j.surg.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Koltz PF, Frey JD, Bell DE, et al. Evolution of abdominal wall reconstruction: Development of a unified algorithm with improved outcomes. Ann Plast Surg. 2013;47:476–480. doi: 10.1097/SAP.0b013e3182a6367f. [DOI] [PubMed] [Google Scholar]
- 6.Xing L, Culbertson EJ, Wen Y, Franz MG. Early laparotomy wound failure as the mechanism for incisional hernia formation. J Surg Res. 2013;182:e35–e42. doi: 10.1016/j.jss.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majno G. The Healing Hand: Man and Wound in the Ancient World. Cambridge, Mass: Harvard University Press; 1975. [Google Scholar]
- 8.Höer J, Fischer L, Schachtrupp A. Laparotomy closure and incisional hernia prevention: What are the surgical requirements? (in German). Zentralbl Chir. 2011;136:42–49. doi: 10.1055/s-0030-1262682. [DOI] [PubMed] [Google Scholar]
- 9.Fink C, Baumann P, Wente MN, et al. Incisional hernia rate 3 years after midline laparotomy. Br J Surg. 2014;101:51–54. doi: 10.1002/bjs.9364. [DOI] [PubMed] [Google Scholar]
- 10.DuBay DA, Wang X, Adamson B, Kuzon WM, Jr, Dennis RG, Franz MG. Progressive fascial wound failure impairs subsequent abdominal wall repairs: A new animal model of incisional hernia formation. Surgery. 2005;137:463–471. doi: 10.1016/j.surg.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Melman L, Jenkins ED, Hamilton NA, et al. Histologic and biomechanical evaluation of a novel macroporous polytetrafluoroethylene knit mesh compared to lightweight and heavyweight polypropylene mesh in a porcine model of ventral incisional hernia repair. Hernia. 2011;15:423–431. doi: 10.1007/s10029-011-0787-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: An anatomic and clinical study. Plast Reconstr Surg. 1990;86:519–526. doi: 10.1097/00006534-199009000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Butler CE, Campbell KT. Minimally invasive component separation with inlay bioprosthetic mesh (MICSIB) for complex abdominal wall reconstruction. Plast Reconstr Surg. 2011;128:698–709. doi: 10.1097/PRS.0b013e318221dcce. [DOI] [PubMed] [Google Scholar]
- 14.Ko JH, Wang EC, Salvay DM, et al. Abdominal wall reconstruction lessons learned from 200 “components separation” procedures. Arch Surg. 2009;144:1047–1055. doi: 10.1001/archsurg.2009.192. [DOI] [PubMed] [Google Scholar]
- 15.Ko JH, Salvay DM, Paul BC, Wang EC, Dumanian GA. Soft polypropylene mesh, but not cadaveric dermis, significantly improves outcomes in midline hernia repairs using the components separation technique. Plast Reconstr Surg. 2009;124:836–847. doi: 10.1097/PRS.0b013e3181b0380e. [DOI] [PubMed] [Google Scholar]
- 16.Janis JE. Use of progressive tension sutures in components separation: Merging cosmetic surgery techniques with reconstructive surgery outcomes. Plast Reconstr Surg. 2012;130:851–855. doi: 10.1097/PRS.0b013e318262f1fd. [DOI] [PubMed] [Google Scholar]
- 17.Breuing K, Butler CE, Ferzoco S, et al. Incisional ventral hernias: Review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148:544–558. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Souza JM, Dumanian GA. An evidence-based approach to abdominal wall reconstruction. Plast Reconstr Surg. 2012;130:116–124. doi: 10.1097/PRS.0b013e318254b18c. [DOI] [PubMed] [Google Scholar]
- 19.Wissing J, van Vroonhoven TJ, Schattenkerk ME, Veen HF, Ponsen RJ, Jeekel J. Fascia closure after midline laparotomy: Results of a randomized trial. Br J Surg. 1987;74:738–741. doi: 10.1002/bjs.1800740831. [DOI] [PubMed] [Google Scholar]
- 20.Sørensen LT, Hemmingsen UB, Kirkeby LT, Kallehave F, Jørgensen LN. Smoking is a risk factor for incisional hernia. Arch Surg. 2005;140:119–123. doi: 10.1001/archsurg.140.2.119. [DOI] [PubMed] [Google Scholar]
- 21.Antoniou GA, Georgiadis GS, Antoniou SA, Granderath FA, Giannoukas AD, Lazarides MK. Abdominal aortic aneurysm and abdominal wall hernia as manifestations of a connective tissue disorder. J Vasc Surg. 2011;54:1175–1181. doi: 10.1016/j.jvs.2011.02.065. [DOI] [PubMed] [Google Scholar]
- 22.Yahchouchy-Chouillard E, Aura T, Picone O, Etienne JC, Fingerhut A. Incisional hernias: I. Related risk factors. Dig Surg. 2003;20:3–9. doi: 10.1159/000068850. [DOI] [PubMed] [Google Scholar]
- 23.Pollock AV, Evans M. Early prediction of late incisional hernias. Br J Surg. 1989;76:953–954. doi: 10.1002/bjs.1800760926. [DOI] [PubMed] [Google Scholar]
- 24.Burger JW, Lange JF, Halm JA, Kleinrensink GJ, Jeekel H. Incisional hernia: Early complication of abdominal surgery. World J Surg. 2005;29:1608–1613. doi: 10.1007/s00268-005-7929-3. [DOI] [PubMed] [Google Scholar]
- 25.Carlson MA. Acute wound failure. Surg Clin North Am. 1997;77:607–636. doi: 10.1016/s0039-6109(05)70571-5. [DOI] [PubMed] [Google Scholar]
- 26.Alexander HC, Prudden JF. The causes of abdominal wound disruption. Surg Gynecol Obstet. 1966;122:1223–1229. [PubMed] [Google Scholar]
- 27.Gislason H, Grønbech JE, Søreide O. Burst abdomen and incisional hernia after major gastrointestinal operations: Comparison of three closure techniques. Eur J Surg. 1995;161:349–354. [PubMed] [Google Scholar]
- 28.Greenburg AG, Saik RP, Peskin GW. Wound dehiscence: Pathophysiology and prevention. Arch Surg. 1979;114:143–146. doi: 10.1001/archsurg.1979.01370260033004. [DOI] [PubMed] [Google Scholar]
- 29.Lythgoe JP. Burst abdomen. Postgrad Med J. 1960;36:388–391. doi: 10.1136/pgmj.36.416.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niggebrugge AH, Hansen BE, Trimbos JB, van de Velde CJ, Zwaveling A. Mechanical factors influencing the incidence of burst abdomen. Eur J Surg. 1995;161:655–661. [PubMed] [Google Scholar]
- 31.Sanders RJ, DiClementi D. Principles of abdominal wound closure: II. Prevention of wound dehiscence. Arch Surg. 1977;112:1188–1191. doi: 10.1001/archsurg.1977.01370100042008. [DOI] [PubMed] [Google Scholar]
- 32.Poole GV, Jr, Meredith JW, Kon ND, Martin MB, Kawamoto EH, Myers RT. Suture technique and wound-bursting strength. Am Surg. 1984;50:569–572. [PubMed] [Google Scholar]
- 33.Franz MG. The biology of hernia formation. Surg Clin North Am. 2008;88:1–15. doi: 10.1016/j.suc.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dumanian GA. The physics of abdominal wall reconstruction. In: Nahabedian MY, Bhanot P, editors. Abdominal Wall Reconstruction. Woodbury, Conn: Cinemed; [Google Scholar]
- 35.Cobb WS, Kercher KW, Heniford BT. The argument for lightweight polypropylene mesh in hernia repair. Surg Innov. 2005;12:63–69. doi: 10.1177/155335060501200109. [DOI] [PubMed] [Google Scholar]
- 36.Rodeheaver GT, Nesbit WS, Edlich RF. Novafil: A dynamic suture for wound closure. Ann Surg. 1986;204:193–199. doi: 10.1097/00000658-198608000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strickland JW. Development of flexor tendon surgery: Twenty-five years of progress. J Hand Surg Am. 2000;25:214–235. doi: 10.1053/jhsu.2000.jhsu25a0214. [DOI] [PubMed] [Google Scholar]
- 38.Dudley HA. Layered and mass closure of the abdominal wall. Br J Surg. 1970;57:664–667. doi: 10.1002/bjs.1800570908. [DOI] [PubMed] [Google Scholar]
- 39.Israelsson LA, Millbourn D. Closing midline abdominal incisions. Langenbecks Arch Surg. 2012;397:1201–1207. doi: 10.1007/s00423-012-1019-4. [DOI] [PubMed] [Google Scholar]
- 40.McLeod RS, Brenneman FD, Rotstein OD, Bhanot P Members of the Evidence-Based Reviews in Surgery Group. Does the size of the stitch length affect surgical site infection? J Am Coll Surg. 2013;217:556–559. doi: 10.1016/j.jamcollsurg.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Grapow MT, Melly LF, Eckstein FS, Reuthebuch OT. A new cable-tie based sternal closure system: Description of the device, technique of implantation and first clinical evaluation. J Cardiothorac Surg. 2012;7:59. doi: 10.1186/1749-8090-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orenstein SB, Saberski ER, Kreutzer DL, Novitsky YW. Comparative analysis of histopathologic effects of synthetic meshes based on material, weight, and pore size in mice. J Surg Res. 2012;176:423–429. doi: 10.1016/j.jss.2011.09.031. [DOI] [PubMed] [Google Scholar]


