Abstract
Case series
Patient: —
Final Diagnosis: Dengue fever infection
Symptoms: Bone pain • fever
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases
Objective:
Unusual or unexpected effect of treatment
Background:
About 400 million cases of dengue, a mosquito-borne disease, are reported annually, but no drug is yet available for treatment. In 1988, at Feng Lin Clinic, Taiwan, we encountered about 10,000 cases and tested various drugs before confirming an antiviral effect of amantadine against dengue virus in vitro. After we administered amantadine to patients for 1–2 days, most achieved full remission. None experienced potentially life-threatening dengue hemorrhagic fever or dengue shock syndrome. Herein, we present 34 cases from recent clinical experience that show amantadine’s unusual effect against dengue virus infection.
Case Report:
We divided 34 patients with symptoms of dengue fever, confirmed by a screening test, into 3 groups: 6 Category 1 patients received amantadine at onset, 21 Category 2 patients received amantadine within 2–6 days, and 7 Contrast group patients received no amantadine because they visited other clinics or were admitted to a large hospital. When Category 1 patients were treated with amantadine 100 mg 3 times per day, all symptoms dramatically subsided within 1–2 days. In Category 2 patients, most symptoms diminished within 1–2 days after starting the same regimen. In the Contrast group, all symptoms persisted 7 days after onset. White blood cell and platelet counts in Category 1 and 2 patients recovered to normal range, but remained below low normal in the Contrast group.
Conclusions:
Amantadine is effective and should be given as soon as possible to stop the disease course if dengue fever is confirmed through screening or clinical signs and symptoms. A well-designed larger sample study is warranted to test this effectiveness.
MeSH Keywords: Amantadine, Dengue Virus, Treatment Outcome
Background
Dengue virus infection, a mosquito-borne disease, is a major cause of illness in the tropics and subtropics, with about 400 million infections reported annually [1]. It often manifests with fever, joint pain, and rash, but to date there is no specific drug available to treat it [2]. In 1988, Kaohsiung City, Taiwan, experienced an epidemic of dengue fever, with more than 50,000 infected citizens and more than 300 mortalities. At the Feng Lin Clinic, we encountered about 10,000 cases. We tested various drugs before confirming an antiviral effect of amantadine [3] that made it effective against dengue virus in vitro [4]. After we administered it to patients for 1–2 days, most achieved full remission. None experienced potentially life-threatening dengue hemorrhagic fever or dengue shock syndrome. Herein, we present 34 cases from more recent clinical experience that demonstrate the unusual effect of amantadine against dengue virus infection.
Case Report
In 2014 and 2015, dengue fever epidemics again threatened south Taiwan, especially Tainan and Kaohsiung [5]. More than 200 mortalities were reported, and almost all hospitals were overwhelmed by the epidemics. From August 2014 to November 2015, we prospectively collected patients suspected of having dengue fever for the amantadine regimen after they provided written informed consent to participate. A total of 36 patients were enrolled in our 3 clinics, of whom 34 (19 males and 15 females, age range 14 to 75 years) tested positive for dengue fever by a screening test (Standard Diagnostics®, Dengue NS1 Ag) and had high fever, generalized bone pain, nausea, vomiting, malaise, and sometimes skin rash. We divided these 34 patients into 3 groups: Category 1, Category 2, and Contrast. We used the same regimen for 3 days for the first 2 patient groups: Enzil® (amantadine 100 mg), one tablet 3 times per day by mouth. Six Category 1 patients received amantadine at onset, and 21 Category 2 patients received it within 2–6 days. Seven patients in the Contrast group who visited our clinics for another problem stated that they had made a prior dengue fever visit at another clinic or had been admitted to a large hospital and did not receive amantadine. Table 1 shows the demographic data of these 34 cases.
Table 1.
Demographic data and percentage of target symptoms during onset, day 3, and day 7 in Category 1, Category 2, and Contrast groups.
| Patient characteristics | Category 1 group | Category 2 group | Contrast group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex (male/female) | 4/2 | 10/11 | 5/2 | ||||||
| Age (mean ±SD) | 39.5±28.0 y | 51.7±4.2 y | 40.1±16.4 y | ||||||
| Symptoms | Onset | Day 3 | Day 7 | Onset | Day 3 | Day 7 | Onset | Day 3 | Day 7 |
| Fever | 1.00 | 0.00 | 0.00 | 0.90 | 0.24 | 0.00 | 0.86 | 0.57 | 0.43 |
| Bone pain | 0.83 | 0.00 | 0.00 | 0.95 | 0.00 | 0.00 | 0.71 | 0.29 | 0.29 |
| Rash | 0.00 | 0.00 | 0.00 | 0.10 | 0.19 | 0.00 | 0.00 | 0.43 | 0.43 |
| GI upset | 0.33 | 0.00 | 0.00 | 0.62 | 0.14 | 0.00 | 0.00 | 0.29 | 0.29 |
| Malaise | 0.33 | 0.00 | 0.00 | 0.14 | 0.05 | 0.00 | 0.29 | 0.14 | 0.14 |
| Other* | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.14 |
Including insomnia, chest tightness, or gingival bleeding.
Among the 6 Category 1 patients, all symptoms – fever (in 6 patients), bone pain (in 5 patients), gastrointestinal (GI) upset (in 2 patients), and malaise (in 1 patient) – dramatically subsided (to 0) within 1–2 days. Among the 21 Category 2 patients, most symptoms diminished within 1–2 days, including fever (from 19 to 2 patients), bone pain (from 20 patients to 1 patient), GI upset (from 13 to 2 patients), and malaise (from 3 patients to 1 patient); skin rash, however, increased (from 2 to 4 patients). No complications were noted in these 2 categories. In the Contrast group, all symptoms persisted 7 days after onset: of these 7 patients, 4 had high fever, 2 had bone pain, 2 had malaise, and 3 had skin rash; in addition, 1 had gingival bleeding (Table 1). Figure 1 shows that the white blood cell (WBC) and platelet counts in Category 1 and 2 patients recovered to normal range (WBCs 4000 to 10,000; platelets 150,000 to 40,000), but remained below low normal in the Contrast group.
Figure 1.
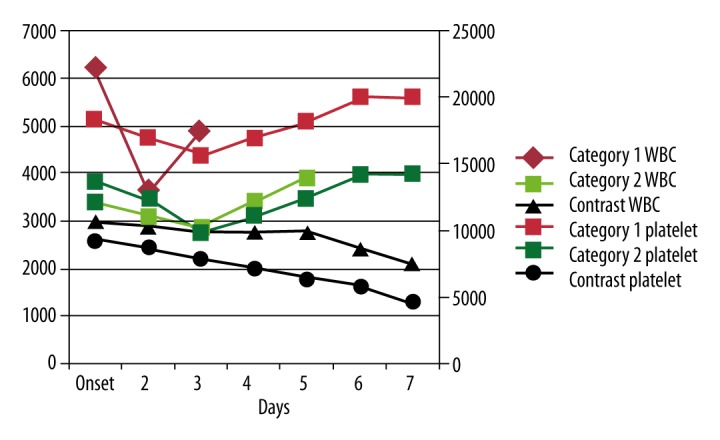
Changes in white blood cell (WBC) and platelet counts in Category 1, Category 2, and Contrast groups at onset and days 2–7 of follow-up. In Category 1 (red) and Category 2 (green), WBCs and platelets recovered to normal within 7 days. However, in Category 3 (black), WBCs and platelets remained abnormal 7 days after onset.
Discussion
This case report is the first to provide preliminary evidence of amantadine treatment effectiveness against dengue fever by clinical trial. Amantadine has been introduced for the prevention and treatment of influenza A [6] and as an anti-parkinsonism drug [7]. Although Koff et al. reported in 1980 that amantadine inhibits dengue virus replication [4], to the best of our knowledge, no physician has since used amantadine to treat dengue fever clinically, other than in 1988 when we used it in about 10,000 cases and found it to be dramatically effective. In this case report, we again show that amantadine is effective against dengue virus infection from the prospectively collected clinical data of patients with dengue fever.
Although dengue virus infection is often mild and self-limited [8], our patients were in great discomfort. When they received amantadine, their symptoms were much improved, but when they did not, their symptoms persisted. We speculate that amantadine can stop the progress of dengue fever to a more severe disease that may lead to mortality, especially in children, older adults, fragile patients, or in those with comorbid chronic diseases, such as diabetes mellitus, hypertension, or cardiovascular disease. A large-scale randomized clinical trial to test this hypothesis is warranted. In 2015, the Centers for Disease Control and Prevention announced that amantadine is not recommended for use as an antiviral drug against influenza because of resistance to it [9], but in the current study, we did not find signs of this phenomenon when amantadine was used to treat dengue virus infection. The most frequently reported side effects of amantadine at the recommended dose include nausea, lightheadedness, and insomnia [10], but we did not observe these complications. Our case report has limitations. First, we used a small sample size and did not perform statistical analyses. We need to repeat the study with 30 patients in each arm in the future, in which we collect full data about comorbidity (hypertension, diabetic mellitus, etc.) and set up a regression model to rule out confounding variables that may contribute to the effectiveness of amantadine. Second, our findings are the result of clinical observation, not a double-blind randomized controlled trial.
Conclusions
Amantadine may be effective for dengue virus infection. We suggest that when dengue fever is confirmed through screening or clinical signs and symptoms, amantadine be given as soon as possible to stop the disease course. In addition, a dengue fever screening test should be performed in high-risk groups, such as co-habiting families of diagnosed patients, to quickly provide treatment. A large-sample, randomized, double-blind study is warranted for testing this effectiveness.
Acknowledgments
We thank Barbara Every, ELS, of BioMedical Editor for English language editing. We also thank Professor Jing-Shiang Hwang, Institute of Statistical Science, Academia Sinica, Taiwan, for statistical advice.
References:
- 1.Centers for Disease Control and Prevention Dengue. [updated 2016 Jan 19; cited 2016 Aug 2]. Available from: https://www.cdc.gov/dengue.
- 2.World Health Organization Dengue. [cited 2016 Aug 2]. Available from: http://www.who.int/topics/dengue/en.
- 3.Davies WL, Grunert RR, Haff RF, et al. Antiviral activity of 1-adamantanamine (amantadine) Science. 1964;144:862–63. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- 4.Koff WC, Elm JL, Halstead SB. Inhibition of dengue virus replication by amantadine hydrochloride. Antimicrob Agents Chemother. 1980;18(1):125–29. doi: 10.1128/aac.18.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control, R.O.C. (Taiwan) As dengue outbreak in Kaohsiung City yet to abate, CECC for Dengue Outbreak continues to provide full support to Kaohsiung City to help bring outbreak under control as soon as possible. 2015. Nov 21, [cited 2016 Jun 8]. Available from: http://www.cdc.gov.tw/english/info.aspx?treeid=bc2d4e89b154059b&nowtreeid=ee0a2987cfba3222&tid=57C3974A3818E7FC.
- 6.Nicholson KG, Wiselka MJ. Amantadine for influenza A. BMJ. 1991;302:425–26. doi: 10.1136/bmj.302.6774.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabey JM, Nissipeanu P, Korczyn AD. Efficacy of memantine, an NMDA receptor antagonist, in the treatment of Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1992;4:277–82. doi: 10.1007/BF02260076. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd SM, Hinfey PB, Shoff WH. Dengue treatment & management. Medscape [updated 2015 Oct 5; cited 2016 Aug 2]. Available from: http://emedicine.medscape.com/article/215840-treatment.
- 9.Centers for Disease Control and Prevention Influenza antiviral drug resistance. [cited 2016 Aug 2]. Available from: http://www.cdc.gov/flu/about/qa/antiviralresistance.htm.
- 10.Cunha JP. Symmetrel FDA prescribing information: side effects (adverse reactions) [cited 2016 Aug 3]. Available from: http://www.rxlist.com/symmetrel-side-effects-drug-center.htm.


