Abstract
OBJECTIVES
The mechanisms that trigger flare in rheumatoid arthritis (RA) are unknown. In murine arthritis models, dysfunctional lymph node (LN) drainage is associated with joint flare. To examine if LN alterations are associated with RA flare, we analyzed the change in LN volume via contrast-enhanced magnetic resonance imaging (CE-MRI) in patients with active joint flare at baseline and 16 weeks after certolizumab pegol (CZP) therapy. We also assessed the changes in popliteal or epitrochlear LN volumes versus the Rheumatoid and Arthritis Outcome Score (RAOS) (knee), or the Michigan Hand Questionnaire (MHQ; wrist/hand), and Disease Activity Score 28 (DAS28), at baseline and 16 weeks.
RESULTS
Total LN volume in 7 of 10 patients with measurable LN on CE-MRI significantly decreased 16 weeks after CZP therapy (mean decrease 37%; P = 0.0019). Improvement in knee pain measured by the RAOS (P = 0.03) inversely correlated with a decrease in total popliteal LN volume (R2 = 0.94). All patients demonstrated significant improvement in DAS28 (mean decrease 1.48; P = 0.0002). For flare in the hand, significant improvement in activities of daily living (ADL) as measured by the MHQ was observed (left hand mean improvement 20%; P = 0.02; right hand mean improvement 37%; P = 0.03).
CONCLUSION
RA patients with the smallest change in LN volume during anti-tumor necrosis factor (anti-TNF) therapy experienced the greatest pain relief in symptomatic knee joints. Moreover, the remarkably linear inverse correlation between LN volume and joint pain observed in this small clinical pilot provides initial evidence to support the concept that dynamic changes in draining LN volume are a biomarker of clinical response to therapy in RA.
Keywords: rheumatoid arthritis, lymph node, tumor necrosis factor inhibitor
Background
Rheumatoid arthritis (RA) is a chronic inflammatory joint disorder that often leads to impaired function and decreased quality of life (QOL).1,2 Since the release of anti-tumor necrosis factor (anti-TNF) therapy, RA patient outcomes have improved considerably, although up to 40% of patients fail to meet the primary endpoints in clinical trials.3–8 Furthermore, even primary responders to biologic agents often experience joint flares over the course of their disease, characterized by pain, swelling, and limited range of motion. To address gaps in our understanding of rheumatoid flare, current studies are focused on elucidating the underlying mechanisms, identifying prognostic biomarkers, and defining novel drug targets for therapeutic intervention.
Our research on arthritic flare utilized longitudinal contrast-enhanced magnetic resonance imaging (CE-MRI) in murine models of RA,9–13 including the TNF-transgenic (TNF-Tg) mouse.9,14 The results from these studies defined distinct and quantitative phenotypes of expanded and collapsed lymph node (LN) draining diseased joints as valid biomarkers of arthritic progression.15–17 Prior to symptomatic disease, draining LNs undergo volume expansion due to increased lymphangiogenesis, lymph egressing from the affected joint, and accumulation of inflammatory cells including a prominent subset of CD23(+)/CD21(hi) B-cells in inflamed nodes (Bin) (expanded phenotype),11,15 which was recently confirmed in RA LN.18 Acute arthritic flare commences when the inflammatory efflux from the diseased joint overwhelms the lymphatic drainage capacity. Treatment with an anti-TNF agent is effective in this model because it reduces inflammation while maintaining lymphatic flow to expanding LNs.10 In contrast, persistent joint inflammation observed in mice with sustained arthritic flare is associated with the collapse of draining LNs (collapsed phenotype) and the interruption of lymphatic flow.12,19 LN phenotypes predictive of flare were validated with prophylactic and therapeutic drug studies in mice.11,12 While early clinical studies that focus on the draining LN as a biomarker of RA disease and response to anti-TNF therapy are under way,20,21 the presence of expanded and collapsed LNs in RA and their potential association with therapeutic response to anti-TNF therapy are not known.
Another major challenge in this field is the absence of objective criteria to define and quantify flare in specific diseased joints in RA patients. To better define flare, the OMERACT Rheumatoid Arthritis Flare Group identified a core domain set that includes pain and function in order to measure RA flare. Furthermore, disease activity indices including the disease activity scale 28 (DAS28) to measure overall activity and the Rheumatoid and Arthritis Outcome Score (RAOS)22 and Michigan Hand Questionnaire (MHQ)23,24 to measure acute knee and wrist synovitis, respectively, are validated tools to determine the impact of rheumatic flare on patient outcomes.25
The preclinical studies outlined above suggest an important interplay between draining LN phenotype and the response to therapy in the flaring joint proximal to the LN. Given that joint-specific outcome measures of RA activity (RAOS and MHQ) are now available, we performed the first pilot study to evaluate the relationship between draining LN volume and pain in patients receiving anti-TNF [certolizumab pegol (CZP)] therapy for RA flare. Furthermore, we examined if the LN biomarker phenotypes identified in the murine studies were also present in human RA by using the same longitudinal CE-MRI approach. Specifically, in RA patients with joint flare, we analyzed change in LN volume pre- and posttreatment with CZP in nodes draining joints with active synovitis.
Methods
Patients
Ten patients with active flare of a single wrist or knee were enrolled. Patients with a diagnosis of RA who presented with new-onset active asymmetric synovitis of the knee or hand were evaluated in the rheumatology clinic at the University of Rochester Medical Center and consecutively recruited to the study. Patients fulfilled the American College of Rheumatology (ACR) 1987 classification criteria for RA26 and were recruited from January 2011 to August 2013. Standard blood tests [including C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)] and a clinical examination were performed at baseline. Disease activity scores were measured in an unblinded fashion. DAS28, RAOS, and MHQ were completed before and after therapy. Patients with lower extremity flare completed the RAOS and patients with upper extremity flare completed the MHQ. Patients were excluded if they had a contraindication to anti-TNF therapy, a glomerular filtration rate <30 mL/min/1.73 m2, or a medical condition or device that precluded performance of study-related procedures. Patients on current anti-TNF therapy were excluded; prior anti-TNF therapy was acceptable if patients had been off the agent for six months. Patients on concomitant disease-modifying antirheumatic drugs (DMARDs) or steroids were included. The study design was approved by the Ethics Committee of the University of Rochester, and subjects’ written informed consent was obtained in accordance with the Declaration of Helsinki.
MRI studies
MRI scans were performed in a 3 T Siemens Trio (Siemens Medical Solutions) at baseline and after 16 weeks of CZP therapy, administered subcutaneously with a loading dose of 400 mg followed by 400 mg monthly. The affected joint was imaged in a dedicated extremity coil on a GE Signa scanner. Standard positioning was adhered to. Multiplane (axial, coronal, and sagittal) images were acquired at 3 T field strength [matrix 320 × 320, field of view (FOV) 14–16 cm] utilizing T1-weighted (2 mm slice thickness), proton density-weighted (2 mm slice thickness), T2-weighted fat-suppressed (3 mm slice thickness), and T1-weighted fast spoiled gradient echo (FSPGR; 0.5 mm slice thickness) sequences before gadolinium. Following the administration of 0.1 mmol/kg of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) delivered manually into a cubital/antecubital vein through a cannula that was inserted before the examination, imaging was again performed, 10 minutes after injection, using 3D T1 FSPGR. Total imaging time was approximately 45 minutes.
CE-MRI analyses
CE-MRI was used to measure synovial and LN contrast enhancement, and Amira (TGS Unit; Mercury Computer Systems) to quantify LN volume as described in Figure 1. In brief, the 3D stack of precontrast scans was aligned with postcontrast scans via automatic registration. Then, a stack of images was generated by subtracting the precontrast scans from the postcontrast scans using the Arithmetic module. The synovial and LN volumes were segmented by manually drawing region of interests (ROIs) on the 3D stack. Contrast enhancement is defined as the synovial or LN signal intensity divided by the mean muscle signal intensity. Two musculoskeletal radiologists (GD and VK) blinded to pre- and posttherapy scans independently determined LN volumes for all nodes entirely captured in the scans. LN measurements were analyzed off axial T1 post gadolinium images, and short axis, long axis, and height were determined for each node. LN volume for each node was calculated and average LN volume was determined by adding all volumes and dividing by the number of nodes (range: 1–7 nodes). Repeated scans and 3D segmentation analyses revealed that LN volume calculations are reliable, and thus were used as a primary outcome measure in this study. Conversely, murine studies demonstrated that 3D rendering of the contrast enhancement of the synovium also includes the synovial fluid space and adjacent soft tissues and that quantification of these volumes is highly susceptible to change from joint positioning in longitudinal CE-MRI. Thus, for this study, the 3D synovial rendering serves as a descriptive biomarker to aid in landmark identification of the LN.
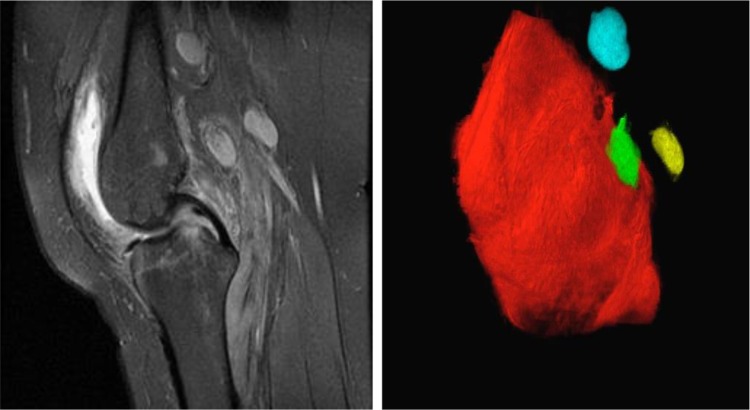
Figure 1.
CE-MRI and 3D volume rendering of asymmetric rheumatoid arthritis knee flare. A representative 2D image of a CE-MRI of the knee (left) is shown to illustrate the primary data used for 3D volume rendering (right) of the synovium (red) and popliteal lymph nodes (blue, green and yellow), which were obtained from a RA patient with asymmetric knee flare prior to CZP therapy.
Statistical analysis
For LN volume measurements, we analyzed interreader reliability by calculating the intraclass correlation coefficient (ICC) for absolute agreement. We applied paired t-tests after confirming normal distribution of data using Kolmogorov-Smirnov (KS) normality test and Wilcoxon signed-rank test for nonnormalized distribution. Correlations between measurements were estimated using Pearson’s correlation coefficient. P-values less than 0.05 were considered significant and P-values less than 0.002 were considered highly significant.
Results
Demographic information of RA patients
Table 1 shows the demographics of the 10 RA patients who completed the 16-week study. Ages ranged from 21 to 71 years, and the majority of patients were female (80%), as expected in a population with RA. None of the patients were previously treated with the long-acting anti-TNF agent CZP; however, 4 of 10 patients had previously received other anti-TNF agents. All patients were on maintenance medications prior to enrollment. Using Amira computer software, a 3D volume rendering of the affected synovium and LNs was obtained (Fig. 1, Supplementary Movie 1). This type of image allows for a more complete visualization of destructive changes to the synovium and localizes LNs not easily detectable on standard MRI imaging.
Table 1.
Characteristics of the study patients.
| PATIENT | AGE (YO) | GENDER | DISEASE DURATION (YR) | MEDICATIONS | COMORBIDITIES | Δ CRP | % Δ DAS28 |
|---|---|---|---|---|---|---|---|
| 1 | 61 | F | 0.5 | Meloxicam, MTX, prednisone, tramadol | Osteoarthritis | +1.12 | −16 |
| 2 | 42 | M | 8 | Hydroxychloroquine, MTX, prednisone, sulfasalazine, tramadol | – | −2.27 | −30 |
| 3 | 64 | F | 7 | Etanercept*, hydroxychloroquine, MTX, prednisone | – | +6.00 | −37 |
| 4 | 28 | F | 1 | MTX | Depression | −8.00 | −38 |
| 5 | 39 | F | 6 | MTX, prednisone | – | −23.00 | −60 |
| 6 | 21 | F | 0.5 | Meloxicam, MTX, prednisone, tramadol | Osteoarthritis learning disability | +10.00 | −17 |
| 7 | 46 | F | 19 | Etanercept*, MTX, naproxen, prednisone, tramadol | – | −25.00 | −18 |
| 8 | 71 | F | 17 | Adalimumab*, etanercept*, hydroxychloroquine, MTX, prednisone | Hyperlipidemia scoliosis congenital spondylolisthesis obstructive sleep apnea chronic peripheral venous insufficiency osteopenia osteoarthritis peripheral neuropathy | −1.00 | −51 |
| 9 | 46 | F | 19 | Etanercept*, MTX, naproxen, prednisone, tramadol | – | −25.00 | −18 |
| 10 | 55 | M | 17 | Prednisone | Hyperlipidemia | +3.00 | −15 |
Note:
Prior use.
Abbreviations: CRP, C-reactive protein; DAS28, Disease Activity Score 28; MTX, methotrexate.
RAOS Pain subscale score correlates with LN volume
All 10 patients treated for 16 weeks with CZP demonstrated significant improvement in DAS28 scores compared to baseline (Supplementary Fig. 1, P = 0.0002). Six of 10 patients experienced a knee flare, and therefore completed the RAOS. Of the subscales measured in this instrument (pain, other symptoms, activities of daily living (ADL), sport and recreational activities, and QOL), only pain improved significantly after therapy (Fig. 2, P = 0.03). Additionally, a trend toward improvement was noted in ADL (P = 0.08) and QOL (P = 0.06). Four of 10 patients had wrist flare and completed the MHQ, which measures separate ADL and total score. Despite the relatively small number of patients, a significant improvement in ADL was observed for both hands (left hand mean improvement 20%; P = 0.02; right hand mean improvement 37%; P = 0.03).
Figure 2.
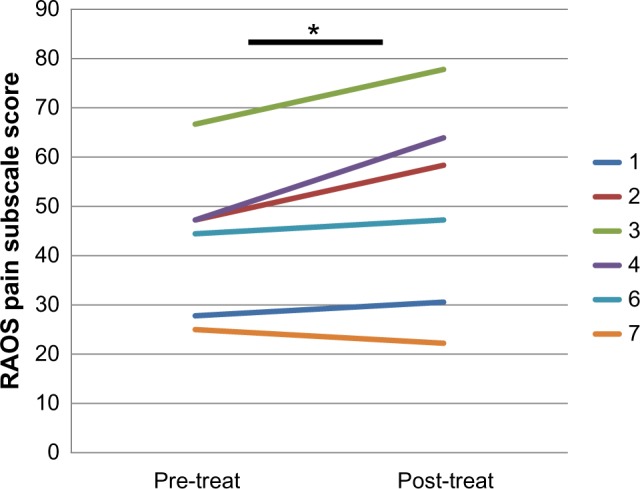
Improved Pain Scores with CZP Therapy. RAOS pain subscale scores (lower score = greater pain) were collected on the six patients with knee arthritis before and after CZP therapy, and the graphed results demonstrate improvement (increased RAOS Pain Subscale) in 5 out of 6 patients after treatment. The legend numbers reflect patient study ID numbers. The mean increase in RAOS Pain Subscale for the group is 13%; *P = 0.03.
Two radiologists measured LN volumes independently, with a very high interreader reliability (ICC = 0.99). Analysis was performed on pooled readings. Three of 10 patients (all with upper extremity involvement) did not have LN visualized on imaging. All 7 of 10 patients with evaluable LNs noted on MRI demonstrated a significant decline in LN volume over the 16-week treatment course (Fig. 3, P = 0.0019). Interestingly, the LN volume decline in flaring knee joints following treatment inversely correlated with the magnitude of pain score improvement in that joint (Fig. 4, P = 0.002). In contrast, regression analysis did not reveal a correlation (direct or inverse) between DAS28 scores and LN volume. Quantification of LN contrast enhancement could not be performed due to coexistent renal insufficiency or the inability to perform repeat CE studies.
Figure 3.
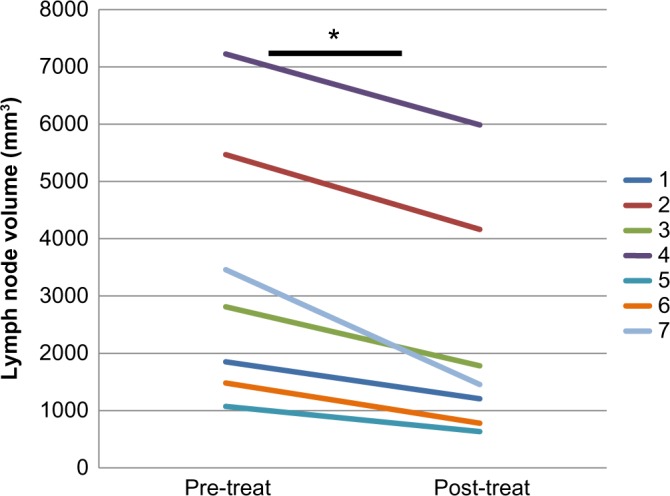
Decreased Draining Lymph Node Volume with CZP Therapy. Lymph node volumes were quantified from CE-MRI pre and post-CZP therapy as described in Figure 1, and the data from the seven patients with evaluable lymph nodes draining flaring RA joints is presented to illustrate the decrease in lymph node volume with CZP therapy. Of note is that three out of the ten patients enrolled did not have detectable LNs on CE-MRI imaging. The mean decrease in lymph node volume for the group is 36% *P = 0.0019.
Figure 4.
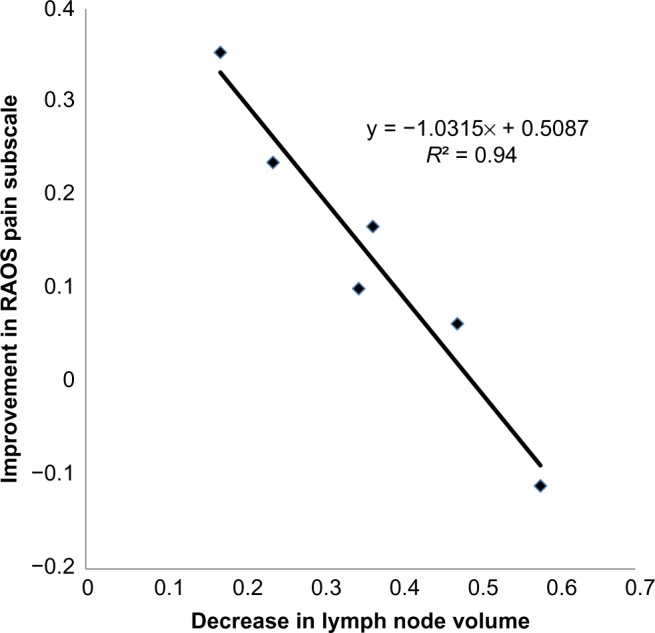
Change in popliteal lymph node volume during anti-TNF therapy inversely correlates with the degree of pain relief in the adjacent knee symptomatic from RA flare. A linear regression analysis was performed to assess the relationship between the change in RAOS Pain Subscale with CZP therapy (data in Fig. 2 plotted as (Pre – Post)/Pre), versus the change in lymph node volume with CZP therapy (data in Fig. 3 plotted as (Pre – Post)/Pre). The data are graphed with the slope and Pearson correlation coefficient.
Discussion
Although Chauffard and Ramon first described LN involvement in RA patients over a century ago,27,28 our understanding of the nature of this relationship remained limited due to the absence of quantitative outcome measures. To address this gap, we describe the first clinical pilot to examine the effect of anti-TNF therapy on LNs draining an actively inflamed, flaring RA joint. We noted that LN volumes, evaluated by MRI, declined in all patients with measurable nodes. Interestingly, this LN volume decline is consistent with our murine studies in which during the early phase of arthritic flare when LN volumes are expanded treatment with anti-TNF agents was associated with a decline in LN volume.10 We found that the decline in LN volume correlated with decreased joint inflammation in the mouse model and parallel events may be operative in human RA. It should be noted that other imaging techniques have recently garnered interest in evaluation and quantification of RA severity including 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography (FDG-PET/CT)29,30 and optical spectral transmission imaging.31 However, we used MR imaging due to the lack of exposure to radiation and because of its specificity in localizing soft tissue changes necessary in evaluating LN changes. It is important to note that the MHQ and RAOS are measures of single joint activity (hand and knee respectively) and even though the DAS28 score decreased in all subjects, it did not correlate highly with improvement in the flaring joint. Thus, inclusion of a local measure of single joint disease activity, such as the RAOS, proved to be critical for interpreting changes in LN volume as a potential response biomarker. Moreover, in the subset of patients with knee arthritis, improvement in knee pain inversely correlated with the extent of decline in LN volume. Specifically, the patients with the greatest pain relief showed the least change in LN volume. Taken together with our preclinical results, we interpret this initial observation to suggest that decrements in LN volume likely are associated with maintenance of LN function and lymph flow and a reduction of synovial inflammation, whereas LNs that undergo high volume loss signify a collapsed phenotype with impaired lymphatic flow. Admittedly, our murine studies show a clear delineation between expanding and collapsed LN phenotype, which we were unable to capture in this initial clinical pilot study.
Another interesting observation was that of the four patients with upper extremity arthritis, three did not have measurable LNs, preventing correlation with clinical parameters of disease activity. Notably, all three of these patients had long-standing disease of more than 10 years (mean disease duration 14.6 versus 6.0 years in the other seven patients). One conceivable explanation is that long-term chronic rheumatoid inflammation may lead to damage of lymphatic vessels and draining LNs from the active joint as was demonstrated in the TNF-Tg mouse model of RA.19 This effect would have greater impact on the upper extremities because of fewer draining nodes in the upper extremity compared to the lower extremity.32 Further studies will be necessary to determine at which time a draining LN becomes nonfunctional.
Our study has two limitations that are currently being addressed as future directions. The first is the small sample size, although we did include subjects with diverse disease characteristics including those with newly diagnosed RA and individuals with long-standing disease. The second limitation is our inability to directly measure lymphatic function due to technical challenges in quantifying LN contrast enhancement. To address these limitations and further elucidate the role of lymphatics in RA flare, we have initiated a study using state-of-the-art near infrared indocyanine green (NIR-ICG) imaging techniques in patients with early disease who flare compared to those with long-standing disease and chronic persistent synovitis. This imaging modality provides accurate images that demonstrate striking lymphatic dysfunction in chronic noninflammatory conditions such as lymphedema,33,34 and we anticipate that it will provide insights into RA lymphatic function.
Conclusions
The results from this pilot study provide preliminary human validation of our findings in murine RA models, which indicate that lymphatic dysfunction modulates joint inflammation. Additional studies will likely delineate the mechanisms underlying lymph flow from inflamed joints and unveil new therapeutic targets to prevent or attenuate joint flare in RA.
Supplementary Materials
Supplemental Figure 1. Improved disease activity score with CZP therapy. All ten patients enrolled in the study showed an improved disease activity score (DAS). Measurements were recorded at baseline (pre-treat) and after 16 weeks of therapy with CZP (post-treat). *P = 0.0002.
Supplementary Movie 1. A 3D volume rendering of affected synovium and LNs.
Acknowledgments
The authors would like to thank Kathleen Green for technical assistance with imaging. Written informed consent was obtained from the patients for publication of their individual details and accompanying images in this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ACR
American College of Rheumatology
- Bin
B-cells in inflamed nodes
- CE
contrast-enhanced
- CRP
C-reactive protein
- CZP
certolizumab pegol
- DAS28
disease activity score 28
- DMARD
disease-modifying antirheumatic drug
- ESR
erythrocyte sedimentation rate
- FSPGR
fast spoiled gradient echo
- ICC
intraclass correlation coefficient
- IL
interleukin
- LN
lymph node
- MRI
magnetic resonance imaging
- NIR-ICG
near infrared indocyanine green
- PLN
popliteal lymph node
- RA
rheumatoid arthritis
- ROI
region of interest
- TNF
tumor necrosis factor
Footnotes
ACADEMIC EDITOR: Chuanju Liu, Editor in Chief
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1714 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by a grant from UCB Pharmaceuticals. These studies were also supported by research grants from the National Institutes of Health PHS awards [T32 AR053459; R01s AR048697, AR053586, and AR056702; P01 AI078907; DP2OD006501; and P30 AR069655 for EMS]. The project described in this publication was supported by the University of Rochester CTSA award (UL1 TR000042) from the National Center for Advancing Translational Sciences of the National Institutes of Health. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: CTR discloses grants from Amgen, UCB, and Abbvie, and consulting fees from Abbvie, Novartis, Janssen, Pfizer, Sun, Sanofi, Regeneron and UCB, all outside the work presented here. Other authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: HR, ES, CTR, RWW. Analyzed the data: HR, EMB, GD, VK, RB, SM. Wrote the first draft of the manuscript: HR, ES, CTR. Contributed to the writing of the manuscript: EMB, GD, VK, RB, SM. Agree with manuscript results and conclusions: HR, ES, CTR, EMB, RWW, GD, VK, RB, SM. Jointly developed the structure and arguments for the paper: HR, ES, CTR, EMB, RWW, GD, VK, RB, SM. Made critical revisions and approved final version: HR, ES, CTR, EMB, RWW, GD, VK, RB, SM. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.da Silva JA, Phillips S, Buttgereit F. Impact of impaired morning function on the lives and well-being of patients with rheumatoid arthritis. Scand J Rheumatol Suppl. 2011;125:6–11. doi: 10.3109/03009742.2011.566434. [DOI] [PubMed] [Google Scholar]
- 2.Khan NA, Yazici Y, Calvo-Alen J, et al. Reevaluation of the role of duration of morning stiffness in the assessment of rheumatoid arthritis activity. J Rheumatol. 2009;36(11):2435–42. doi: 10.3899/jrheum.081175. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann R, Cutolo M, Genovese MC, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64(3):617–29. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 5.Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 6.Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med. 2000;343(22):1594–602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 7.Schiff M, Keiserman M, Codding C, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67(8):1096–103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–97. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 9.Proulx ST, Kwok E, You Z, et al. MRI and quantification of draining lymph node function in inflammatory arthritis. Ann N Y Acad Sci. 2007;1117:106–23. doi: 10.1196/annals.1402.016. [DOI] [PubMed] [Google Scholar]
- 10.Proulx ST, Kwok E, You Z, et al. Longitudinal assessment of synovial, lymph node, and bone volumes in inflammatory arthritis in mice by in vivo magnetic resonance imaging and microfocal computed tomography. Arthritis Rheum. 2007;56(12):4024–37. doi: 10.1002/art.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Kuzin I, Moshkani S, et al. Expanded CD23(+)/CD21(hi) B cells in inflamed lymph nodes are associated with the onset of inflammatory-erosive arthritis in TNF- transgenic mice and are targets of anti-CD20 therapy. J Immunol. 2010;184(11):6142–50. doi: 10.4049/jimmunol.0903489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Ju Y, Bouta EM, et al. Efficacy of B cell depletion therapy for murine joint arthritis flare is associated with increased lymphatic flow. Arthritis Rheum. 2012;65(1):130–8. doi: 10.1002/art.37709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Q, Guo R, Wood R, et al. Vascular endothelial growth factor C attenuates joint damage in chronic inflammatory arthritis by accelerating local lymphatic drainage in mice. Arthritis Rheum. 2011;63(8):2318–28. doi: 10.1002/art.30421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10(13):4025–31. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Zhou Q, Wood R, et al. CD23+/CD21hi B cell translocation and ipsilateral lymph node collapse is associated with asymmetric arthritic flare in TNF-Tg mice. Arthritis Res Ther. 2011;13(4):R138. doi: 10.1186/ar3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouta EM, Ju Y, Rahimi H, et al. Power Doppler ultrasound phenotyping of expanding versus collapsed popliteal lymph nodes in murine inflammatory arthritis. PLoS One. 2013;8(9):e73766. doi: 10.1371/journal.pone.0073766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouta EM, Li J, Ju Y, et al. The role of the lymphatic system in inflammatory-erosive arthritis. Semin Cell Dev Biol. 2015;38:90–7. doi: 10.1016/j.semcdb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuzin II, Kates SL, Ju Y, et al. Increased numbers of CD23 CD21 Bin-like B cells in human reactive and rheumatoid arthritis lymph nodes. Eur J Immunol. 2016;46(7):1752–57. doi: 10.1002/eji.201546266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouta EM, Wood RW, Brown EB, Rahimi H, Ritchlin CT, Schwarz EM. In vivo quantification of lymph viscosity and pressure in lymphatic vessels and draining lymph nodes of arthritic joints in mice. J Physiol. 2014;592(pt 6):1213–23. doi: 10.1113/jphysiol.2013.266700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manzo A, Caporali R, Vitolo B, et al. Subclinical remodelling of draining lymph node structure in early and established rheumatoid arthritis assessed by power Doppler ultrasonography. Rheumatology (Oxford) 2011;50(8):1395–400. doi: 10.1093/rheumatology/ker076. [DOI] [PubMed] [Google Scholar]
- 21.Benaglio F, Vitolo B, Scarabelli M, et al. The draining lymph node in rheumatoid arthritis: current concepts and research perspectives. Biomed Res Int. 2015;2015:420251. doi: 10.1155/2015/420251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bremander ABI, Petersson IF, Roos E. Validation of the rheumatoid arthritis outcome score (RAOS) – for the lower extremity. Ann Rheum Dis. 2003;62:364. doi: 10.1186/1477-7525-1-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durmus D, Uzuner B, Durmaz Y, Bilgici A, Kuru O. Michigan hand outcomes questionnaire in rheumatoid arthritis patients: relationship with disease activity, quality of life, and handgrip strength. J Back Musculoskelet Rehabil. 2013;26(4):467–73. doi: 10.3233/BMR-130408. [DOI] [PubMed] [Google Scholar]
- 24.Waljee JF, Chung KC, Kim HM, et al. Validity and responsiveness of the Michigan hand questionnaire in patients with rheumatoid arthritis: a multicenter, international study. Arthritis Care Res (Hoboken) 2010;62(11):1569–77. doi: 10.1002/acr.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fransen J, Hauselman H, Michel BA, Caravatti M, Stucki G. Responsiveness of the self-assessed rheumatoid arthritis disease activity index to a flare of disease activity. Arthritis Rheum. 2001;44(1):53–60. doi: 10.1002/1529-0131(200101)44:1<53::AID-ANR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 26.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 27.Young A, Koduri G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21(5):907–27. doi: 10.1016/j.berh.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Robertson MD, Hart FD, White WF, Nuki G, Boardman PL. Rheumatoid lymphadenopathy. Ann Rheum Dis. 1968;27(3):253–60. doi: 10.1136/ard.27.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suto T, Okamura K, Yonemoto Y, Okura C, Tsushima Y, Takagishi K. Prediction of large joint destruction in patients with rheumatoid arthritis using F-18-FDG PET/CT and disease activity score. Medicine (Baltimore) 2016;95(7):e2841. doi: 10.1097/MD.0000000000002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudhari AJ, Ferrero A, Godinez F, et al. High-resolution F-18-FDG PET/CT for assessing disease activity in rheumatoid and psoriatic arthritis: findings of a prospective pilot study. Br J Radiol. 2016;89(1063):20160138. doi: 10.1259/bjr.20160138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Onna M, Ten Cate DF, Tsoi KL, et al. Assessment of disease activity in patients with rheumatoid arthritis using optical spectral transmission measurements, a non-invasive imaging technique. Ann Rheum Dis. 2016;75(3):511–8. doi: 10.1136/annrheumdis-2015-207315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catalano O, Nunziata A, Saturnino PP, Siani A. Epitrochlear lymph nodes: anatomy, clinical aspects, and sonography features. Pictorial essay() J Ultrasound. 2010;13(4):168–74. doi: 10.1016/j.jus.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams KE, Rasmussen JC, Darne C, et al. Direct evidence of lymphatic function improvement after advanced pneumatic compression device treatment of lymphedema. Biomed Opt Exp. 2010;1(1):114–25. doi: 10.1364/BOE.1.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen JC, Tan IC, Marshall MV, Fife CE, Sevick-Muraca EM. Lymphatic imaging in humans with near-infrared fluorescence. Curr Opin Biotechnol. 2009;20(1):74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Improved disease activity score with CZP therapy. All ten patients enrolled in the study showed an improved disease activity score (DAS). Measurements were recorded at baseline (pre-treat) and after 16 weeks of therapy with CZP (post-treat). *P = 0.0002.
Supplementary Movie 1. A 3D volume rendering of affected synovium and LNs.