Abstract
Background
Activated Cdc42 kinase1 (ACK1) is a non-receptor tyrosine kinase which is critical for cell survival, proliferation, and migration. Genomic amplification of ACK1 has been reported in multiple human cancers. We aimed to investigate ACK1 protein expression in colorectal mucosa with inflammation and neoplasm, and to evaluate its correlation with disease activity and severity.
Material/Methods
A total of 250 individuals who underwent total colonoscopy were collected randomly from January 2007 to May 2013 in Nanfang Hospital, Guangzhou, China. Colorectal mucosal biopsy specimens were obtained by endoscopy from 78 patients with ulcerative colitis (UC), 22 with Crohn’s disease (CD), 20 with infectious colitis, 26 with non-IBD and noninfectious colitis, 16 with sporadic adenomas, 4 with dysplasia-associated lesions or masses, 10 with sporadic colorectal cancer (CRC), 4 with UC-related CRC, 10 with hyperplastic polyps, and 60 without colonic abnormalities. ACK1 protein levels were determined immunohistochemically. The correlations of ACK1 expression with disease activity and severity were also evaluated.
Results
Significantly increased ACK1 expression was observed in epithelial cells of colorectal mucosa with inflammation and dysplasia compared to controls (P<0.05). ACK1 expression correlated with clinical activity in IBD (χ2=4.57, P=0.033 for UC; χ2=5.68, P=0.017 for CD), as well as grade of dysplasia in preneoplastic lesions (P<0.05). No significant differences in ACK1 expression were found between UC and CD, or between IBD and non-IBD conditions (P>0.05).
Conclusions
ACK1 protein is increased extensively in colitis and colorectal dysplasia. ACK1 overexpression may play a role in colorectal inflammation and neoplasms.
MeSH Keywords: Activated Cdc42 kinase1, inflammatory bowel disease, colitis, colorectal neoplasms
Background
Activated Cdc42 kinase (ACK1, also known as ACK or TNK2) is a ubiquitously expressed non-receptor tyrosine kinase which was originally identified by its ability to bind CDC42 in active GTP-bound form [1]. Genomic amplification and overexpression of ACK1 have been shown in a variety of human tumors. In cell lines of epithelial origin, ACK1 integrates extracellular growth factor stimuli from multiple ligand-activated activated receptor tyrosine kinases, to initiate intracellular signaling cascades that are critical for cell survival, cell proliferation, cell differentiation, and cell migration [2–10].
Our study group recently demonstrated significantly increased ACK1 protein expression in the colorectal mucosa of ulcerative colitis (UC) compared to that in people without intestinal diseases, by using integrated strategy in proteomics [11]. UC is a clinical subtype of inflammatory bowel disease (IBD). The expression levels of ACK1 expression in other colorectal inflammatory conditions except UC have not been examined. The diagnosis of IBD is usually based on a combination of endoscopy, laboratory indices, and histological and clinical examination, but approximately 10% of IBD still cannot be discriminated if patients present with similar manifestations. Several markers have been suggested to facilitate the differentiation of IBD, with a low specificity and sensitivity, or weak correlation with disease activity [12,13]. Whether ACK1 protein is of help in differentiating between UC and other colorectal inflammatory diseases is unclear. UC patients with long disease duration have an increased risk of developing colorectal cancer (CRC). Several different molecular abnormalities have been found between UC-associated and sporadic CRC sequences [14–17]. Although copy numbers, as well as mRNA, of ACK1 gene have been found to be altered in colorectal tumors, the expression levels of ACK1 protein during different kinds and stages of CRC progression have not been characterized.
Therefore, the aims of our study were to investigate the expression of ACK1 at the protein level in colorectal mucosa from patients with different colorectal inflammatory, as well as neoplastic conditions, and to evaluate its correlation with disease activity or severity.
Material and Methods
Patient sample analysis
A total of 250 colorectal biopsy specimens were collected randomly from individuals who underwent total colonoscopy from January 2007 to May 2013 in Nanfang Hospital, Guangzhou, China. The colorectal specimens consisted of 78 UC, 22 CD, 20 infectious colitis (such as bacterial dysentery, pseudomembranous enteritis and viral enteritis), 26 non-IBD and noninfectious colitis(such as microscopic colitis, diversion colitis, diverticular colitis, eosinophilic colitis, ischemic colitis and radiation colitis) [18], 16 sporadic adenomas (SAs) (6 UC-associated and 10 non-UC-associated), 4 dysplasia-associated lesion or mass (DALM), 10 sporadic CRC, 4 UC-related CRC, 10 hyperplastic polyps, and 60 normal tissues obtained from people free of intestinal diseases. The diagnosis of IBD was based on clinical, endoscopic, and pathohistological criteria as described by Lennard-Jones [19]. All biopsy specimens were fixed in 4% paraformaldehyde (Sangon Biotech Co., Ltd., Shanghai, China) for at least 6 h and embedded in paraffin (Sangon Biotech Co., Ltd., Shanghai, China) until use.
The following parameters for all patients were obtained from medical records: age, sex, course of disease, colitis location, laboratory serological indices (white blood cells [WBC], erythrocyte sedimentation rate [ESR], C-reactive protein [CRP]), endoscopic features, and pathological features.
For patients with UC, the clinical activity index (CAI) was evaluated using the Mayo score [20]. For patients with CD, the Crohn’s disease activity index (CDAI) was measured according to CDAI of Best et al. [21]. The endoscopic activity was assessed according to the endoscopic part of the Rachmilewitz score [22,23]. The histological activity of colitis severity was graded according to Geboes criteria [24]. Dysplasia was defined according to modified Vienna criteria as either low-grade dysplasia (LGD) or high-grade dysplasia (HGD) [25]. The collection of serum indexes and colonoscopy was completed within the same 7-day period for all patients.
The present study was approved by the Institute Research Medical Ethics Committee of the Affiliated Nanfang Hospital, Southern Medical University. All participants provided written informed consent prior to involvement in the investigation [26].
Immunohistochemistry
The protein expression of ACK1 was measured by immunohistochemistry in colorectal tissue specimens. The paraffin-embedded blocks were cut into 4-μm sections, heated at 60°C for 30 min, deparaffinized in xylene, and rehydrated through graded alcohol solutions (100%, 95%, 90%, 80%, and 70%; Guangzhou Chemical Reagent Co., Ltd, Guangdong, China) for 3 min each, at room temperature. The sections were then washed with double-distilled water 3 times for 10 min, and blocked by 3% H2O2 (Guangzhou Chemical Reagent Co., Ltd., Guangdong, China) in absolute methanol for 10 min at room temperature to bleach endogenous peroxidase activity. After that, antigen retrieval was performed by microwave-treating in 0.1 mol/L sodium citrate buffer (pH6.0, Wuhan Boster Biological Technology, Ltd. Wuhan, China) for 20 min at 800 watts and cooling for 60 min. The slides were incubated with a rabbit polyclonal antibody against human ACK1 (sc-323, 1: 100; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. After washing with phosphate-buffered saline (PBS)/Tween 3 times for 10 min, the slides were incubated with a 1: 500 dilution of the biotinylated secondary antibody (Zhongshan Goldenbridge Biotechnology Co., Ltd., Beijing, China) for 30 min. After another washing with PBS for 3 times, they were incubated for 1 min with the avidin-biotin complex reagent at room temperature. The colorimetric reaction was performed with 3, 3′-diaminobenzidine (Beyotime Institute of Biotechnology Co., Ltd., Haimen, Jiangsu, China) as the chromogen and nickel chloride enhancement. The slides were then counterstained with hematoxylin (Beyotime Institute of Biotechnology Co., Ltd., Haimen, Jiangsu, China) and mounted with Permount. Finally, they were observed under a light microscope (Olympus BX-51 microscope, Olympus DP71 digital camera, Tokyo, Japan).
The degree of staining was independently assessed by 2 pathologists and graded semiquantitatively using criteria previously described: 0, no expression of ACK1; 1+, mild expression of ACK1; 2+, moderate expression of ACK1; and 3+, strong expression of ACK1.
Statistical analysis
Statistical analysis was performed using SPSS software version 13.0. Descriptive data are expressed as mean ± standard deviation. Differences between 2 and multiple groups of non-parametric data were compared by the Mann-Whitney and Kruskal Wallis tests, respectively. The t test was used for parametric numerical data. The distribution of ACK1 positive rates and categorical variables describing patient demographics was compared using the χ2 test. The correlation between 2 defined parameters was determined by calculating Spearman’s correlation coefficient. Two-tailed P values of 0.05 or less were regarded as indicating the presence of a statistically significant difference.
Results
Expression of ACK1 protein in normal colonic and IBD mucosa
A total of 78 patients with UC (49 females, 29 males, with a mean age of 40.18±13.35 years old) and 22 patients with CD (15 females, 7 males, with a mean age of 35.67±9.69 years old) were analyzed. There were no significant differences in demographic details between these 2 groups of patients (χ2=0.09, P=0.817 for sex; t=1.186, P=0.690 for mean age).
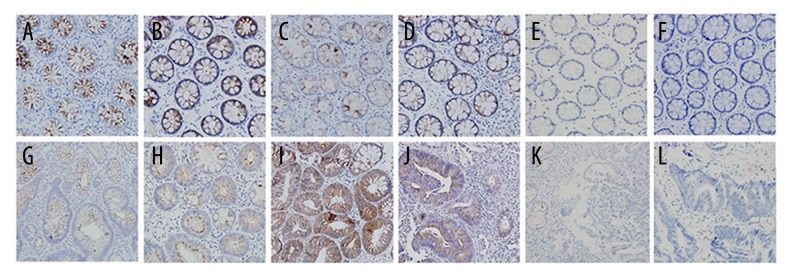
ACK1 protein was exclusively expressed in cytoplasm of colorectal epithelial cells, but not in inflammatory cells (Figure 1A, 1B, 1E). The expression of ACK1 was lacking or very weak in colonic biopsies from healthy controls. Markedly elevated levels of ACK1 expression were observed in IBD patients as compared to controls (P<0.000; Table 1), and the up-regulation of this protein was similar in UC and CD (P=0.254; Table 1).
Figure 1.
Immunohistochemical staining of ACK1 protein in colorectal mucosal biopsies (×200). ACK1 protein was exclusively expressed in cytoplasm of colorectal epithelial cells. (A) UC, (B) CD, (C) non-IBD and noninfectious colitis, (D) infectious colitis, (E) controls, (F) hyperplastic polyps, (G) non-UC-associated SAs of LGD, (H) UC-associated SAs, (I) non-UC-associated SAs of HGD, (J) DALM, (K) sporadic CRC, (L) UC-related CRC. UC – ulcerative colitis, CD – Crohn’s disease, SA – sporadic adenomas, DALM – dysplasia-associated lesion mass, CRC – colorectal cancer, HGD – high-grade dysplasia, LGD – low-grade dysplasia
Table 1.
ACK1 positive expression in colitis and healthy control.
| n | Staining | Positive rate (%) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 1+ and 2+ and 3+/all | 2+ and 3+/all | ||
| UC | 78 | 14 | 25 | 11 | 28 | 82.1 | 50 |
| Active UC | 54 | 10 | 15 | 8 | 21 | 81.5 | 53.7 |
| CD | 22 | 6 | 8 | 2 | 6 | 63.6 | 27.3 |
| Non-IBD and noninfectious colitis | 26 | 11 | 15 | 0 | 0 | 57.7 | 0 |
| Infectious Colitis | 20 | 5 | 4 | 8 | 3 | 75.0 | 55.0 |
| Control | 60 | 52 | 8 | 0 | 0 | 14.3 | 0 |
ACK1 – activated Cdc42 kinase1; UC – ulcerative colitis; CD – Crohn’s disease; 0 – no expression of ACK1; 1+ – mild expression of ACK1; 2+ – moderate expression of ACK1; 3+ – severe expression of ACK1.
ACK1 protein expression was further compared between subgroups classified by disease activities (Table 2). IBD patients with mild clinical activity showed significantly lower ACK1 expression than those with moderate-to-severe activity (P=0.033 for UC, P=0.017 for CD). For both UC and CD, ACK1 protein was expressed at equal levels in moderate clinical grading and severe grading (P=0.458 for UC, P=0.423 for CD). Only weak and moderate positive correlations between ACK1 expression and clinical activity were found in UC (r=0.384, P=0.030) and CD patients (r=0.661, P=0.010), respectively. In contrast, there were no significant differences in ACK1 expression when patients were classified by histological activity (P=0.869 for UC, P=0.799 for CD). Moreover, IBD patients with and without endoscopically active inflammation showed similar ACK1 expression (P=0.355), whereas the expression of this protein was significantly higher in endoscopic active colorectal mucosa than in endoscopic normal mucosa in the same IBD patient (P<0.05) (data not shown). No correlations of ACK1 expression with histological inflammatory activity (r=0.076, P=0.603 for UC; r=−0.073, P=0.748 for CD), endoscopic activity (r=0.110, P=0.359), serum parameters (such as CRP, ESR and WBC) and disease extent were observed in either UC or CD (P>0.05 for all; Table 3).
Table 2.
Association of ACK1 expression with clinical, endoscopic and histological grading in IBD.
| UC | CD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Staining | Z/χ2 (P) | Staining | Z/χ2 (P) | |||||||
| 0 | 1+ | 2+ | 3+ | 0 | 1+ | 2+ | 3+ | |||
| Clinical grading | −2.138 (0.033)a −0.742 (0.458)b |
−2.382 (0.017)a −0.802 (0.423)b |
||||||||
| Mild | 2 | 4 | 1 | 1 | 2 | 3 | 0 | 0 | ||
| Moderate | 1 | 1 | 0 | 6 | 0 | 4 | 0 | 3 | ||
| Severe | 3 | 2 | 2 | 9 | 0 | 0 | 1 | 1 | ||
| Endoscopic grading | −0.925 (0.355) | – | ||||||||
| Non-active | 4 | 7 | 2 | 5 | ||||||
| Active | 10 | 15 | 8 | 21 | 6 | 8 | 2 | 6 | ||
| Histological grading | 0.281 (0.869) | 0.448 (0.799) | ||||||||
| Mild | 1 | 3 | 2 | 2 | 0 | 0 | 1 | 0 | ||
| Moderate | 3 | 2 | 1 | 6 | 1 | 2 | 0 | 1 | ||
| Severe | 6 | 6 | 4 | 13 | 5 | 6 | 1 | 5 | ||
0 – no expression of ACK1; 1+ – mild expression of ACK1; 2+ – moderate expression of ACK1; 3+ – severe expression of ACK1; ACK1 – activated Cdc42 kinase1; IBD – inflammatory bowel disease; UC – ulcerative colitis; CD – Crohn’s disease.
Mild vs. moderate and severe;
moderate vs. severe.
Table 3.
Correlation between ACK1 expression and clinical characteristics, endoscopic and histological grading in IBD.
| UC | CD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Staining | r (P) | Staining | r (P) | |||||||
| 0 | 1+ | 2+ | 3+ | 0 | 1+ | 2+ | 3+ | |||
| Clinical grading | 0.384 (0.030) | 0.661(0.010) | ||||||||
| Mild | 2 | 4 | 1 | 1 | 2 | 3 | 0 | 0 | ||
| Moderate and severe | 4 | 3 | 2 | 15 | 0 | 4 | 1 | 4 | ||
| Endoscopic grading | 0.110 (0.359) | – | ||||||||
| Non-active | 4 | 7 | 2 | 5 | ||||||
| Active | 10 | 15 | 8 | 21 | 6 | 8 | 2 | 6 | ||
| Histological grading | 0.076 (0.603) | −0.073 (0.748) | ||||||||
| Mild | 1 | 3 | 2 | 2 | 1 | 2 | 2 | |||
| Moderate | 3 | 2 | 1 | 6 | ||||||
| Severe | 6 | 6 | 4 | 13 | 5 | 6 | 6 | |||
| Disease extent | – | −0.059 (0.804) | ||||||||
| CRP | 0.114 (0.565) | 0.267 (0.402) | ||||||||
| ESR | 0.024 (0.908) | 0.415 (0.180) | ||||||||
| WBC | −0.008 (0.966) | −0.205 (0.463) | ||||||||
0 – no expression of ACK1; 1+ – mild expression of ACK1; 2+ – moderate expression of ACK1; 3+ – severe expression of ACK1; ACK1 – activated Cdc42 kinase1; IBD – inflammatory bowel disease; UC – ulcerative colitis; CD – Crohn’s disease; CRP – C-reactive protein; ESR – erythrocyte sedimentation rate; WBC – white blood cells.
Expression of ACK1 in other colitis mucosa
It is sometimes difficult to distinguish endoscopically active IBD from infectious colitis or non-IBD and noninfectious colitis if their manifestations are similar, so the expression levels of ACK1 expression among these colorectal inflammatory conditions were also compared (Table 1). A total of 26 patients with non-IBD and noninfectious colitis (13 females, 13 males, with a mean age of 41.46±13.79 years), 20 patients with infectious colitis (7 females, 13 males, with a mean age of 38.15±10.42 years), 54 UC patients with active endoscopic inflammation (19 females, 35 males, with a mean age of 40.08±13.74 years), and 22 CD patients (15 females, 7 males, with a mean age of 35.67±9.69 years) were analyzed. There were no significant differences in demographic details among these groups of patients (t=0.232, P=0.874 for mean age; χ2=1.75, P=0.625 for sex).
Both infectious colitis and chronic colitis exhibited some degree of cytoplasmic staining for ACK1 protein (Figure 1C, 1D; Table 1). The highest ACK1 expressions were found in endoscopically active UC, followed by infectious colitis, then CD, then non-IBD and noninfectious colitis, and finally controls (χ2=72.96, P=0.000). Significantly different ACK1 expression were found between infectious colitis and controls (P=0.020), between non-IBD and noninfectious colitis and controls (P=0.000), between non-IBD and noninfectious colitis and infectious colitis (P=0.003), between non-IBD and noninfectious colitis and CD (P=0.020), and between endoscopically active UC and non-IBD and noninfectious colitis (P=0.000). However, the expression levels of ACK1 protein were similar between infectious colitis and endoscopically active UC (P=0.298), between infectious colitis and CD (P=0.755), and between endoscopically active UC and CD (P=0.209).
The histological grade of inflammation was significantly higher in endoscopically active IBD than in non-IBD and noninfectious colitis (P=0.000 for both UC and CD) and infectious colitis (P=0.000 for both UC and CD). The inflammatory degrees in non-IBD and noninfectious colitis and infectious colitis were similar to each other (P=0.707). No significant differences in ACK1 expression were observed when patients were classified by histological activity (χ2=2.63, P=0.150 for chronic colitis; χ2=0.16, P=0.921 for infectious colitis; χ2=2.31, P=0.316 for endoscopically active UC). ACK1 expression did not correlate with histological inflammatory degree in any of these 2 groups of colitis (r=0.324, P=0.106 for non-IBD and noninfectious colitis; r=0.034, P=0.887 for infectious colitis) (Table 4).
Table 4.
Association between ACK1 expression and histological grade of inflammation in non-IBD and noninfectious colitis, infectious colitis as well as endoscopically active UC.
| Staining | Non-IBD and noninfectious colitis | Infectious colitis | Endoscopically active UC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | Mild | Moderate | Severe | |
| 0 | 8 | 3 | 0 | 4 | 1 | 0 | 0 | 1 | 6 |
| 1+ | 6 | 9 | 0 | 2 | 1 | 1 | 0 | 2 | 5 |
| 2+ | 0 | 0 | 0 | 2 | 4 | 2 | 0 | 1 | 4 |
| 3+ | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 7 | 11 |
0 – no expression of ACK1; 1+ – mild expression of ACK1; 2+ – moderate expression of ACK1; 3+ – evere expression of ACK1; ACK1 – activated Cdc42 kinase1; IBD – inflammatory bowel disease; UC – ulcerative colitis.
The sensitivity, specificity, and accuracy of ACK1 protein expression for diagnosis of IBD
Because ACK1-positive rates were significantly different among colitis and controls (χ2=60.057, P<0.001), we further evaluate its usefulness in distinguishing IBD patients from healthy controls, as well as from patients with intestinal inflammatory conditions (Table 1).
ACK1-positive status had 88.9% sensitivity, 78.8% specificity, and 84.06% accuracy in discriminating between active UC and controls. ACK1 positivity as a predictor of CD had a sensitivity of 66.6%, a specificity of 90%, and an accuracy of 83.33%. Including healthy people and patients with non-IBD gastrointestinal inflammation as the control group resulted in somewhat higher specificity (83.70%) but lower sensitivity (65.31%) and accuracy (73.91%) for UC, and higher specificity (92.30%) but lower sensitivity (32.00%) for CD.
Of the ACK1 moderately-to-strongly positive colitis cases, 29 were active UC (53.7%), 11 were infectious colitis (55%), 11 were CD (27.3%), and none were chronic colitis or controls. ACK1 moderately-to-strongly positive status had a diagnostic potential in distinguishing UC from controls with 100% sensitivity, 70.5% specificity, and 77.5% accuracy. Including healthy people and patients with non-IBD gastrointestinal inflammation as the control group resulted in somewhat higher specificity (79.17%) and similar accuracy (77.5%) but lower sensitivity (72.5%).
Expression of ACK1 in patients during CRC progression
Moderate-to-strong ACK1 expression was detected in the cytoplasm of not only DALM but also SA cells, whereas CRCs were negative for ACK1 staining. In addition, ACK1 was weakly expressed only in 1 of 10 hyperplastic polyps (Table 5; Figure 1F–1L).
Table 5.
Expression of ACK1 in colorectal neoplasms and hyperplastic polyps.
| Staining | Hyperplastic polyps | Non-UC-associated SAs | UC-associated SAs | DALM | CRC | UC-CRC | ||
|---|---|---|---|---|---|---|---|---|
| LGD | HGD | LGD | HGD | HGD | ||||
| 0 | 9 | 0 | 0 | 0 | 0 | 0 | 10 | 4 |
| + | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2+ | 0 | 5 | 1 | 2 | 0 | 0 | 0 | 0 |
| 3+ | 0 | 0 | 4 | 0 | 4 | 4 | 0 | 0 |
0 – no expression of ACK1; 1+ – mild expression of ACK1; 2+ – moderate expression of ACK1; 3+ – severe expression of ACK1; SA – sporadic adenomas; UC – ulcerative colitis; DALM – dysplasia-associated lesion mass; CRC – colorectal cancer; HGD – high grade dysplasia; LGD – low grade dysplasia.
There were no significant differences in ACK1 expression between UC-associated and non-UC-associated SAs, or between UC-associated SAs and DALM with regard to the degree of dysplasia (P>0.05). For both UC-associated and non-UC-associated SAs, ACK1 expression was higher in HGD than in LGD (P<0.05) (Table 5; Figure 1G–1L).
Discussion
This is the first study exploring the expression of ACK1 protein in different colorectal diseases. We have previously reported ACK1 overexpression in colorectal epithelial cells of UC. Our findings here further showed that the expression level of ACK1 protein was upregulated not only in patients with IBD but also in those with other types of colitis, as compared to controls. We observed a higher ACK1 expression in endoscopically active colorectal mucosa relative to endoscopically normal mucosa in the same IBD patient. Moreover, we observed that endoscopically active and endoscopically quiescent IBD present equivalent expression levels of ACK1 protein, indicating a potential role of ACK1 overexpression in the remission period of IBD. Thus, ACK1 overexpression is not a distinctive feature of UC but is rather simply a concomitant of inflammation in the intestinal tract, and may play an important role in the pathogenesis of colorectal inflammatory process.
We found that ACK1-positive rates were different among colitis cases and controls. However, the sensitivity or specificity of ACK1 positivity for the diagnosis of IBD was far from perfect. Additionally, the expression level of this protein was similar in cases of UC and CD. These results indicate ACK1 is of limited value in distinction between UC and CD, or between IBD and non-IBD inflammatory conditions.
We further found ACK1 expression correlated only with clinical activity of IBD, but not with either endoscopic activity or histological activity. These different correlations may be due to the poor agreement among clinical, endoscopic and histological activity in IBD patients [27]. Numerous studies have shown the correlation of disease activity with inflammatory markers was much stronger for CD than for UC [14,28,29]. Similarly, we found ACK1 expression correlated less well with clinical activity in UC relative to CD. Previous studies have shown increased secretion of proinflammatory cytokines (such as TNF-α, IL-6, and IL-1) by colonic epithelial cells from IBD patients, and the absence of association of TNF-α production with either histologically or endoscopically inflammatory degree has been reported in IBD as well [30]. We failed to find associations between ACK1 expression and serum level of inflammatory reactants (such as CRP, ESR, and WBC). It is possible that ACK1 expression is associated with some inflammatory mediators (such as TNF-α, IL-6, and IL-1) released directly by the gut mucosal cells, but not acute-phase proteins (such as CRP) produced by hepatocytes, or serum inflammatory proteins (such as ESR and WBC) increased by various conditions other than gut inflammation [31].
Long-term UC patients are at high risk for developing CRC compared with the general population. The pathogenic sequences of sporadic CRC and UC-related CRC progression are quite different. UC-related CRC progresses from flat dysplastic lesions and especially DALM, while the dysplastic precursor of sporadic CRC is usually an adenomatous polyp [14–17]. In the present study, we demonstrated that ACK1 protein expression was significantly increased in SAs as well as in DALM, but not in hyperplastic polyps, which carried no risk of neoplastic transformation. We also found associations between ACK1 expression and dysplasia grade in both types of precursor lesions for CRC. This is in accordance with studies of several other tissues which showed ACK1 expression correlated with malignant progression [2–6]. By contrast, we found both UC-related CRC and sporadic CRC were negative for ACK1 protein expression. Our findings suggest that ACK1 overexpression may play a contributory role during early stages of colorectal neoplastic transformation, whether UC-related or not. The absence of ACK1 expression in later stages of colorectal carcinogenesis may indicate a trend toward silencing ACK1 expression during epithelial dedifferentiation of CRC.
Coexistence of DALM and SAs is not rare in patients with UC-related neoplasms. One subtype of DALM consists of an “adenoma-like” polypoid dysplastic lesion that morphologically resembles SAs. The clinical distinction between DALMs and SAs that arise in UC patients is extremely important because their management is quite different. The presence of DALM is an indication for colectomy, whereas both UC-associated and non-UC-associated SAs that are pathobiologically related are usually treated by polypectomy only. However, it is difficult to differentiate between these 2 dysplasias based on routine histologic evaluation. Recent studies have reported several differences in the onset and frequency of molecular alternations between DALMs and SAs. For instance, DALM shows a significantly higher proportion of p53 immunostaining when compared with SAs, while lack of bcl2 and nuclear β-catenin expression appear to favor a diagnosis of DALM over SAs [14–17]. Here, we observed ACK1 protein was expressed uniformly between DALM and SAs, indicating that ACK1 expression may not be useful in distinguishing these 2 types of lesions. Furthermore, we found that the ACK1 expression pattern in SAs arising in UC patients resembled those in non-UC-associated ones, supporting the notion that these 2 forms of dysplasia share similar molecular alternations and are pathobiologically related.
Although Ack1 overexpression has been observed in multiple cancers, including digestive system tumors, most functional studies of ACK1 signaling pathways are focused on the respiratory and reproductive system [2–5]. Our study revealed extensively increased expression of ACK1 protein in colorectal inflammation and dysplasia, but the potentially functional role and molecular mechanism for ACK1 overexpression in these colorectal diseases are currently completely unknown. Abnormal hyperproliferation as well as decreased apoptosis of intestinal epithelial cells contribute to the promotion and progression of colorectal tumors [16]. ACK1 has been shown to promote proliferation and protect against apoptosis in many types of epithelial cells, and, thereby, ACK1 overexpression in intestinal epithelial cells may play a role in the development of colorectal dysplasia. The activation of cellular proliferation pathways is also crucial for maintaining mucosal integrity in inflammatory conditions [32]. Another critical component in mucosal wound healing and regeneration is through epithelial cell migration, and ACK1 also has the ability to enhance epithelial cell spreading and migration [33]. These findings indicate that ACK1 may be involved in maintaining colorectal epithelial barrier integrity in inflammatory disorders. It has been suggested that inflammation can give rise to malignancy. A common denominator for both colonic neoplasias and colitis is the activation of signaling pathways that regulate cellular proliferation, apoptosis, and inflammation [34,35]. AKT, one of the major downstream targets of ACK1 signaling, has been shown to play important roles in the pathogenesis of both inflammation and tumorigenesis. Besides its well-known ability to affect cellular proliferation, apoptosis, and migration, the AKT signaling pathway also regulates the production of proinflammatory cytokines, not only in mononuclear cells, but also in epithelial cells [36,37]. ACK1 has recently been reported to activate AKT in hepatocellular and pancreatic cancer. We therefore speculate that ACK1 signaling may link inflammation and dysplasia transformation via AKT in colorectal epithelial cells. However, due to the possible abilities of ACK1 to maintain colorectal mucosa integrity, as well as mediate inflammation, it is difficult to predict whether ACK1 overexpression exerts a beneficial or detrimental effect in the wound healing process, and this question needs further investigation in cell models as well as in the diseased mucosa.
There are some limitations to the present study. We only assessed correlations of ACK1 expression with disease activity and severity. Whether this protein may be able to predict disease courses and outcomes needs to be evaluated in future studies. Due to the limited number of patients, some associations with ACK1 protein expression should be interpreted with caution. In addition, some studies suggest a different pathway of molecular events between flat sporadic colorectal adenomas and their more common polyploidy counterparts [38]. “Adenoma-like” DALM was also found to show some molecular differences from “non-adenoma-like” DALM [39]. Further investigations with larger sample size and more types of precursor lesions for CRCs are needed to better elucidate ACK1 alternations during CRC progression.
Conclusions
Taken together, we have demonstrated for the first time that ACK1 protein expression was increased extensively in mucosal epithelial cells of colitis and colorectal dysplasia. The expression of ACK1 was correlated positively with both disease activity of IBD and disease severity of colorectal dysplasia. We conclude that ACK1 may play a role in colorectal inflammatory process and in neoplastic progression. Further studies are needed to elucidate the exact molecular mechanisms by which ACK1 is involved in colitis and colorectal neoplasms.
Footnotes
Disclosure of conflict of interest
None.
Source of support: This work was supported in part by Guangdong Provincial Science and Technology Foundation (5004770) to Fachao Zhi
References
- 1.Mahajan K, Mahajan NP. ACK1 tyrosine kinase: Targeted inhibition to block cancer cell proliferation. Cancer Lett. 2013;338:185–92. doi: 10.1016/j.canlet.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie B, Zen Q, Wang X, et al. ACK1 promotes hepatocellular carcinoma progression via downregulating WWOX and activating AKT signaling. Int J Oncol. 2015;46:2057–66. doi: 10.3892/ijo.2015.2910. [DOI] [PubMed] [Google Scholar]
- 3.Gelman IH. Androgen receptor activation in castration-recurrent prostate cancer: The role of Src-family and Ack1 tyrosine kinases. Int J Biol Sci. 2014;10:620–26. doi: 10.7150/ijbs.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei X, Li YF, Chen GD, et al. Ack1 overexpression promotes metastasis and indicates poor prognosis of hepatocellular carcinoma. Oncotarget. 2015;6:40622–41. doi: 10.18632/oncotarget.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahajan K, Mahajan NP. ACK1/TNK2 tyrosine kinase: Molecular signaling and evolving role in cancers. Oncogene. 2015;34:4162–67. doi: 10.1038/onc.2014.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahajan K, Coppola D, Challa S, et al. Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its activation. PLoS One. 2010;5:e9646. doi: 10.1371/journal.pone.0009646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahajan K, Challa S, Coppola D, et al. Effect of Ack1 tyrosine kinase inhibitor on ligand-independent androgen receptor activity. Prostate. 2010;70:1274–85. doi: 10.1002/pros.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan DS, Haaland B, Gan JM, et al. Bosutinib inhibits migration and invasion via ACK1 in KRAS mutant non-small cell lung cancer. Mol Cancer. 2014;13:13. doi: 10.1186/1476-4598-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen ZL, Chang S, Li BZ, et al. Correlation study of the overexpression of activated Cdc42-associated kinase 1 and the stage and prognosis of esophageal squamous cell carcinoma. Zhonghua Yi Xue Za Zhi. 2011;91:166–70. [PubMed] [Google Scholar]
- 10.Mahajan K, Coppola D, Chen YA, et al. Ack1 tyrosine kinase activation correlates with pancreatic cancer progression. Am J Pathol. 2012;180:1386–93. doi: 10.1016/j.ajpath.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, Kang B, Lu C, et al. Evaluation of p38 MAPK pathway as a molecular signature in ulcerative colitis. J Proteome Res. 2011;10:2216–25. doi: 10.1021/pr100969w. [DOI] [PubMed] [Google Scholar]
- 12.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut. 2006;55:426–31. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Üstün Y, Kilincalp S, Çoban Ş, et al. Evaluation of early atherosclerosis markers in patients with inflammatory bowel disease. Med Sci Monit. 2016;22:3943–50. doi: 10.12659/MSM.898160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shigaki K, Mitomi H, Fujimori T, et al. Immunohistochemical analysis of chromogranin A and p53 expressions in ulcerative colitis-associated neoplasia: Neuroendocrine differentiation as an early event in the colitis-neoplasia sequence. Hum Pathol. 2013;44:2393–99. doi: 10.1016/j.humpath.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Shivakumar BM, Chakrabarty S, Rotti H, et al. Comparative analysis of copy number variations in ulcerative colitis associated and sporadic colorectal neoplasia. BMC Cancer. 2016;16:271. doi: 10.1186/s12885-016-2303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorsteinsdottir S, Gudjonsson T, Nielsen OH, et al. Pathogenesis and biomarkers of carcinogenesis in ulcerative colitis. Nat Rev Gastroenterol Hepatol. 2011;8:395–404. doi: 10.1038/nrgastro.2011.96. [DOI] [PubMed] [Google Scholar]
- 17.Averboukh F, Ziv Y, Kariv Y, et al. Colorectal carcinoma in inflammatory bowel disease: A comparison between Crohn’s and ulcerative colitis. Colorectal Dis. 2011;13:1230–35. doi: 10.1111/j.1463-1318.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen OH, Vainer B, Rask-Madsen J. Non-IBD and noninfectious colitis. Nat Clin Pract Gastroenterol Hepatol. 2008;5:28–39. doi: 10.1038/ncpgasthep1005. [DOI] [PubMed] [Google Scholar]
- 19.Ooi CJ, Fock KM, Makharia GK, et al. The Asia-Pacific consensus on ulcerative colitis. Gastroenterol Hepatol. 2010;25:453–68. doi: 10.1111/j.1440-1746.2010.06241.x. [DOI] [PubMed] [Google Scholar]
- 20.Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: Definitions and diagnosis. J Crohns Colitis. 2012;6:965–90. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Van Assche G, Dignass A, Panes J, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI) Gastroenterology. 1979;77:843–46. [PubMed] [Google Scholar]
- 23.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–25. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 24.Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827–51. doi: 10.1016/j.crohns.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–55. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giordano S. The 2008 Declaration of Helsinki: Some reflections. J Med Ethics. 2010;36:598–603. doi: 10.1136/jme.2009.034132. [DOI] [PubMed] [Google Scholar]
- 27.Regueiro M, Rodemann J, Kip KE, et al. Physician assessment of ulcerative colitis activity correlates poorly with endoscopic disease activity. Inflamm Bowel Dis. 2011;17:1008–14. doi: 10.1002/ibd.21445. [DOI] [PubMed] [Google Scholar]
- 28.Cappello M, Morreale GC. The role of laboratory tests in Crohn’s disease. Clin Med Insights Gastroenterol. 2016;9:51–62. doi: 10.4137/CGast.S38203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwak MS, Kim KJ, Park SH, et al. Elevated C-reactive protein is associated with disease progression in patients with mild Crohn’s disease. Springerplus. 2016;5:878. doi: 10.1186/s40064-016-2606-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reimund JM, Wittersheim C, Dumont S, et al. Increased production of tumour necrosis factor-alpha, interleukin-1 beta, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn’s disease. Gut. 1996;39:684–89. doi: 10.1136/gut.39.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solem CA, Loftus EV, Jr, Tremaine WJ, et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–12. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 32.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 33.Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 34.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Sun L, An Y, Shen X. Cabozantinib, a Novel c-Met Inhibitor, Inhibits Colorectal Cancer Development in a Xenograft Model. Med Sci Monit. 2015;21:2316–21. doi: 10.12659/MSM.893590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee DF, Kuo HP, Chen CT, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–55. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 38.van Wyk R, Slezak P, Hayes VM, et al. Somatic mutations of the APC, KRAS, and TP53 genes in nonpolypoid colorectal adenomas. Genes Chromosomes Cancer. 2000;27:202–8. [PubMed] [Google Scholar]
- 39.Itzkowitz SH, Present DH Crohn’s and Colitis Foundation of America Colon Cancer in IBD Study Group. Crohn’s and Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–21. doi: 10.1097/01.mib.0000160811.76729.d5. [DOI] [PubMed] [Google Scholar]