Supplemental Digital Content is Available in the Text.
Key Words: d-amphetamine, lisdexamfetamine, pharmacokinetics, yogurt, orange juice
Abstract
Background:
This open-label, crossover study examined lisdexamfetamine dimesylate (LDX) and d-amphetamine pharmacokinetics in healthy adults after administration of an intact LDX capsule or after the capsule was emptied into orange juice or yogurt and the contents consumed.
Methods:
Healthy adult volunteers (N = 30) were administered a 70-mg LDX capsule or the contents of a 70-mg capsule mixed with yogurt or orange juice using a 3-way crossover design. Blood samples were collected serially for up to 96 hours after dose. Pharmacokinetic endpoints included maximum plasma concentration (Cmax) and area under the plasma concentration versus time curve from zero to infinity (AUC0–∞) or to last assessment (AUClast). Relative LDX and d-amphetamine bioavailabilities from the contents of a 70-mg LDX capsule mixed with orange juice or yogurt were compared with those from the intact LDX capsule using bioequivalence-testing procedures.
Results:
Geometric least squares mean ratios (90% confidence intervals [CIs]) for d-amphetamine (active moiety) were within the prespecified bioequivalence range (0.80–1.25) when the contents of a 70-mg LDX capsule were mixed with orange juice [Cmax: 0.971 (0.945, 0.998); AUC0–∞: 0.986 (0.955, 1.019); AUClast: 0.970 (0.937, 1.004)] or yogurt [Cmax: 0.970 (0.944, 0.997); AUC0–∞: 0.945 (0.915, 0.976); AUClast: 0.944 (0.912, 0.977)]. Geometric least squares mean ratios (90% CIs) for LDX (inactive prodrug) were below the accepted range when the contents of a 70-mg LDX capsule were mixed with orange juice [Cmax: 0.641 (0.582, 0.707); AUC0–∞: 0.716 (0.647, 0.792); AUClast: 0.708 (0.655, 0.766)]; the lower 90% CI for Cmax [0.828 (0.752, 0.912)] was below the accepted range when the contents of a 70-mg LDX capsule were mixed with yogurt.
Conclusions:
Relative bioavailability of d-amphetamine (the active moiety) did not differ across administrations, which suggests that emptying an LDX capsule into orange juice or yogurt and consuming it is an alternative to intact capsules.
INTRODUCTION
Lisdexamfetamine dimesylate (LDX), a prodrug of d-amphetamine,1 is approved in the United States and other countries for use in patients aged 6 years and older with attention-deficit/hyperactivity disorder (ADHD) and only in the United States for adults with moderate to severe binge eating disorder.2 The pharmacokinetic profiles of orally administered LDX and its active metabolite, d-amphetamine, have been examined in several studies.3–7 In 2 studies, after administration of a single 70-mg LDX dose to healthy adults under fasting conditions, which were initiated 10–11 hours before dose, d-amphetamine mean maximum plasma concentration (Cmax) ranged from approximately 70 to 80 ng/mL, area under the plasma concentration versus time curve from zero to infinity (AUC0–∞) ranged from 1110 to 1342 ng·h−1·mL−1, mean time to maximum concentration (tmax) ranged from 3 to 4 hours, and terminal half-life (t1/2) ranged from 9.7 to 10.4 hours.3,5 In another study, mean Cmax and AUC0–∞ for d-amphetamine in healthy adults under fasting conditions, which were initiated 10 hours before dose, increased dose-proportionally as the LDX dosage increased from 50 to 250 mg, with mean values ranging from approximately 45 ng/mL and 818 ng·h−1·mL−1, respectively, after 50-mg LDX and to 246 ng/mL and 5133 ng·h−1·mL−1 after 250-mg LDX.4 In a fourth study, after 7 days of 70-mg LDX in adults, the mean steady-state Cmax and AUC0–∞ levels for d-amphetamine were 90 ng/mL and 1110 ng·h−1·mL−1, respectively, with an average observed d-amphetamine concentration of approximately 46 ng/mL.6 Finally, in children with ADHD, under fasting conditions, which were initiated at least 8 hours before dose, mean Cmax and AUC0–∞ ranged from approximately 53 to 134 ng/mL and 845 to 2157 ng·h−1·mL−1, respectively, after single doses of 30, 50, or 70-mg LDX; mean tmax and t1/2 ranged from 3.41 to 3.58 hours and 8.61 to 8.90 hours, respectively.7
Parents of children with ADHD have reported that their children have difficulty or dislike taking their medication when it is administered as a tablet.8 Furthermore, it is reasonable to speculate that some adults with ADHD have difficulty taking their ADHD medication because a proportion of the adult population reports difficulty in swallowing medications.9 Therefore, having alternate methods to administer LDX could ease administration across individuals of any age group. LDX is currently approved to be administered as an intact capsule or the contents of the capsule may be consumed immediately after being emptied and completely dispersed in water, orange juice, or yogurt.2 The primary objective of this study was to compare the pharmacokinetic profiles of LDX and d-amphetamine between 2 modes of oral administration in healthy, fasted adults: when the contents of a 70-mg LDX capsule were mixed with a soft food (yogurt) or orange juice and when an intact 70-mg LDX capsule was swallowed whole. This study served as the basis for the recommendation that LDX could be mixed with orange juice or yogurt when administered to any age group. These vehicles were chosen because they are generally appealing to individuals across age groups. A secondary objective was to assess the safety and tolerability of a single dose of LDX from a capsule mixed with yogurt or orange juice compared with those of LDX from an intact capsule.
MATERIALS AND METHODS
Study Design and Treatment
This open-label, randomized, 3-period crossover study (ClinicalTrials.gov: NCT01890785) was conducted by Clinical Pharmacology of Miami, Inc. (Miami, FL), from July 15, 2013, to August 22, 2013. The study consisted of a screening phase that ended at least 28 days before the first treatment dose; 3 single-dose, 5-day treatment periods separated by a 7-day washout between treatments; and a follow-up period. Healthy men or women (18–55 years old) were admitted to the clinical research center 1 day before the initiation of each treatment period (day –1) and remained at the center through completion of all day 5 assessments for the treatment period.
The treatments consisted of (1) administration of LDX after the contents of a 70-mg capsule were mixed with 4 ounces of orange juice; (2) administration of LDX after the contents of a 70-mg capsule were mixed with 4 ounces of yogurt; or (3) administration of an intact 70-mg LDX capsule with 240 mL of room temperature water. At the beginning of the first treatment period, participants were randomly assigned to receive each of 3 treatments in 1 of 6 treatment sequences in a 1:1:1:1:1:1 ratio. Participants were assigned a 4-digit randomization number immediately before dosing on day 1 of the first treatment period after the eligibility had been determined. The randomization schedule was produced and held by the clinical research organization PRA Health Sciences (Raleigh, NC). For all treatments, participants were required to consume each treatment within a 3-minute period, to fast overnight (approximately 10 hours) before dosing and for another 4 hours after dosing, and to refrain from fluid intake for 4 hours before and 2 hours after dosing. Additionally, participants were not permitted to lie down for 4 hours after dosing. A follow-up phone call was made 7 ± 2 days after the final treatment to identify any ongoing or new adverse events (AEs) and concomitant medications taken since discharge.
The protocol was approved by the institutional review board (Aspire IRB, Santee, CA) before the study initiation, and the study was conducted in accordance with the International Conference on Harmonization and Good Clinical Practice and the Declaration of Helsinki. All participants provided written informed consent before participating in the study.
Participants
Men and nonpregnant, nonlactating women aged 18–55 years old who were healthy (ie, with no evidence of active or chronic disease after a detailed review of medical and surgical histories and a complete physical examination) were eligible to participate. Additionally, participants were required to have a body mass index between 18.5 and 30 kg/m2 at the screening visit and hemoglobin values ≥12 g/dL at screening and on day –1 of treatment period 1. Study participants also needed to be able to swallow a dose of the study drug according to the study conditions and to comply with study procedures.
Exclusion criteria included current or recurrent disease (eg, cardiovascular, renal, liver, gastrointestinal, malignancy, or other conditions) that could affect the pharmacokinetics or pharmacodynamics of the study drug or clinical and laboratory evaluations; current or relevant physical or psychiatric illness that may make the participant unable to comply with study requirements, or complete the study, or that presented undue risk to the participant; in the investigator's judgment, significant illness within 2 weeks of the first dose of the study drug or significant history of anxiety, tension, or agitation; and currently considered a suicide risk or demonstrating suicidal ideation or had a previous suicide attempt. Individuals with histories or the presence of structural abnormalities, syncope, cardiac conduction problems, exercise-related cardiac events, clinically relevant bradycardia, symptomatic cardiovascular disease, advanced arteriosclerosis, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, transient ischemic attack, stroke, and vulnerability to sympathomimetic effects of stimulants were excluded, as were those with a family history of sudden cardiac death or ventricular arrhythmia, a history of controlled or uncontrolled hypertension, or with resting supine systolic blood pressure (SBP) >139 mm Hg or diastolic blood pressure (DBP) >89 mm Hg. Individuals were also excluded if they had known or suspected intolerance or hypersensitivity to the study drug, to closely related compounds, or to orange juice or vanilla yogurt; if they had used another investigational product within 30 days before receiving the first dose of the study drug; or if they had an inability to follow a standardized diet and meal schedule, or inability to fast. Individuals could not be enrolled if they used any medication that was known to inhibit or induce the cytochrome P450 enzymes responsible for metabolism of the study drug within 14 days of the first dose of the study drug.
Study Endpoints
Pharmacokinetics
Based on the previous LDX pharmacokinetic assessments4 and the dose of LDX used in this study, blood samples for pharmacokinetic assessment were collected on treatment days 1 (30 minutes before dose and 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, and 12 hours after dose), 2 (24 and 36 hours after dose), 3 (48 hours after dose), 4 (72 hours after dose), and 5 (96 hours after dose) during each treatment period.
Pharmacokinetic endpoints for LDX and d-amphetamine included tmax, Cmax, AUC0–∞, t1/2, area under the curve from the time of dosing to the last measurable concentration (AUClast), first-order rate constant associated with the terminal (log-linear) portion of the curve ( ), total body clearance for extravascular administration divided by the fraction of the absorbed dose (CL/F), and the volume of distribution associated with the terminal slope after extravascular administration divided by the fraction of dose absorbed (Vz/F).
), total body clearance for extravascular administration divided by the fraction of the absorbed dose (CL/F), and the volume of distribution associated with the terminal slope after extravascular administration divided by the fraction of dose absorbed (Vz/F).
Bioanalytical Methodology
Plasma concentrations of LDX and d-amphetamine were measured using validated liquid chromatography–tandem mass spectrometry methods. For the analysis, 50 μL of internal standard was added to a 100-μL aliquot of plasma. Proteins were precipitated by the addition of 500 μL of a chilled acetonitrile/formic acid (100:5; volume:volume) solution. After vortex and centrifugation, the supernatant was removed, evaporated under nitrogen at 40°C, and reconstituted to a volume of 300 μL. A 10-μL sample was injected into the liquid chromatography–tandem mass spectrometry system and analyzed using an API 4000 mass spectrometer (AB SCIEX, Framingham, MA) coupled with a Shimadzu LC system (Shimadzu, Kyoto, Japan).
Plasma concentrations were calculated using an 8-point standard curve. The lower limit of quantification was 1 ng/mL for LDX and 2 ng/mL for d-amphetamine; calibration standards in human plasma ranged from 1 to 100 ng/mL for LDX and from 2 to 200 ng/mL for d-amphetamine (QPS, Newark, DE). Quality control samples for LDX (3, 20, and 80 ng/mL) and d-amphetamine (6, 40, and 160 ng/mL) were prepared in separate batches and stored at −20°C. In regard to assay performance, coefficients of variation and biases for LDX, respectively, were 7.0% and −5.0% at 3 ng/mL, 5.3% and −6.0% at 20 ng/mL, and 4.4 and −2.8 at 80 ng/mL. For d-amphetamine, coefficients of variation and biases, respectively, were 5.7% and −4.0% at 6 ng/mL, 2.4% and −5.3% at 40 ng/mL, and 2.1% and −2.5% at 160 ng/mL.
Safety and Tolerability
Safety assessments included recording of AEs, physical examinations, vital signs, 12-lead electrocardiograms (ECGs), and clinical laboratory evaluations. AEs were recorded at screening, on all days during each treatment period, and at follow-up. Clinical laboratory evaluations and physical examinations were conducted at screening, day –1, and day 5 for each treatment period. Vital signs were assessed at screening, day –1, 30 minutes before dose and 0.5, 2, 4, 8, 12, 24, 48, 72, and 96 hours after dose during each treatment period. Twelve-lead ECGs were performed at screening, day –1, 30 minutes before dose, and 96 hours after dose of each treatment period.
Other Measurements
A 5-point Likert scale (1 = “bad” to 5 = “excellent”) was used to rate the overall taste of LDX in the orange juice or yogurt. Participants completed the questionnaire within 2 minutes of ingestion on day 1 of treatment.
Data Analysis
The sample size was estimated using nQuery 6.0 (Statistical Solutions, Boston, MA) for a 2 × 2 crossover design and converted to a 3 × 3 crossover, as previously described.10 To achieve 85% power (1-sided α = 0.05; true mean ratio = 1.05), 24 participants (4 per sequence) were required to complete the study based on the estimate of the true within-subject SD for d-amphetamine of 0.215.4
Pharmacokinetic analyses were based on the pharmacokinetic analysis set (all participants in the safety analysis set for whom primary pharmacokinetic data were sufficient and interpretable). All statistical analyses were performed using SAS (SAS Institute, Cary, NC) Version 9.1.3 or higher. Descriptive statistics were calculated for all pharmacokinetic parameters. Log-transformed pharmacokinetic parameters for LDX and d-amphetamine among the 3 treatments were compared using analysis of variance for a crossover design with fixed factors for sequence, treatment, and period and random factors for subject within sequence.
Relative bioavailability of the 2 test treatments (LDX in orange juice and in yogurt) compared with the reference treatment (intact LDX capsule) was determined for d-amphetamine (the active moiety) and LDX (the pharmacologically inactive prodrug) using bioequivalence-testing procedures. Point estimates and 90% confidence interval [CI] for the comparisons of Cmax, AUC0–∞, and AUClast (LDX in orange juice versus the intact LDX capsule; LDX in yogurt vs. the intact LDX capsule) were calculated. If the 90% CIs of the geometric mean ratios were within the interval of 0.8–1.25, equivalence was assumed. If the 90% CIs were not fully contained within the 0.8 to 1.25 interval, a difference between the test and reference treatments could not be excluded, and the mean ratio estimates and their CIs were assessed. Safety endpoints were summarized using descriptive statistics from the safety analysis set (participants taking ≥1 study drug dose and having ≥1 postdose safety assessment).
RESULTS
Participant Disposition and Demographics
Thirty participants were enrolled in the study; all participants were included in the randomized, pharmacokinetic, and safety analysis sets (see Figure, Supplemental Digital Content 1, http://links.lww.com/TDM/A166). One participant requested to be withdrawn the day after receiving LDX mixed with orange juice during treatment period 3; no AEs were reported for this participant. Participant demographics are summarized in Table 1.
TABLE 1.
Baseline Demographic Characteristics, Safety Analysis Set

Pharmacokinetics
Linear scale plots for mean ± SD plasma concentrations over time by treatment for LDX and d-amphetamine are shown in Figures 1A, B. Across treatments, LDX concentrations reached a maximum at 1 hour after dose and returned to predose levels after 4 hours. Mean peak LDX plasma concentrations were generally comparable across treatments but tended to be higher after administration of the intact capsule compared with LDX mixed with orange juice or yogurt. Plasma concentration versus time profiles for d-amphetamine were similar; mean peak plasma concentrations were observed at 4 hours after dose for all treatments and returned to pretreatment levels after 72 hours.
FIGURE 1.

Plasma concentration versus time plots for (A) LDX and (B) d-Amphetamine by treatment regimen, pharmacokinetic analysis set.
Descriptive statistics for all pharmacokinetic parameters are summarized in Table 2. Mean values for LDX Cmax and AUClast were the lowest after LDX was mixed with orange juice and the highest after administration of the intact LDX capsule. Mean values for LDX AUC0–∞ were lowest after LDX was mixed with orange juice and similar when LDX was mixed with yogurt or administered as an intact capsule. Mean t1/2 and tmax values for LDX were similar across all 3 treatments; the mean LDX CL/F value was higher after LDX was mixed with orange juice compared with being mixed with yogurt or administered as an intact capsule. For d-amphetamine, mean values for Cmax, AUClast, CL/F, t1/2, and tmax were similar across all of the treatments. The mean AUC0–∞ value for d-amphetamine was slightly lower when LDX was mixed with yogurt compared with being mixed with orange juice or administered as an intact capsule.
TABLE 2.
Pharmacokinetic Parameters (Mean ± SD) by Treatment, Pharmacokinetic Analysis Set

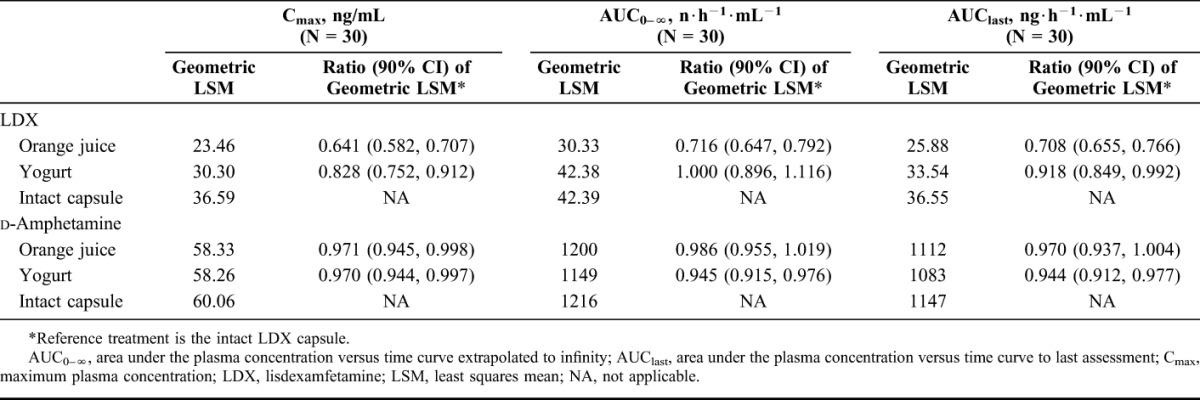
Geometric least squares mean (LSM) and the ratios of the geometric LSM for Cmax, AUC0–∞, and AUClast are summarized in Table 3. For LDX (the inactive prodrug), geometric LSM ratios and their respective 90% CIs for Cmax, AUC0–∞, and AUClast fell entirely below the lower limit of the prespecified range when mixed with orange juice compared with the intact LDX capsule. Geometric LSM ratios and the respective 90% CIs for LDX AUC0–∞ and AUClast fell within the prespecified range when mixed with yogurt compared with the intact LDX capsule; the lower 90% CI for LDX Cmax fell below the lower limit of the prespecified range. For d-amphetamine (the active moiety), the geometric LSM ratios and their 90% CIs for Cmax, AUC0–∞, and AUClast all fell entirely within the prespecified range when LDX was mixed with orange juice or yogurt compared with administration as an intact capsule.
TABLE 3.
Geometric Means for Cmax, AUC0–∞, and AUClast by Treatment, Pharmacokinetic Analysis Set
The observed total variability for d-amphetamine for all 3 treatment groups in the pharmacokinetic analysis set was 24.6% (95% CI, 19.5%–33.4%) for log-transformed AUC0–∞ and 17.3% (95% CI, 13.7%, 23.2%) for log-transformed Cmax. Intrasubject variability (95% CI) for log-transformed AUC0–∞ and Cmax was 7.6% (6.4%, 9.3%) and 6.3% (5.4%, 7.8%), respectively. Intersubject variability (95% CI) for log-transformed AUC0–∞ and Cmax was 23.4% (18.2%, 33.0%) and 16.1% (12.4%, 22.8%), respectively.
Safety and Tolerability
Across all treatment regimens, 73.3% of participants (22/30) reported a treatment-emergent AE (TEAE); the frequency of TEAEs by treatment is summarized in Table 4. Only 1 TEAE was not considered to be related to the study drug. There were no serious or severe TEAEs; no TEAEs led to study discontinuation. All TEAEs were categorized as mild in severity. TEAEs reported in ≥1 participant in any treatment regimen are summarized in Table 4. The TEAEs reported most frequently across all treatments were decreased appetite, dry mouth, and headache.
TABLE 4.
Summary of TEAEs,* Safety Analysis Set

There were no apparent differences between treatment groups for changes in pulse, DBP, or SBP (Table 5). Mean pulse increased to maximum levels at 12 hours after treatment and returned to pretreatment levels by 48 hours after dose. Mean SBP increased to maximum levels at 4 hours after treatment and returned to pretreatment levels by 48 hours after dose. Mean DBP increased to maximum levels at 2 hours after treatment and returned to pretreatment levels by 48 hours after dose. Mean changes from baseline in ECG heart rates and interval were small, with no apparent differences between treatments (data not shown).
TABLE 5.
Mean ± SD Change From Baseline in Pulse Rate, SBP, and DBP, Safety Analysis Set

Ratings of Taste
Most participants rated the taste of LDX when mixed with orange juice [good, 15/30 (50.0%); excellent, 9/30 (30.0%)] or yogurt [good, 10/30 (33.3%); excellent, 16/30 (53.3%)] as good or excellent.
DISCUSSION
The purpose of this study was to compare the relative bioavailability of LDX and d-amphetamine when LDX from a capsule is mixed with soft food or liquid, such as yogurt or orange juice, versus when LDX is administered as an intact capsule. The main findings demonstrate that the relative bioavailability of the active moiety, d-amphetamine, based on Cmax and AUC, is not different from that of an intact LDX capsule when LDX is mixed with orange juice or yogurt. The pharmacokinetic profile of d-amphetamine, including tmax and t1/2, observed when LDX was mixed with yogurt or orange juice in this study was comparable to previous reports in single-dose studies of healthy adults.3–5 Intrasubject variability for d-amphetamine was low and within one third to one half of the corresponding intersubject variability. These findings indicated that LDX was suitable for use in adults when swallowed as an intact capsule, when mixed with a soft food such as yogurt, or when mixed with a liquid such as orange juice. Although these data supported a subsequent label change for LDX in the United States,2 this is the first detailed description of these findings.
The ability to mix the contents of LDX capsules in soft food or liquids for the purpose of administration is consistent with reports for other ADHD medications.11–14 Metadate CD (extended-release methylphenidate HCl capsules; UCB, Inc., Smyrna, GA),12 Adderall XR (extended-release mixed amphetamine salts; Shire US Inc., Wayne, PA),13 and Focalin XR (extended-release dexmethylphenidate hydrochloride; Novartis Pharmaceuticals Corp., East Hanover, NJ)14 are approved to be swallowed whole or sprinkled on applesauce and consumed immediately. Using standard bioequivalence-testing procedures, studies of Adderall XR15 and Metadate CD16 reported comparable relative bioavailability of the active ingredients under fasting conditions in healthy adults when the contents of a capsule were sprinkled over a small amount of applesauce compared with the intact capsule swallowed whole.
In contrast to the findings for d-amphetamine, relative bioavailability of LDX when mixed with orange juice or yogurt was lower compared with that from an intact LDX capsule, with geometric LSM ratios and 90% CIs for Cmax, AUC0–∞, and AUClast falling below the prespecified bioequivalence range when LDX was mixed with orange juice and the lower CI for Cmax falling below the lower limit of the prespecified bioequivalence range when LDX was mixed with yogurt. The reason for this discrepancy between the bioavailabilities of d-amphetamine and LDX is not known. However, as d-amphetamine is pharmacologically active whereas LDX is not1 and the reduced bioavailability of LDX when mixed with orange juice or yogurt does not result in reduced d-amphetamine bioavailability, the observed changes in overall LDX concentrations may not have clinical significance.
The safety and tolerability profile of LDX did not substantially differ across treatments, and no unexpected TEAEs or vital sign changes were observed. There were no serious or severe TEAEs; all TEAEs were considered mild in severity. Among the most frequently reported TEAEs (decreased appetite, dry mouth, and headache), dry mouth and headache have been previously reported in healthy adults administered LDX3–5; decreased appetite, dry mouth, and headache have also been reported in clinical studies of LDX in children, adolescents, and adults with ADHD.17–19 The patterns of changes in pulse and blood pressure are also consistent with previously published findings in healthy adults.3,4
These data should be considered in light of several potential limitations. First, findings related to safety and tolerability from this phase 1 study should be interpreted with caution because the sample size was small and participants were predominantly male. Second, data related to the taste acceptability of LDX mixed with orange juice or yogurt were exploratory in nature. Third, because this is a single-dose study, it is not known whether differences in bioavailability would be observed if lower doses of LDX were administered or if LDX was mixed with different foods or liquids.
CONCLUSIONS
This was the first study to compare the relative bioavailability of LDX when the contents of a capsule were mixed with soft food or liquids with that of an intact LDX capsule when swallowed whole in healthy adults. The relative bioavailability of d-amphetamine did not differ when LDX was mixed with orange juice or yogurt compared with LDX being taken as an intact capsule. These results support the administration of LDX in orange juice or soft foods such as yogurt as an appropriate alternative to swallowing intact capsules. Most of the participants rated the taste of LDX in orange juice or yogurt as acceptable, good, or excellent. This suggests that the administration of LDX in these vehicles may not negatively affect LDX use in individuals who require an alternative mode of administration to swallowing an intact LDX capsule.
ACKNOWLEDGMENTS
Under the direction of the authors, writing assistance was provided by Stefan Kolata, PhD (a former employee of CHC), and Craig Slawecki, PhD (a current employee of CHC). Editorial assistance in the form of proofreading, copyediting, and fact checking was also provided by CHC. Shailesh Desai, PhD, from Shire, reviewed and edited the manuscript for scientific accuracy.
Footnotes
Supported by the sponsor, Shire Development LLC (Lexington, MA). Shire Development LLC provided funding to Complete Healthcare Communications, LLC (Chadds Ford, PA), for support in writing and editing this manuscript.
J. Ermer is a former employee of Shire and holds stock and/or stock options in Shire. M. Corcoran and P. T. Martin are employees of Shire and hold stock and/or stock options in Shire. K. Lasseter conducted the study with support from Shire.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.drug-monitoring.com).
REFERENCES
- 1.Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vyvanse. Lisdexamfetamine Dimesylate. Wayne, PA: Shire US Inc; 2015. Available at: pi.shirecontent.com/PI/PDFs/Vyvanse_USA_ENG.pdf. Accessed October 10, 2016. [Google Scholar]
- 3.Krishnan S, Zhang Y. Relative bioavailability of lisdexamfetamine 70-mg capsules in fasted and fed healthy adult volunteers and in solution: a single-dose, crossover pharmacokinetic study. J Clin Pharmacol. 2008;48:293–302. [DOI] [PubMed] [Google Scholar]
- 4.Ermer J, Homolka R, Martin P, et al. Lisdexamfetamine dimesylate: linear dose-proportionality, low intersubject and intrasubject variability, and safety in an open-label single-dose pharmacokinetic study in healthy adult volunteers. J Clin Pharmacol. 2010;50:1001–1010. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan SM, Pennick M, Stark JG. Metabolism, distribution and elimination of lisdexamfetamine dimesylate: open-label, single-centre, phase I study in healthy adult volunteers. Clin Drug Investig. 2008;28:745–755. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan SM, Stark JG. Multiple daily-dose pharmacokinetics of lisdexamfetamine dimesylate in healthy adult volunteers. Curr Med Res Opin. 2008;24:33–40. [DOI] [PubMed] [Google Scholar]
- 7.Boellner SW, Stark JG, Krishnan S, et al. Pharmacokinetics of lisdexamfetamine dimesylate and its active metabolite, d-amphetamine, with increasing oral doses of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder: a single-dose, randomized, open-label, crossover study. Clin Ther. 2010;32:252–264. [DOI] [PubMed] [Google Scholar]
- 8.Charach A, Skyba A, Cook L, et al. Using stimulant medication for children with ADHD: what do parents say? A brief report. J Can Acad Child Adolesc Psychiatry. 2006;15:75–83. [PMC free article] [PubMed] [Google Scholar]
- 9.Fields J, Go JT, Schulze KS. Pill properties that cause dysphagia and treatment failure. Curr Ther Res Clin Exp. 2015;77:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu RP, Zheng H. Sample size calculation for bioequivalence studies with high-order crossover designs. Control Clin Trials. 2003;24:436–439. [DOI] [PubMed] [Google Scholar]
- 11.Fischer R, Schutz H, Grossmann M, et al. Bioequivalence of a methylphenidate hydrochloride extended-release preparation: comparison of an intact capsule and an opened capsule sprinkled on applesauce. Int J Clin Pharmacol Ther. 2006;44:135–141. [DOI] [PubMed] [Google Scholar]
- 12.Metadate CD. Methylphenidate HCL. Smyrna, GA: UCB, Inc; 2015. Available at: www.ucb.com/_up/ucb_com_products/documents/Metadate_CD_COL_02_2015.pdf. Accessed October 10, 2016. [Google Scholar]
- 13.Adderall XR. Mixed Salts of a Single-entity Amphetamine Product. Wayne, PA: Shire US Inc; 2015. Available at: pi.shirecontent.com/PI/PDFs/AdderallXR_USA_ENG.PDF. Accessed October 10, 2016. [Google Scholar]
- 14.Focalin XR. Dexmethylphenidate Hydrochloride. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015. Available at: pharma.us.novartis.com/product/pi/pdf/focalinXR.pdf. Accessed October 10, 2016. [Google Scholar]
- 15.Tulloch SJ, Zhang Y, McLean A, et al. SLI381(Adderall XR), a two-component, extended-release formulation of mixed amphetamine salts: bioavailability of three test formulations and comparison of fasted, fed, and sprinkled administration. Pharmacotherapy. 2002;22:1405–1415. [DOI] [PubMed] [Google Scholar]
- 16.Pentikis HS, Simmons RD, Benedict MF, et al. Methylphenidate bioavailability in adults when an extended-release multiparticulate formulation is administered sprinkled on food or as an intact capsule. J Am Acad Child Adolesc Psychiatry. 2002;41:443–449. [DOI] [PubMed] [Google Scholar]
- 17.Adler LA, Goodman DW, Kollins SH, et al. Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69:1364–1373. [DOI] [PubMed] [Google Scholar]
- 18.Findling RL, Childress AC, Cutler AJ, et al. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:395–405. [DOI] [PubMed] [Google Scholar]
- 19.Coghill D, Banaschewski T, Lecendreux M, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23:1208–1218. [DOI] [PubMed] [Google Scholar]


