Abstract
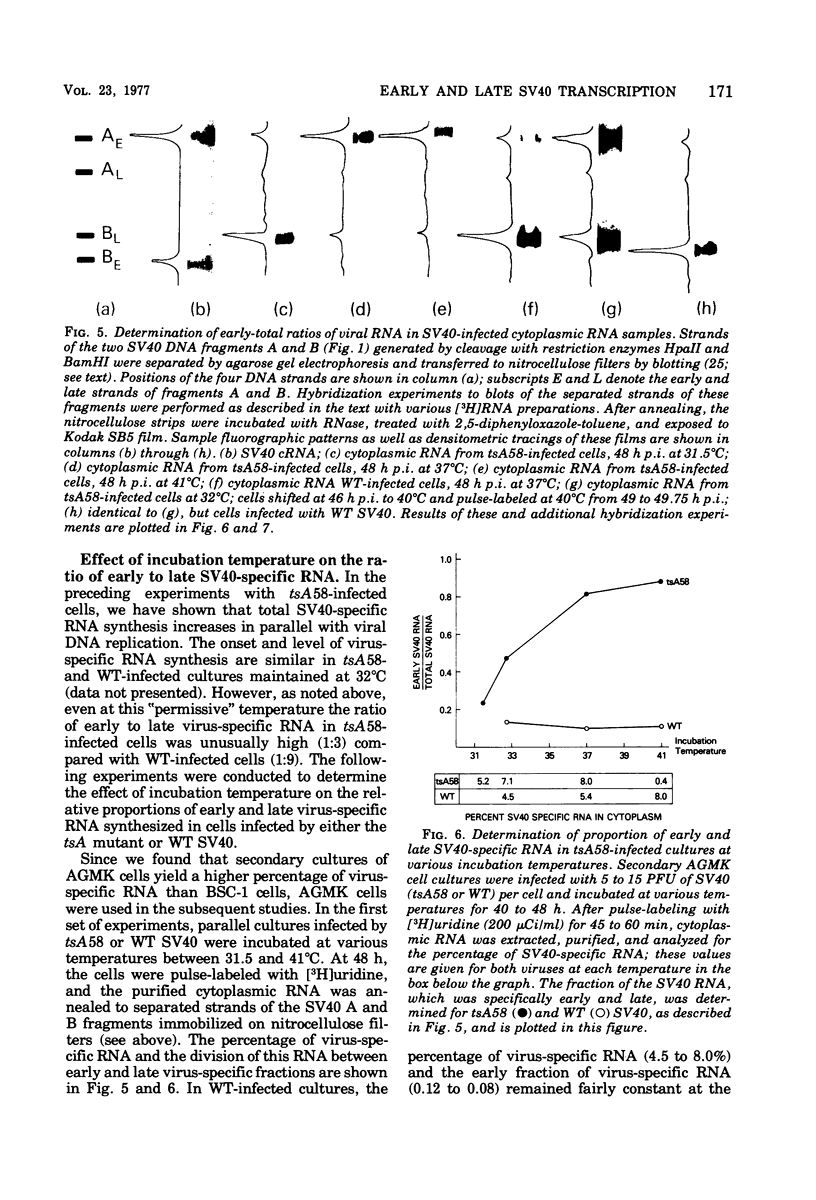
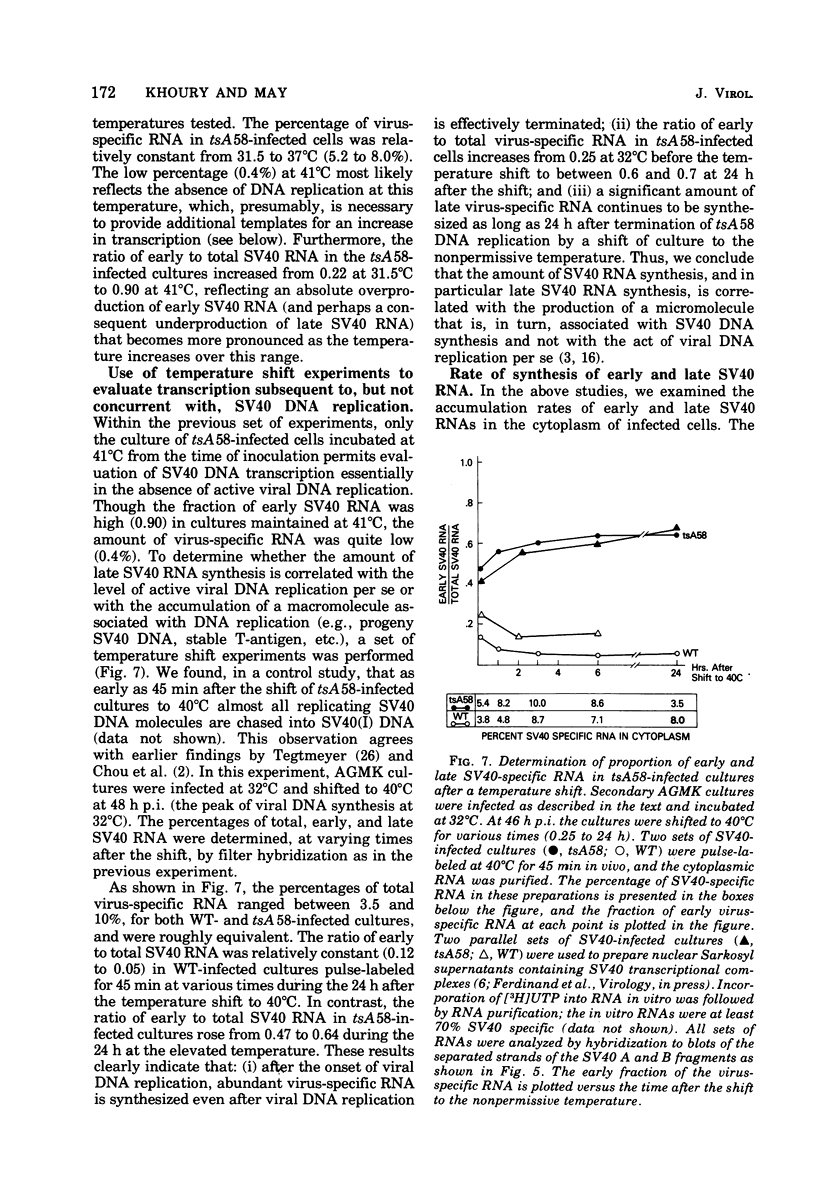
Virus-specific RNA synthesized in monkey cells after infection by both wild-type simian virus 40 (SV40) and the early SV40 temperature-sensitive mutant tsA58 has been analyzed. The fraction of SV40-specific RNA increased throughout infection with either wild-type SV40 or with tsA58 in direct proportion to the accumulation of progeny DNA molecules, suggesting their role in the late transcriptional process. Cytoplasmic fractions from cells infected at various temperatures (31.5 to 41°C) by wild-type virus and harvested 48 h later contained 4 to 8% virus-specific RNA, of which 5 to 10% was early SV40 RNA. In contrast, though 5 to 8% of the cytoplasmic RNA from tsA 58-infected cells incubated at 31.5 to 37°C for 48 h was virus specific, the percentage of early virus-specific RNA ranged from 25 to 80% as the incubation temperature increased. In tsA58-infected cultures incubated for 48 h at 41°C (a temperature at which essentially no tsA 58 DNA synthesis occurred), only 0.4% of the cytoplasmic RNA was virus specific, but at least 90% of this RNA was early. In experiments where cells were inoculated at 32°C and shifted at 48 h postinfection to 40°C for various times, the percentage of virus-specific pulse-labeled RNA varied from 3.5 to 10.0%. Of the virus-specific RNA, early SV40 RNA ranged from 14 to 65% in tsA 58-infected cultures. Analogous studies with Sarkosyl-extracted viral transcription complexes to incorporate label into nascent (unprocessed) viral RNA yielded essentially identical results. This finding strongly suggests that the overproduction of early SV40 RNA occurs at the level of synthesis. While cytosine arabinoside effectively terminated most viral DNA replication in wild-type-infected cells, the ratio of early to late viral RNA remained less than 1:9. These results demonstrate that: (1) the amount of virus-specific RNA synthesized depends directly on the amount of viral DNA available for use as templates; once viral DNA replication has occurred, presumably providing progeny SV40 DNA molecules for templates, the level of transcription remains high; (ii) termination of viral DNA replication does not terminate late SV40 transcription; (iii) early SV40 RNA is overproduced by tsA 58 at all temperatures, but especially at higher temperatures; and (iv) overproduction of early SV40 RNA appears to be correlated with defectiveness of the tsA mutant T-antigen. These results suggest that T-antigen may regulate its own production either by repressing the synthesis of early viral RNA or by stimulating the synthesis of late SV40 RNA or both.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter T. H., Ginsberg H. S. Viral transcription in KB cells infected by temperature-sensitive "early" mutants of adenovirus type 5. J Virol. 1976 Apr;18(1):156–166. doi: 10.1128/jvi.18.1.156-166.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Avila J., Martin R. G. Viral DNA synthesis in cells infected by temperature-sensitive mutants of simian virus 40. J Virol. 1974 Jul;14(1):116–124. doi: 10.1128/jvi.14.1.116-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan K., Tegtmeyer P., Anthony D. D. Relationship of replication and transcription of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1927–1930. doi: 10.1073/pnas.70.7.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Mousset S. Isolation and partial characterization of a nuclear RNA polymerase - SV40 DNA complex. FEBS Lett. 1975 Aug 1;56(1):149–155. doi: 10.1016/0014-5793(75)80130-x. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Aviv H. Preferential synthesis of viral late RNA by nuclei isolated from SV40 lytically infected cells. Cell. 1976 Apr;7(4):567–573. doi: 10.1016/0092-8674(76)90207-5. [DOI] [PubMed] [Google Scholar]
- Hayward G. S. Gel electrophoretic separation of the complementary strands of bacteriophage DNA. Virology. 1972 Jul;49(1):342–344. doi: 10.1016/s0042-6822(72)80042-4. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Khoury G., Howley P., Nathans D., Martin M. Posttranscriptional selection of simian virus 40-specific RNA. J Virol. 1975 Feb;15(2):433–437. doi: 10.1128/jvi.15.2.433-437.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Martin M. A., Lee T. N., Danna K. J., Nathans D. A map of simian virus 40 transcription sites expressed in productively infected cells. J Mol Biol. 1973 Aug 5;78(2):377–389. doi: 10.1016/0022-2836(73)90123-x. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Mapping temperature-sensitive mutants of simian virus 40: rescue of mutants by fragments of viral DNA. Virology. 1974 Aug;60(2):466–475. doi: 10.1016/0042-6822(74)90340-7. [DOI] [PubMed] [Google Scholar]
- Laub O., Aloni Y. Transcription of simian virus 40. V. Regulattion of simian virus 40 gene expression. J Virol. 1975 Nov;16(5):1171–1183. doi: 10.1128/jvi.16.5.1171-1183.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manteuil S., Girard M. Inhibitors of DNA synthesis: their influence on replication and transcription of simian virus 40 DNA. Virology. 1974 Aug;60(2):438–454. doi: 10.1016/0042-6822(74)90338-9. [DOI] [PubMed] [Google Scholar]
- May E., Kopecka H., May P. Mapping the transcription site of the SV40-specific late 16 S mRNA. Nucleic Acids Res. 1975 Oct;2(10):1995–2005. doi: 10.1093/nar/2.10.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B. J., Kleid D. G., Ptashne M. Lambda repressor turns off transcription of its own gene. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4785–4789. doi: 10.1073/pnas.72.12.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Ferguson J., Davis R. W., Stark G. R. T antigen binds to simian virus 40 DNA at the origin of replication. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1605–1609. doi: 10.1073/pnas.72.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L. J. Isolation and characterization of poly(A)-containing polyoma "early" and "late" messenger RNAs. Nucleic Acids Res. 1976 Mar;3(3):661–676. doi: 10.1093/nar/3.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Sharp P. A., Keller W. Transcription of Simian virus 40. I. Separation of the strands of SV40 DNA and hybridization of the separated strands to RNA extracted from lytically infected and transformed cells. J Mol Biol. 1972 Sep 14;70(1):57–71. doi: 10.1016/0022-2836(72)90163-5. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]