Abstract
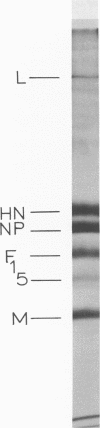
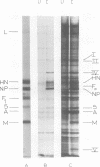
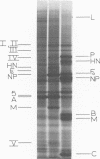
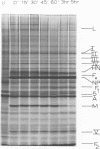
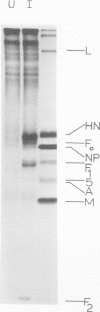
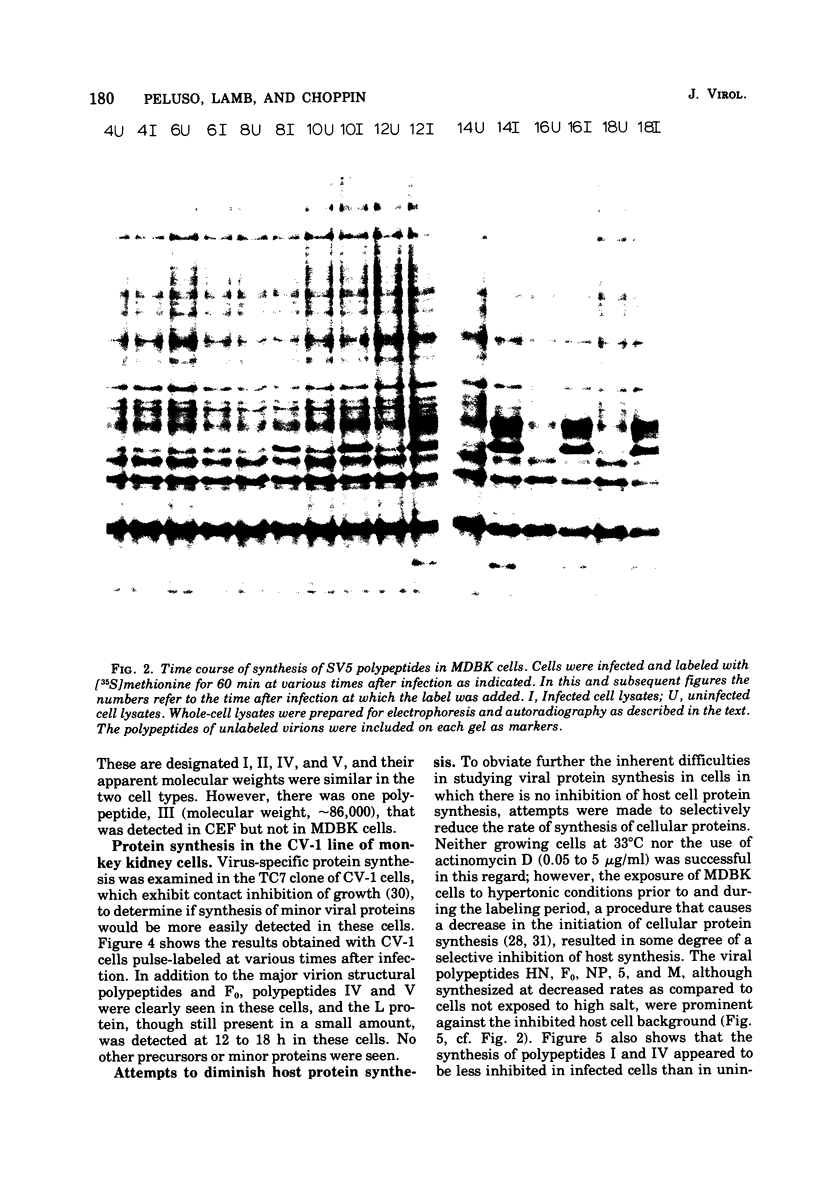
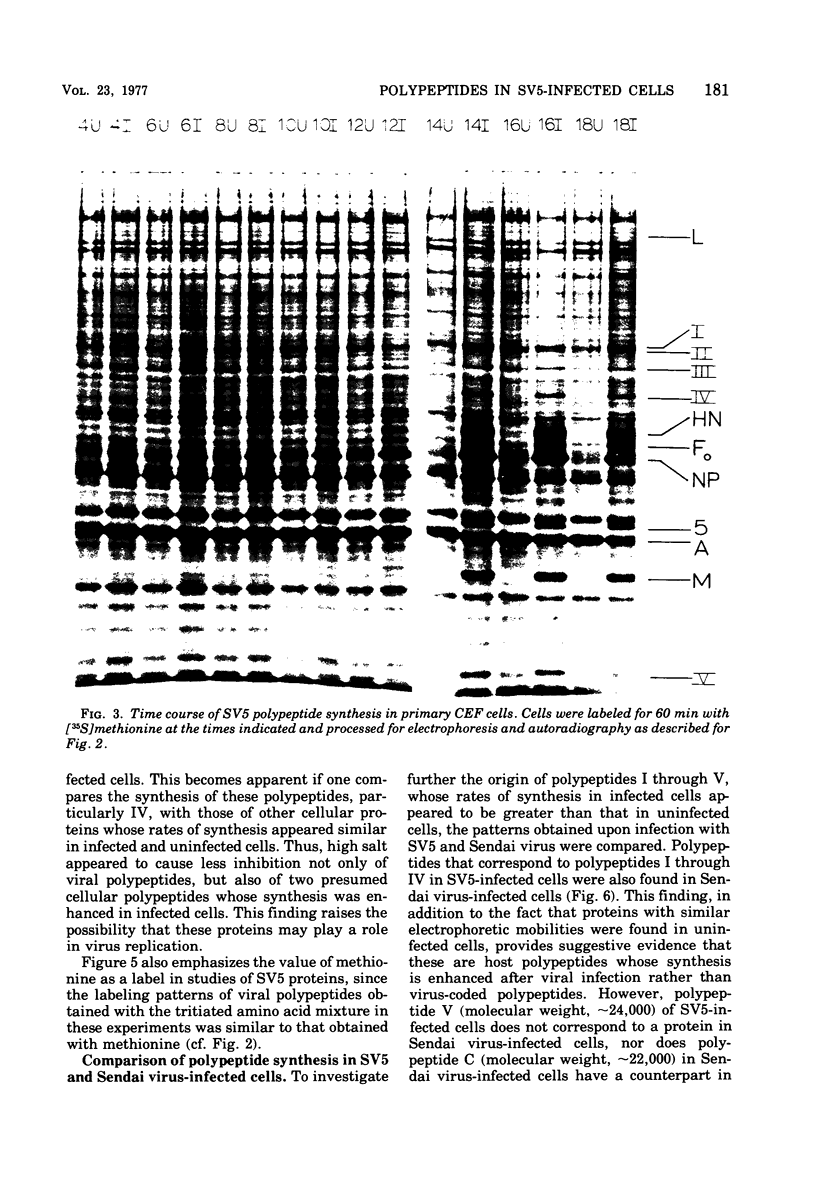
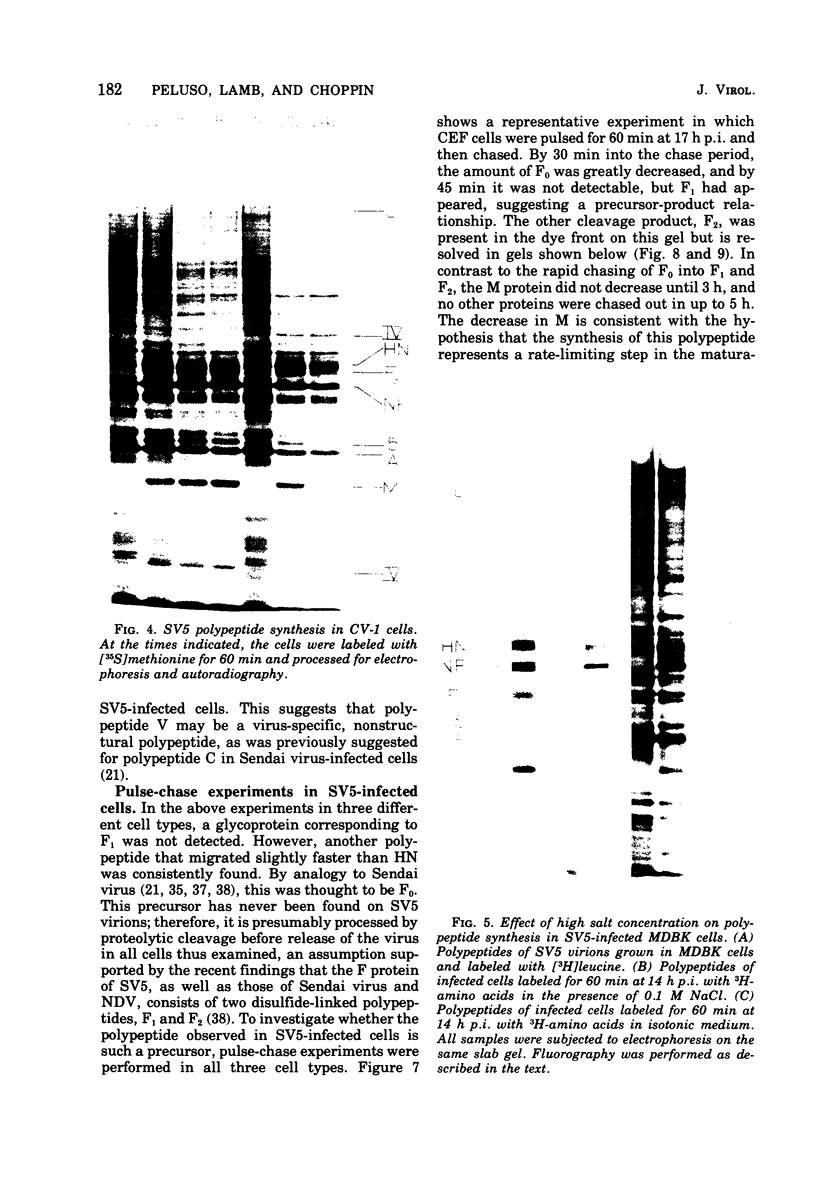
Polypeptide synthesis in three different cell types infected with simian virus 5 has been examined using high-resolution polyacrylamide slab gel electrophoresis, and all of the known viral polypeptides have been identified above the host cell background. The polypeptides were synthesized in infected cells in unequal proportions, which are approximately the same as they are found in virions, suggesting that their relative rates of synthesis are controlled. The nucleocapsid polypeptide (NP) was the first to be detected in infected cells, and by 12 to 14 h the other virion structural polypeptides were identified, except for the polypeptides comprising the smaller glycoprotein (F). However, a glycosylated precursor (F0) with a molecular weight of 66,000 was found in each cell type, and pulse-chase experiments suggested that this precursor was cleaved to yield polypeptides F1 and F2. No other proteolytic processing was found. In addition to the structural polypeptides, the synthesis of five other polypeptides, designated I through V, has been observed in simian virus 5-infected cells. One of these (V), with a molecular weight of 24,000, was found in all cells examined and may be a nonstructural viral polypeptide. In contrast, there are polypeptides present in uninfected cells that correspond in size to polypeptides I through IV, and similar polypeptides have also been detected in increased amounts in cells infected with Sendai virus. These findings, and the fact that the synthesis of all four of these polypeptides is not increased in every cell type, suggest that they represent host polypeptides whose synthesis may be enhanced upon infection. When a high salt concentration was used to decrease host cell protein synthesis in infected cells, polypeptides IV and (to a lesser extent) I were synthesized in relatively greater amounts than other cellular polypeptides, as were the viral polypeptides. The possibility that these polypeptides may play some role in virus replication is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Inhibition of Sindbis virus replication by zinc ions. Virology. 1976 Jul 1;72(1):272–277. doi: 10.1016/0042-6822(76)90330-5. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Korant B. D. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J Virol. 1974 Aug;14(2):282–291. doi: 10.1128/jvi.14.2.282-291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPPIN P. W. MULTIPLICATION OF A MYXOVIRUS (SV5) WITH MINIMAL CYTOPATHIC EFFECTS AND WITHOUT INTERFERENCE. Virology. 1964 Jun;23:224–233. doi: 10.1016/0042-6822(64)90286-7. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Klenk H. D., Choppin P. W. The proteins of the parainfluenza virus SV5. 1. Separation of virion polypeptides by polyacrylamide gel electrophoresis. Virology. 1969 Nov;39(3):460–466. doi: 10.1016/0042-6822(69)90094-4. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Compans R. W. Phenotypic mixing of envelope proteins of the parainfluenza virus SV5 and vesicular stomatitis virus. J Virol. 1970 May;5(5):609–616. doi: 10.1128/jvi.5.5.609-616.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W., Holmes K. V. Replication of SV5 RNA and the effects of superinfection with poliovirus. Virology. 1967 Nov;33(3):442–451. doi: 10.1016/0042-6822(67)90119-5. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Hightower L. E., Bratt M. A. Protein synthesis in Newcastle disease virus-infected chicken embryo cells. J Virol. 1974 Apr;13(4):788–800. doi: 10.1128/jvi.13.4.788-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Caliguiri L. A., Choppin P. W. The proteins of the parainfluenza virus SV5. II. The carbohydrate content and glycoproteins of the virion. Virology. 1970 Oct;42(2):473–481. doi: 10.1016/0042-6822(70)90290-4. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Chemical composition of the parainfluenza virus SV5. Virology. 1969 Jan;37(1):155–157. doi: 10.1016/0042-6822(69)90321-3. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Lipids of plasma membranes of monkey and hamster kidney cells and of parainfluenza virions grown in these cells. Virology. 1969 Jun;38(2):255–268. doi: 10.1016/0042-6822(69)90367-5. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Plasma membrane lipids and parainfluenza virus assembly. Virology. 1970 Apr;40(4):939–947. doi: 10.1016/0042-6822(70)90140-6. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Butterworth B. E. Inhibition by zinc of rhinovirus protein cleavage: interaction of zinc with capsid polypeptides. J Virol. 1976 Apr;18(1):298–306. doi: 10.1128/jvi.18.1.298-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus proteins in infected cells: translation of viral polypeptides, including three P polypeptides, from RNA produced by primary transcription. Virology. 1976 Oct 15;74(2):504–519. doi: 10.1016/0042-6822(76)90356-1. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Lackland H., Choppin P. W. Isolation and characterization of the nonglycosylated membrane protein and a nucleocapsid complex from the paramyxovirus SV5. Virology. 1975 Oct;67(2):365–374. doi: 10.1016/0042-6822(75)90438-9. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Caliguiri L. A., Choppin P. W. Nucleocapsid protein subunits of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1970 Nov;6(5):677–684. doi: 10.1128/jvi.6.5.677-684.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Choppin P. W. Proteins and glycoproteins of paramyxoviruses: a comparison of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1971 Jan;7(1):47–52. doi: 10.1128/jvi.7.1.47-52.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D. Activation of precursors to both glycoporteins of Newcastle disease virus by proteolytic cleavage. Virology. 1977 Mar;77(1):125–134. doi: 10.1016/0042-6822(77)90412-3. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Regulatory events in the synthesis of Sendai virus polypeptides and their assembly into virions. Virology. 1976 Aug;73(1):79–88. doi: 10.1016/0042-6822(76)90062-3. [DOI] [PubMed] [Google Scholar]
- Rovera G., Mehta S., Maul G. Ghost monolayers in the study of the modulation of transcription in cultures of CV1 fibroblasts. Exp Cell Res. 1974 Dec;89(2):295–305. doi: 10.1016/0014-4827(74)90793-9. [DOI] [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Samson A. C., Fox C. F. Selective inhibition of Newcastle disease virus-induced glycoprotein synthesis by D-glucosamine hydrochloride. J Virol. 1974 Apr;13(4):775–779. doi: 10.1128/jvi.13.4.775-779.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Protease activation mutants of sendai virus. Activation of biological properties by specific proteases. Virology. 1976 Jan;69(1):265–277. doi: 10.1016/0042-6822(76)90213-0. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. The hemagglutinating and neuraminidase protein of a paramyxovirus: interaction with neuraminic acid in affinity chromatography. Virology. 1974 Nov;62(1):125–133. doi: 10.1016/0042-6822(74)90308-0. [DOI] [PubMed] [Google Scholar]