Abstract
Background
The aim of this study was to explore the mechanism by which cervical cancer is inhibited by promoting IGFBP7 expression using ellagic acid from pomegranate peel extract.
Material/Methods
HeLa cells were divided into 6 groups: control group (NC), blank control group (BL), and IGFBP7 overexpression group (IGFBP7), and 2.5 uM, 5. 0 uM, and 10.0 uM ellagic acid-treated groups. The cell proliferation ability was detected and the degree of invasion in the 6 groups was measured by Transwell assay. The expression levels of IGFBP7 and AKT/mTOR in the 6 groups of cells were detected by RT-PCR technique.
Results
Compared with NC and BL groups, The IGFBP7 gene expressions of the IGFPB7 and ellagic acid-treated groups were significantly increased (P<0.05). There was a dose-effect dependence in the ellagic acid-treated groups. The invasion ability of the IGFBP7 group and ellagic acid-treated groups was significantly lower than that of NC and BL groups in HeLa cells (P<0.05). The apoptosis rate of the IGFBP7 group and ellagic acid-treated groups was significantly higher than that of the NC and BL groups in HeLa cells (P<0.05). AKT and mTOR mRNA and protein expressions of the IGFBP7 group and ellagic acid-treated groups were significantly lower than that of the NC and BL groups (P<0.05). There was a dose-effect dependence in the ellagic acid-treated groups.
Conclusions
The ellagic acid in pomegranate peel extract can inhibit the AKT/mTOR signaling pathway by enhancing the expression level of IGFBP7, which can inhibit the HeLa cells in cervical cancer.
MeSH Keywords: Ellagic Acid, HeLa Cells, Nephroblastoma Overexpressed Protein, Oncogene Protein v-akt
Background
Pomegranates have edible, medicinal, and health-related properties, and are commonly grown in some regions of China. Cancer, as well as cardiovascular and inflammatory diseases, can be prevented and treated with pomegranate extract by virtue of its abundant and diverse polyphenols, including ellagic acid, which has antibacterial, antifungal, antiviral, convergence hemostatic, anti-inflammatory, antioxidant, and anti-tumor properties [1–4]. Recent studies have shown that polyphenols can inhibit tumor invasion and infiltration [5,6]. To date, the mechanism by which tannic acid acts on cervical cancer cells has not been explained in detail. In view of this, the present study investigated the effects of different concentrations of the acid on the stimulation of cervical cancer HeLa cells.
Material and Methods
Cell line
HeLa cells were purchased from the Chinese Academy of Sciences Shanghai Cell Bank.
Experimental drugs and related reagents
Ellagic acid was purchased from Dalian Meilun Biological Technology Co., Ltd., with a mass fraction of 99%. Fetal bovine serum, DMEM glucose medium, and trypsin were purchased from the HyClone Company (USA). The DNA purification kit and Plasmid Extraction kit were purchased from Sigma Company (Germany). The Effectene transfection kit was purchased from Qiagen Company (USA), and the rabbit anti-human IGFBP7 was purchased from Abcam Corporation (UK). The RT-PCR kit and cDNA reverse transcription kit was purchased from Japan Toyobo Corporation (Japan).
Experimental method
HeLa cells were divided into 6 groups: the control group (NC), the blank control group (BL), the IGFBP7 overexpression group (IGFBP7), and the 2.5 uM, 5 uM, and 10.0 uM ellagic acid-treated groups.
HeLa cells of all groups were treated with DMEM high-glucose medium and the BL group was transfected with empty plasmid into HeLa cells. The IGFBP7 group was transfected with IGFBP7 overexpression into HeLa cells. The ellagic acid-treated groups just used 2.5 uM, 5.0 uM, and 10.0 uM ellagic acid in DMEM. Each group was incubated at 5.0% CO2 and 37°C for 24 h with culture medium.
Cell invasion assay
We used a 50-ul Transwell chamber coated with 1 mg/ml of Matrigel incubated for 30 min at 37°C, according to the 1 x 105/ml concentration. In the next chamber, we added 600 ul of 10% of fetal calf serum DMEM high-glucose medium, incubated at 37°C and 5% CO2 for 48 h, fixed with 4% formaldehyde. We calculated the percentage of cells passing through the membrane.
MTT experiment
HeLa cells in logarithmic growth phase were seeded into 96-well plates and cultured for 24 h. The NC group, BL group, and IGFBP7-transfected group were cultured using MEM culture medium, and the treatment group used 2.5 ug/ul, 10.0 ug/ml, and 5.0 ug/ml tannic acid for 24 h. The cell apoptosis rate was detected.
Western blotting
Total proteins were extracted from cells of all groups. Protein concentrations were determined by BCA. The sample was separated by 50 SDS-PAGE with a concentration of 12%, which was transferred to the membrane. The membrane was closed and added for the night. We washed the membrane, added 2 antibodies, and incubated it at room temperature incubation for 1 h with TBST after washing the membrane. ImageJ software was used to measure the gray value of the strip.
RT-PCR
The expression levels of IGFBP7 and AKT/mTOR signaling pathway were detected in the 6 groups of cells by RT-PCR. GAPDH used as a reference index for PCR detection, and the primers were designed as follows:
IGFBP7: F: 5′-TGCCATGCATCCAATTCCCA-3′
R: 5′-TGGAGGTTTATAGCTCGGCA-3′
Akt: F: 5′-TGCATTGCCGAGTCCAGAA-3′
R: 5′-GCATCCGAGAAACAAAACATCA-3′
mTOR: F: 5′-ACAGCCCGTCACAATGCA-3′
R: 5′-GCTACCCGAATCAGCTCTTCA-3′
GAPDH: F: 5′-TGCACCACCAACTGCTTAGC-3′
R: 5′-GGCATGGACTGTGGTCATGAG-3′
Reaction conditions were set at: 95 min, 95 s, 30°C, 58°C, 30 s, 42 cycles, with the last cycle at 95°C for 10 min.
Statistical processing and analysis
Data are presented as the mean ± standard deviation (SD) values. Statistical analyses comparing the 2 groups were evaluated using one-way ANOVA with SPSS 19.0 (SPSS Inc., Chicago, IL). P<0.05 was considered statistically significant.
Results
Invasion of HeLa cells
The cell invasion in the 6 groups was measured by Matrigel membrane cell number counting. The invasion in the IGBP7 group and ellagic acid-treated groups (2.5 uM, 5.0 uM, and 10.0 uM) were significantly lower than in the BC and NC groups. There was a significant dose-effect relationship in the 3 ellagic acid-treated groups. The data are shown in Table 1.
Table 1.
Hela cell invasion of 6 groups (χ̄±s).
| Group | n | Cell transmission rate (%) |
|---|---|---|
| NC group | 5 | 97.56±6.43 |
| BL group | 5 | 95.41±5.44 |
| IGFBP7 group | 5 | 57.31±8.71*,# |
| 2.5 uM group | 5 | 76.43±7.59*,# |
| 5.0 uM group | 5 | 65.54±8.16*,#,** |
| 10.0 uM group | 5 | 56.44±8.11*,#,**,*** |
P<0.05, Compared with NC group;
P<0.05, Compared with BL group;
P<0.05, Compared with 2.5 uM group;
P<0.05, Compared with 5.0 uM group.
MTT test
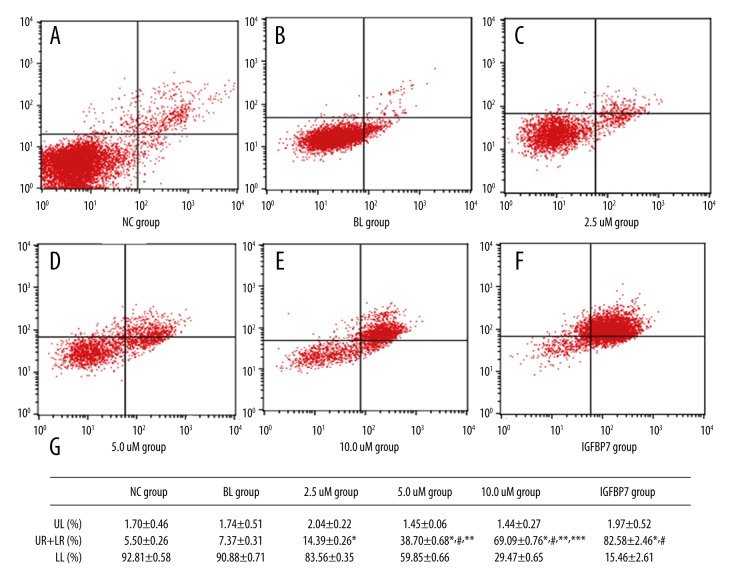
Compared with the NC and BL groups, the apoptosis rates of the 3 ellagic acid-treated groups were significantly increased (P<0.05) and there were significant dose-effect relationships in the ellagic acid-treated groups. The data are shown in Figure 1.
Figure 1.
The apoptosis in the 6 groups. (A) The apoptosis in the NC group. (B) The apoptosis in the BL group. (C) The apoptosis in the 2.5 uM group. (D) The apoptosis in the 5.0 uM group. (E) The apoptosis in the 10.0 uM group. (F) The apoptosis in the IGFBP7 group. (G) Differences in apoptosis among the 6 groups.
IGFBP7 gene and protein expressions in the 6 groups
IGFBP7 protein and mRNA expression of IGFBP7 and the 3 ellagic acid-treated groups were significantly higher than in the NC and BL groups (P<0.05). The data are shown in Figure 2.
Figure 2.
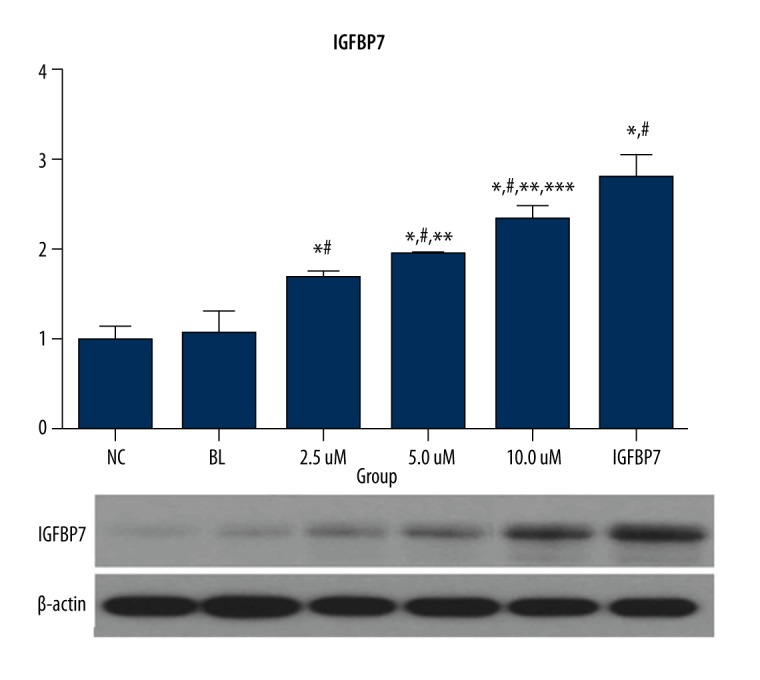
The gene and protein expression of IGFBP7 in the 6 groups (fold-change).
AKT gene and protein expressions in the 6 groups
AKT protein and mRNA expression of the IGFBP7 and ellagic acid-treated groups were significantly lower than in NC and BL groups (P<0.05). The data are shown in Figure 3.
Figure 3.
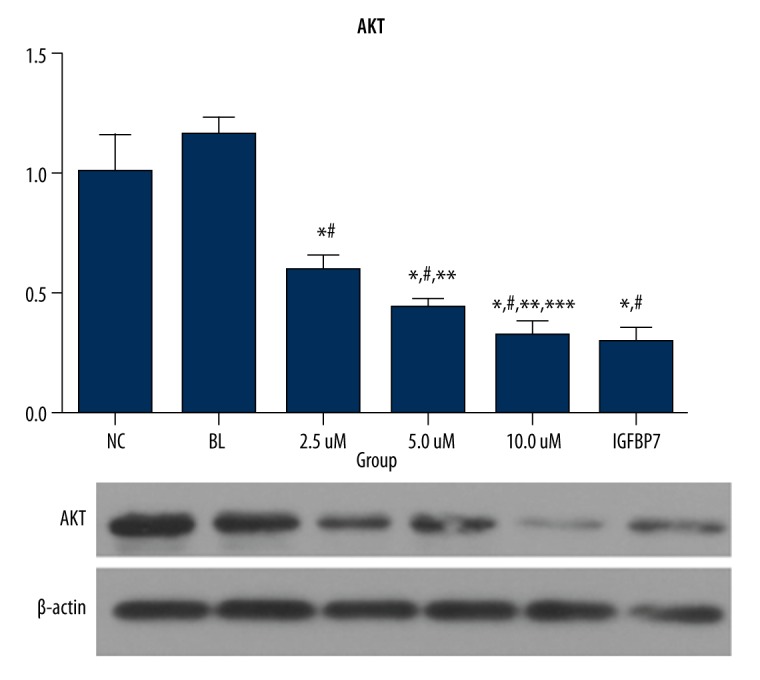
The gene and protein expression of AKT in the 6 groups (fold-change).
mTOR gene and protein expressions in the 6 groups
mTOR protein and mRNA expression of the IGFBP7 and ellagic acid-treated groups were significantly lower than in the NC and BL groups (P<0.05). The data are shown in Figure 4.
Figure 4.
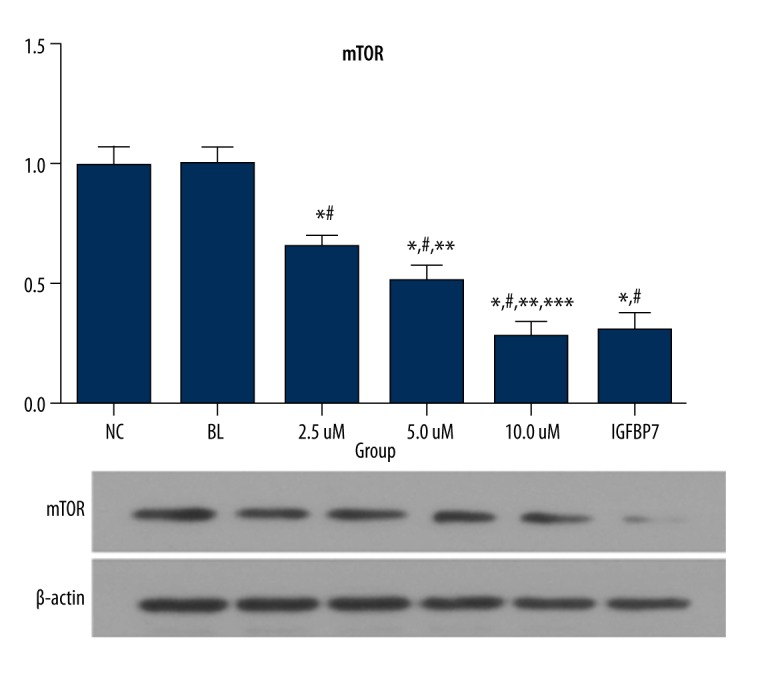
The gene and protein expression of mTOR in the 6 groups (fold-change).
Discussion
Cervical cancer is one of the most common gynecologic malignant tumors, and it is a serious threat to women’s health [7]. The cervical cancer mortality rate is high, mainly due to the high rates of growth, recurrence, and metastasis [8]. Therefore, the key problems to be solved are how to inhibit tumor growth, recurrence, and metastasis, reduce mortality, prolong the disease-free survival of patients, and improve the quality of life [9]. However, at present, most cancer treatment drugs cause serious adverse reactions and have limited efficacy. Therefore, the development of anti-cancer drugs that have low toxicity and high efficacy has become an important focus in research on cancer treatment. Traditional Chinese herbal medicines have anti-inflammatory and anti-tumor pharmacological activity, and the cervical cancer clinical drug treatment is effective [10].
Recent studies have found that polyphenols from natural sources have an important role in inhibiting the occurrence and development of tumor cells, but there are some differences in mechanism of action [11,12]. In the present study, we found that the use of different concentrations of ellagic acid affect the cervical cancer HeLa cells, and can stimulate the expression of IGFBP7 and thus inhibit the AKT/mTOR signaling pathway. We also found that it can inhibit the invasion of HeLa cells, and that there was a significant dose-effect relationship among the 3 different concentrations.
The results of this study show that the invasion of HeLa cells in the 3 ellagic acid-treated and the IGFBP7 group were significantly lower than in the NC and BL groups. The results suggest that the expression of HeLa and the expression of ellagic acid and the overexpression of IGFBP7 can significantly decrease the invasion ability of the cells, and the molecular mechanism of the results was further analyzed.
The AKT/motor signaling pathway plays a key role in cell proliferation, differentiation, apoptosis, and invasion, and it is an important signaling pathway in the occurrence and development of tumors [13,14]. In AKT and mTOR gene and protein expression level, the results of this study suggested that the expression level in the IGFBP7 group and the 3 ellagic acid-treated group was significantly lower than that of the NC and BL groups. The results confirmed that the decrease in HeLa cell invasion was due to the inhibition of the AKT/mTOR signaling pathway. Many studies had confirmed that the expression of IGFBP7 is closely related to the occurrence of many kinds of cancer [16–18]. Expression level and cervical cancer-specific correlation research is relatively limited, so in this study we transfected IGFBP7 into HeLa cells as the positive control group. The results confirmed that the gene expression levels of IGFBP7 in the 3 ellagic acid-treated groups and the IGFBP7 group were significantly increased, which shows that the effect of the inhibition of the AKT/mTOR signaling pathway was achieved by stimulating the IGFBP7 expression.
We also found that the effects of invasion and apoptosis of HeLa cells were different in different concentrations, and there was a significant dose-dependent relationship among the 3 concentrations. The present study has certain limitations. The concentrations of ellagic acid were relatively low due to consideration of the toxicity of ellagic acid to the cells. Whether 10 uM is the most effective concentration needs further study.
Conclusions
Ellagic acid, a polyphenol extracted from pomegranate peel, inhibits tumor proliferation by regulating IGBPF7 expression in a dose-dependent manner.
Footnotes
Source of support: Departmental sources
References
- 1.Damas J, Remade VG. Ther thrombopenic effect of ellagic acid in the rat, another model of platelet stimulation in vivo. Thromn Res. 1987;45:153–63. doi: 10.1016/0049-3848(87)90169-1. [DOI] [PubMed] [Google Scholar]
- 2.Takuo K, Ishida H, Yokota M, et al. Studies on antihemorrhagic substances in herbs classified as hemostatics in chinese medicine III on the antihemorrhagic principle in Sanguisorba officinallis L. Chem Pharm Bull (Tokyo) 1984;32(11):4478–81. doi: 10.1248/cpb.32.4478. [DOI] [PubMed] [Google Scholar]
- 3.Wu L, Jin L, Zhang W, et al. Roles of long non-coding RNA CCAT2 in cervical cancer cell growth and apoptosis. Med Sci Monit. 2016;22:875–79. doi: 10.12659/MSM.897754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin F, Pan L, Li L, et al. Effects of a simulated CO2 pneumoperitoneum environment on the proliferation, apoptosis, and metastasis of cervical cancer cells in vitro. Med Sci Monit. 2014;20:2497–503. doi: 10.12659/MSM.891179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudan S, Rupasinghe HF. Flavonoid-enriched apple fraction AF4 induces cell cycle arrest, DNA topoisomerase II inhibition, and apoptosis in human liver cancer HepG2 cells. Nutr Cancer. 2014;66(7):1237–46. doi: 10.1080/01635581.2014.951733. [DOI] [PubMed] [Google Scholar]
- 6.Lall RK, Syed DN, Adhami VM, et al. Dietary polyphenols in prevention and treatment of prostate cancer. Int J Mol Sci. 2015;16(2):3350–76. doi: 10.3390/ijms16023350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bazhenov AG, Guseĭnov KD, Khadzhimba AV, et al. Results of treatment for recurrent cancer of the uterine cervix. Vopr Onkol. 2009;55(3):319–26. [PubMed] [Google Scholar]
- 8.Yan H, Liu Z, Fu X, et al. Long-term outcomes of radical vaginal trachelectomy and laparoscopic pelvic lymphadenectomy after neoadjuvant chemotherapy for the IB1 cervical cancer: A series of 60 cases. Int J Surg. 2016;29:38–42. doi: 10.1016/j.ijsu.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Bach A, Bender-Sigel J, Schrenk D, et al. The antioxidant quercetin inhibits cellular proliferation via HIF-1-dependent induction of p21WAF. Antioxid Redox Signal. 2009;9(2):138–61. doi: 10.1089/ars.2009.3000. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Zhang W, Lv Q, et al. The critical role of quercetin in autophagy and apoptosis in HeLa cells. Tumour Biol. 2016;37(1):925–29. doi: 10.1007/s13277-015-3890-4. [DOI] [PubMed] [Google Scholar]
- 11.Fetoni AR, Paciello F, Mezzogon D, et al. Molecular targets for anti-cancer redox chemotherapy and cisplatin-induced ototoxicity: The role of curcumin on pSTAT3 and Nrf-2 signalling. Br J Cancer. 2015;113(10):1434–44. doi: 10.1038/bjc.2015.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigacci S, Micell C, Nediani C, et al. Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: A mechanistic insight. Oncotarget. 2015;6(34):35344–57. doi: 10.18632/oncotarget.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Q, Liang X, Dai J, et al. Prostaglandin transporter, SLCO2A1, mediates the invasion and apoptosis of lung cancer cells via PI3K/AKT/mTOR pathway. Int J Clin Exp Pathol. 2015;8(8):9175–81. [PMC free article] [PubMed] [Google Scholar]
- 14.Riquelme I, Tapia O, Sandoyal A, et al. miR-101-2, miR-125b-2 and miR-451a act as potential tumor suppressors in gastric cancer through regulation of the PI3K/AKT/mTOR pathway. Cell Oncol (Dordr) 2016;39(1):23–33. doi: 10.1007/s13402-015-0247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Hou Y, Yuan J, et al. Twist promotes reprogramming of glucose metabolism in breast cancer cells through PI3K/AKT and p53 signaling pathways. Oncotarget. 2015;6(28):25755–69. doi: 10.18632/oncotarget.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki H, Igarashi S, Nojima M, et al. IGFBP7 is a p53-responsive gene specifically silenced in colorectal cancer with CpG island methlator phenotype. Carcinogenesis. 2010;31(3):342–49. doi: 10.1093/carcin/bgp179. [DOI] [PubMed] [Google Scholar]
- 17.Vizioli MG, Sensi M, Miranda C, et al. IGFB7: An onco-suppressor gene in thyroid carcinogenesis. Oncogene. 2010;29(26):3835–44. doi: 10.1038/onc.2010.136. [DOI] [PubMed] [Google Scholar]
- 18.Kim EY, Kim A, Kim SK, et al. Inhibition of mTORC1 induces loss of E-cadherin through AKT/GSK-3β signaling-mediated up-regulation of E-cadherin repressor complexes in non-small cell lung cancer cells. Respir Res. 2014;15:26. doi: 10.1186/1465-9921-15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]