Abstract
Background:
Therapeutic approaches to addressing insufficient lactation are available but remain poorly understood. Current trends in maternal health, such as increasing rates of obesity, delayed age at childbearing, and high rates of cesarean section, may be associated with physiological challenges for lactation that cannot be managed by counseling alone. Women who have not had success with counseling alone, including adoptive mothers seeking to induce lactation, may use galactagogues (pharmaceutical and herbal compounds used to increase lactation). We present a review of selected studies of galactagogues and data indicating popular demand for such products.
Methods:
A systematic search was conducted for published studies on the use of galactagogues for breast-feeding. The following databases were searched: MEDLINE (PubMed), EBSCO (Academic Search Complete), and EMBASE. The search was conducted between July 15, 2015, and August 18, 2015; only English language articles were included, and we imposed no restrictions on publication date. Two authors independently reviewed the studies and extracted data.
Results:
Blinded, placebo-controlled clinical trials of 2 pharmaceutical galactagogues (domperidone and metoclopramide) and 5 popular herbal galactagogues (shatavari, fenugreek, silymarin, garlic, and malunggay) were identified. All of the studies identified for domperidone showed a significant difference in milk production between the treatment and placebo groups. Of the 6 trials of metoclopramide, only 1 study showed a significant difference in milk production compared to placebo. Results of the clinical trials on herbal galactagogues were mixed. Our review of the evidence for the efficacy of popular pharmaceutical and herbal galactagogues revealed a dearth of high-quality clinical trials and mixed results.
Conclusion:
Health providers face the challenge of prescribing or recommending galactagogues without the benefit of robust evidence. Given the suboptimal rates of exclusive breast-feeding worldwide and the availability and demand for medical and herbal lactation therapies, controlled trials and analyses investigating these medicines are urgently warranted.
Keywords: Breast feeding, galactogogues, health policy, lactation
INTRODUCTION
Breast-feeding newborns and infants provides optimal nutrition along with immune support and a host of lifelong health benefits to mother and child.1-3 Yet significant barriers to breast-feeding persist, and women, particularly those living in low-income settings, experience social, psychological, and physiological difficulties that impact breast-feeding.4,5 In the United States, for example, less than half of infants receive any breast milk at 6 months (49.4%), and approximately one-quarter are breast-fed at 1 year (26.7%).5
Professional lactation support should be the cornerstone of health-service support for postpartum mothers to achieve breast-feeding practices widely recognized as optimum for child health and development, including prompt initiation and exclusive breast-feeding during the first 6 months of life.3,6,7 Improving access to support and counseling is vitally important to improving breast-feeding rates. As of 2013, there were only 3.5 international board-certified lactation consultants and 3.8 certified lactation counselors per 1,000 live births in the United States.5 Counseling may not fully address physiological barriers to breast-feeding, such as primary lactation insufficiency.8,9
Delayed childbearing, high rates of cesarean section, stressful labor lasting >1 hour, and obesity can create physiological barriers to the establishment of lactation.3,10,11 For example, delayed onset of lactogenesis II, defined as the increase in lactation observed within 72 hours of delivery, was reported to affect 22%-31% of women according to studies that took place in California and Conneticut, respectively.10,11 Risk factors for delayed onset of lactogenesis II include primiparity, high body mass index, cesarean section, and stress during labor or prolonged labor.10,11 Other causes of low milk production may be generally related to mammary hypoplasia12,13 and use of analgesics during labor, as well as a family history of alcohol dependence and obesity, both of which can inhibit the prolactin response required for milk production immediately postpartum.14-17 When the onset of milk production is delayed, breast-feeding is more likely to be halted early, and newborns are at greater risk of excess neonatal weight loss,17-19 prompting supplementation with infant formula.
Women with insufficient milk production who do not respond to lactation counseling, as well as adoptive parents seeking to induce lactation, may pursue therapy with herbal and pharmaceutical galactagogues. Available information suggests that demand for pharmaceutical20,21 and herbal galactagogues22 is increasing, but guidance on their use is not widely available. The Academy of Breastfeeding Medicine currently cites insufficient evidence to recommend any specific pharmacologic or herbal galactagogues.23 The purpose of our review is to describe the current evidence on herbal and pharmaceutical galactagogues in light of the apparent demand for such products.
METHODS
Between July 15, 2015, and August 18, 2015, we searched the following databases for published studies of galactagogues used for breast-feeding and lactation: MEDLINE (PubMed), EBSCO (Academic Search Complete), and EMBASE. We did not restrict publication dates for inclusion. The search strategy consisted of the following terms solo and in combination: galactagogue, galactogogue, lactagogue, lactogogue, herbal, pharmaceutical, domperidone, metoclopramide, and lactation. Studies of herbal galactagogues were identified by searching the Latin or English name and lactation. The search terms for herbal galactagogues included milk thistle, Silybum marianum, fenugreek, Trigonella foenum-graecum, malunggay, Moringa oleifera, garlic, Allium sativum, shatavari, and Asparagus racemosus. Other popular galactagogues were searched: blessed thistle, Cnicus benedictus, goat's rue, Galega officinalis, fennel, and Foeniculum vulgare. The reference lists of peer-reviewed publications were also searched by hand.
Following review and consultation with clinician experts, we included studies of the pharmaceutical galactagogues most commonly prescribed (off-label in the United States) for the specific purpose of augmenting lactation: domperidone and metoclopramide. These drugs are also the most commonly studied in relation to lactation, although other pharmaceuticals, such as some antipsychotic medications,24 are also known to affect prolactin production and potentially to induce or augment lactation. Only articles in English were reviewed. Studies included were restricted to blinded, placebo-controlled, randomized trials on human subjects. Studies were only included if estimated milk production, infant weight gain, or time to relactation was an outcome of interest, as these were considered the most useful for clinical guidance and research strategy.
To develop inclusion criteria for herbal galactagogues, we consulted experts (lactation counselors) on the most commonly recommended or used products that are generally commercially available from the recorded sources of data described below. Herbal products that were ranked among the top 40 best-selling herbal dietary supplements by the American Botanical Council25 and were most commonly used for inducing lactation in population-based surveys26,27 were included. Inclusion criteria for review of studies describing herbal galactagogues were blinded, placebo-controlled trials, with treatment and control groups comparable at baseline, either through randomization or assignment, using only human subjects. Studies were only included if estimated milk production, infant weight gain, serum prolactin levels, or time to relactation was an outcome of interest, as these were considered the most useful for clinical guidance and research strategy.
We conducted a Google Trends analysis to ascertain the apparent consumer demand for products to increase breast milk production among users of the Google search engine. Google Trends is a freely available, publicly accessible application of Google Inc. (available at https://www.google.com/trends) that provides data based on Google searches to show how often a particular search term is entered relative to the total search volume across various regions of the world and in various languages. Google Trends only analyzes data for popular terms, so search terms with low volume appear as 0, and it eliminates repeated searches from the same internet protocol address that are performed in a short period of time. Google Trends adjusts search data to facilitate comparisons between terms. Without this feature, geographic regions with the most search volume would always be ranked highest. Each data point is divided by the total searches of the geography and time range it represents to compare the relative popularity. The resulting numbers are presented in a scale range of 0-100. We conducted our analysis on August 18, 2015, using the search term increase breast milk. Other terms analyzed were galactagogue and increase lactation, but we excluded these results because of insufficient search volume and clarity of trend results.
RESULTS
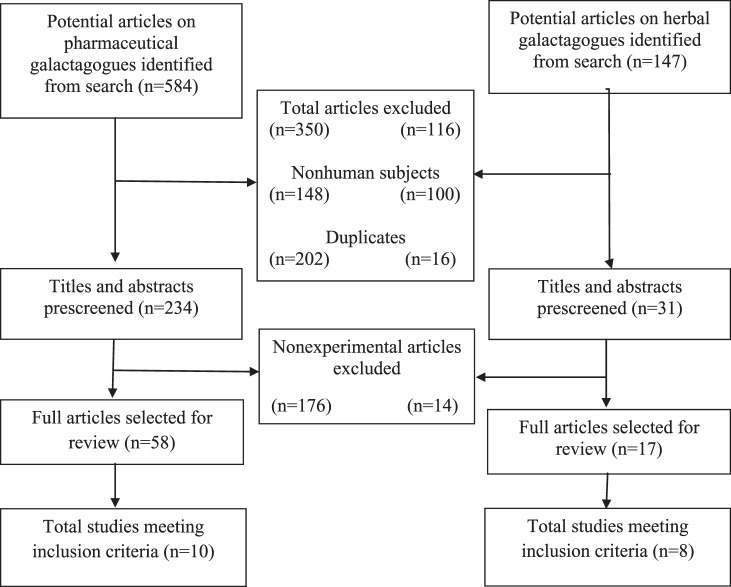
A total of 731 articles (n=584 on pharmaceutical galactagogues and n=147 on herbal galactagogues) were identified in the initial search, outlined in Figure 1. A total of 466 articles were excluded (n=350 pharmaceutical and n=116 herbal) because of use of nonhuman subjects (n=148 and n=100, respectively) and duplication (n=202 and n=16, respectively). The titles and abstracts of 265 articles (n=234 and n=31, respectively) were prescreened. Reviews, case studies, and other nonexperimental studies were then excluded (n=176 and n=14, respectively), and the full text of the remaining 75 studies was retrieved for review. A total of 18 studies met the inclusion criteria (n=10 and n=8, respectively).
Figure 1.
Flow chart of selection of studies.
Pharmaceutical Galactagogues
Domperidone
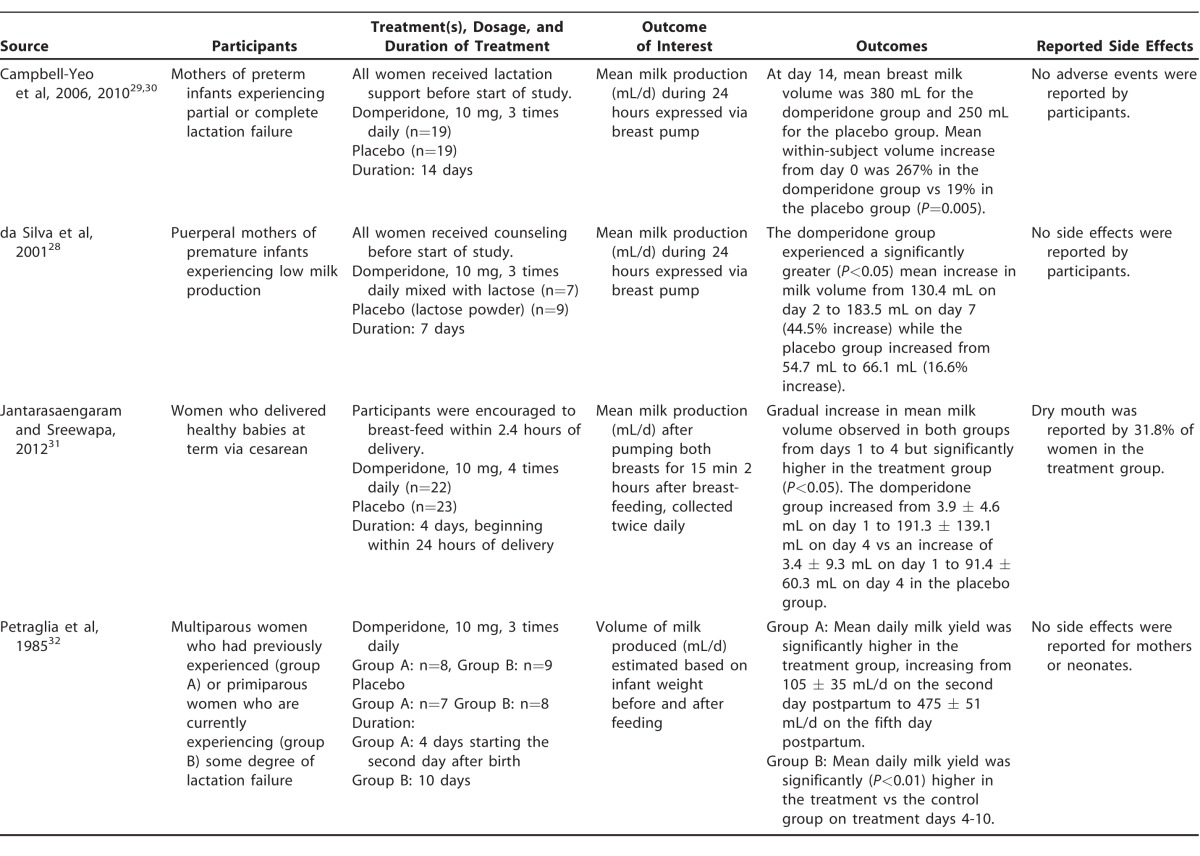
Table 1 summarizes the 4 studies of domperidone that we identified (Campbell-Yeo et al published their protocol separately).28-32 Several differences were noted in terms of method of breast milk collection, criteria for participation, counseling/support provided to mothers, and duration of treatment. Two studies28-30 analyzed the volume of breast milk expressed via a mechanical pump during a period of 24 hours, while in another study,31 mothers collected milk via mechanical pump from each breast for a period of 15 minutes twice daily, 2 hours after they breast-fed their infant. Another study32 estimated milk volume by test-weighing infants before and after a feeding. Two of the studies31,32 included participants who delivered at term: the former included infants delivered via cesarean section whose mothers showed no evidence of insufficient lactation, and the latter included infants delivered normally whose mothers had experienced complete or partial lactation failure. Two studies included participants who delivered prematurely and were experiencing insufficient lactation.28-30 In the same 2 studies, counseling on breast-feeding practices was reported to have been provided to all participants regardless of assignment before participants entered the study,28-30 and participants of another study received encouragement to breast-feed within 24 hours of delivery.31 One study began treatment on the second day postpartum.32 A high attrition rate among participants in the treatment group of 1 study was noted.28 All studies sufficiently described the randomization process except for Petraglia et al, in which participants were randomly selected, but the method of allocation into study arms was not well described.32 The total number of participants in all studies analyzed was 131.
Table 1.
Summary of Selected Studies on Domperidone
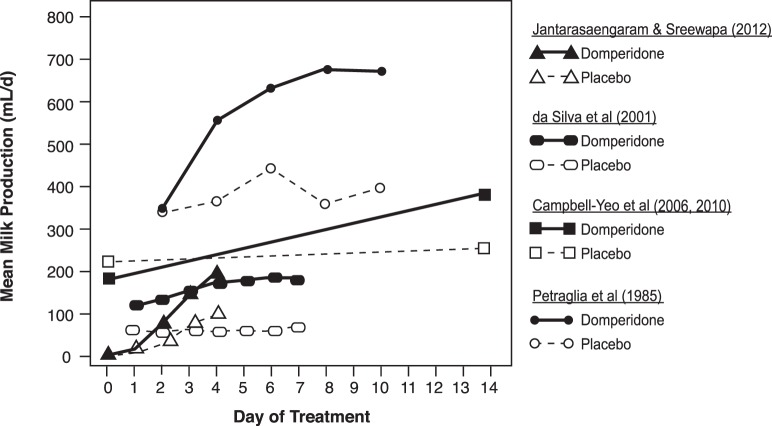
Despite the differences in methodology, the results of all included studies indicate significantly more milk output in mothers treated with 10 mg of domperidone 3 times per day28-30,32 or 4 times per day31 for 4-14 days compared to those given a placebo. Figure 2 shows mean milk production by day of treatment for participants given domperidone vs placebo. Although the collection protocols were different, and thus the results are not directly comparable, an overall trend of higher milk production among participants treated with domperidone vs placebo is apparent, and the mean volume of milk produced appears to continue to increase with the number of days of treatment. The difference in mean milk volume between treatment vs placebo groups was statistically significant within each study at endpoint. A mild side effect (dry mouth) was reported by participants in 1 study.31
Figure 2.
Mean breast milk production results in select randomized controlled trials of domperidone and placebo.28-32
Metoclopramide
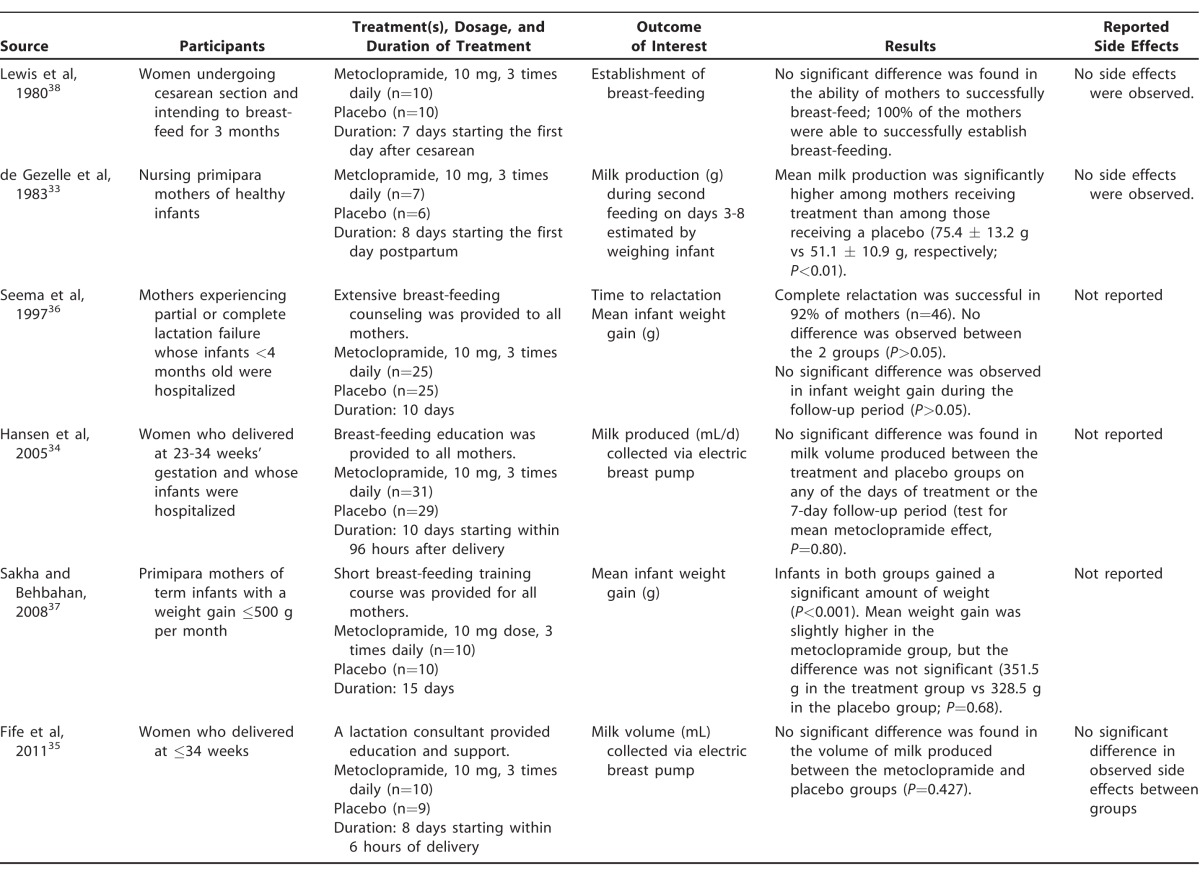
Table 2 summarizes the 6 randomized, double-blind, placebo-controlled trials of metoclopramide we identified, each using 10 mg 3 times daily for a period of 7-15 days.33-38 Study methodology varied and recruitment criteria were not uniform. Participants in each study varied: studies included mothers of healthy infants not experiencing insufficient lactation,33 mothers described as not experiencing any lactation failure,34,35 mothers of hospitalized infants experiencing partial or complete lactation failure,36 mothers of infants who were failing to gain weight (used as a proxy for lactation failure),37 and mothers who had elective or emergency cesarean section.38 Outcomes of interest also varied: 2 studies measured volume of milk produced in mL,34,35 1 estimated milk production using infant weight gain by the difference in weight before and after feeding,33 and 2 measured mean infant weight gain in grams.36,37 In addition to mean infant weight gain, 1 study also evaluated time to relactation,36 and 1 study only assessed the ability to successfully establish breast-feeding.38 Lactation counseling was reportedly provided to all mothers in 4 of the studies at the initiation of the study.34-37 The total number of participants in all studies analyzed was 182.
Table 2.
Summary of Selected Studies on Metoclopramide
Of the 6 studies reviewed, 5 showed no significant difference in volume of milk production, mean infant weight gain, or time to relactation between the 2 study groups.34-38 The 2 studies that looked at ability to successfully establish lactation or relactation found that the majority of mothers in both the treatment and control groups were able to do so.36,38 A single study with a small sample size33 found that infants of mothers treated with metoclopramide consumed significantly more milk than infants of mothers treated with a placebo. Side effects were either not reported by authors,34,36,37 not reported by participants,33,38 or, when reported, were not significantly different between treatment and control groups.35
Herbal Galactagogues
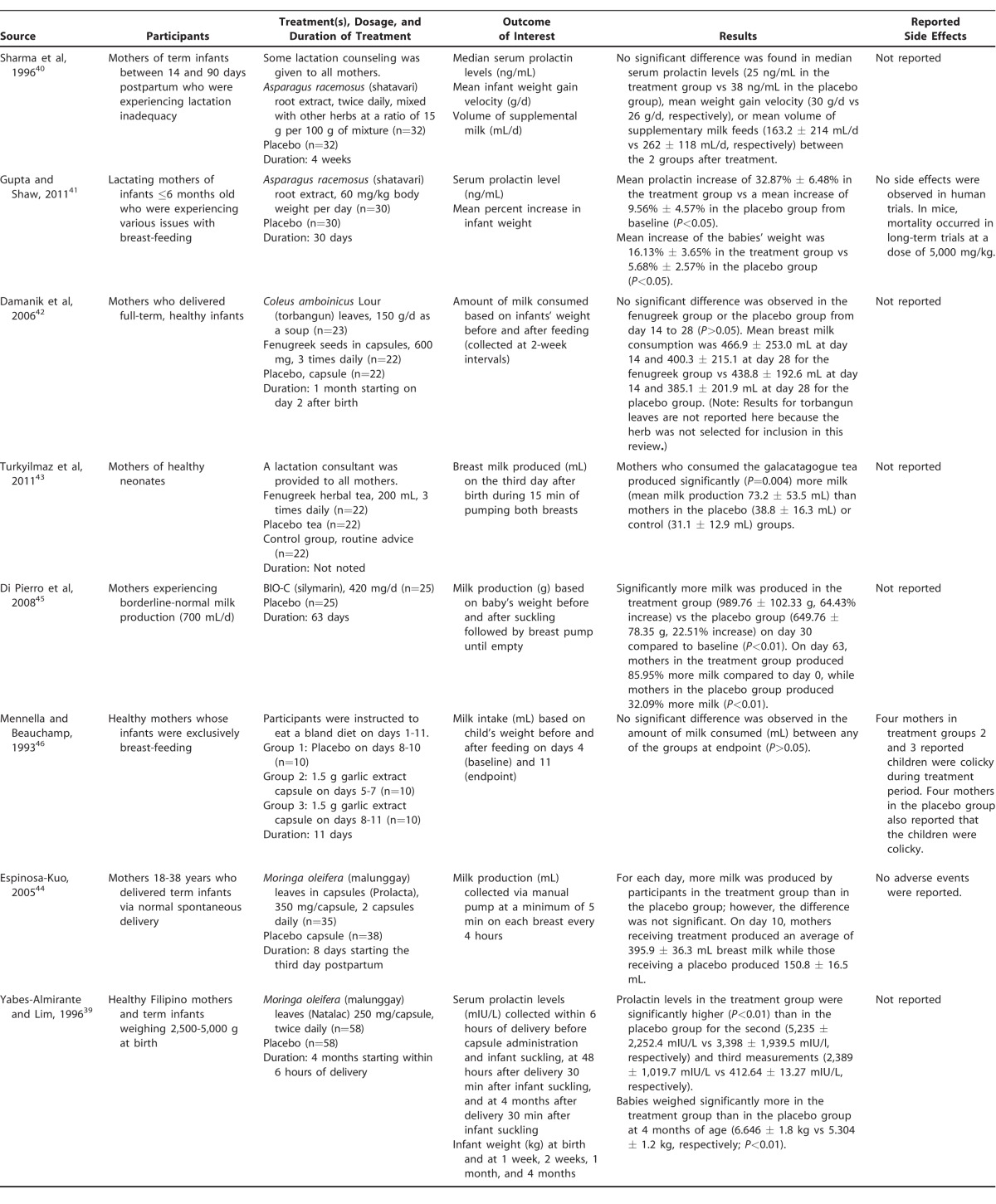
Table 3 summarizes the identified studies of common herbal galactagogues.39-46 Eight blinded, placebo-controlled trials of herbal galactagogues were identified; 2 for Trigonella foenumgraecum (fenugreek),42,43 2 for Asparagus racemosus (shatavari),40,41 1 for a micronized form of silymarin45 (a standardized extract of Silybum marianum [milk thistle]), 1 for Allium sativum (garlic),46 and 2 for Moringa oleifera (malunggay).39,44 Results for torbangun leaves in the Damarik et al study42 are not reported here because the substance is not an outcome of interest in our review. The total number of participants in all studies was 526 including the group given torbangun42 and 503 excluding this group. Although other herbs were identified as commonly used to induce or augment lactation, such as Cnicus benedictus (blessed thistle), Galega officinalis (goat's rue), and Foeniculum vulgare (fennel), no peer-reviewed publications on these herbal preparations were identified that met inclusion criteria.
Table 3.
Summary of Selected Studies on Herbal Galactagogues
Five of the studies were double-blind, randomized, placebo-controlled trials,39-43 1 study was a single-blind, randomized, placebo-controlled trial,44 1 study was double-blind and placebo-controlled but not randomized,45 and 1 study was single-blind and placebo-controlled with randomization not reported by the authors,46 although characteristics of the treatment and control groups were not statistically different at baseline in the latter 2 studies. Three studies recruited women who were specifically identified as experiencing inadequate lactation or borderline-normal milk production,40,41,45 4 studies recruited mothers who delivered at term and began treatment soon after delivery,39,42-44 and 1 study recruited mothers who were exclusively breast-feeding their infants.46 The herbal galactagogues were either prepared as a tea or given as a capsule, and the form of the placebo matched that of the treatment in each study. No information was provided on methodology to mask the taste and/or smell of the herbal galacatagogues (which may result in unblinding of participants).
Milk production (mL or g) was estimated in 5 of the studies, 3 of which used infant weight gain after feeding as a proxy,42,45,46 while the other 2 measured the volume of milk produced pumping each breast.43,44 Three studies measured serum prolactin and increase in infant weight.39-41
Of the 2 studies of shatavari root extract,40,41 only the study by Gupta and Shaw41 reported a significant effect on serum prolactin levels and infant weight gain. Both studies provided the supplement for a similar amount of time; however, in the study by Sharma et al,40 the shatavari root extract was mixed with other herbs at a ratio of 15 g per 100 g of mixture, and participants consumed 2 teaspoons twice daily for 4 weeks. Participants in the Gupta and Shaw study reporting an effect consumed 60 mg/kg body weight of unmixed shatavari root daily for 30 days.41 Both studies had a similarly small sample size (32 and 30 in the treatment groups, respectively). All participants in the study by Sharma et al in which no effect was seen40 were reportedly provided with lactation counseling, while Gupta and Shaw41 did not report lactation support. Human side effects were not reported by either study; however, Gupta and Shaw tested high doses in mice and saw evidence of mortality at a dose of 5,000 mg/kg body weight.41
Two studies were identified that explored the effects of fenugreek on breast milk production, both of which recruited mothers of neonates who delivered at term.42,43 The studies had similar sample sizes, but the methodology differed significantly. Turkyilmaz et al43 provided the fenugreek as an herbal tea (200 mL, 3 times daily) to group 1, an apple tea as a placebo to group 2, and no supplement to group 3; participants in all 3 groups received support from a lactation consultant. Breast milk was collected on the third day after delivery via an electronic breast pump to estimate volume (mL). Damanik et al42 employed a 3-armed design including fenugreek seeds in capsule form (600 mg, 3 times daily) in 1 arm, torbangun leaves as a soup in another arm (torbangun is not a galactagogue of interest for this review, so the results of this arm are not discussed), and a placebo in capsule form in another arm. Lactation support was not reported. Milk consumed was estimated by weighing the infants before and after feeding at 2-week intervals. Turkyilmaz et al43 reported that mothers who consumed the fenugreek tea produced significantly more milk than mothers in the other 2 groups (P=0.004), while Damarik et al42 reported no difference in the amount of milk consumed from mothers in the fenugreek capsule group vs the placebo group.
The single study evaluating the efficacy of micronized silymarin included mothers experiencing borderline-normal milk production (approximately 700 mL/d) and assigned them to a placebo or treatment group to receive 420 mg/d.45 Mothers taking silymarin produced significantly (P<0.01) more milk than the mothers taking a placebo on day 30 of treatment (64.43% increase from baseline vs 22.51% increase, respectively) and on day 63 of treatment (85.95% increase from baseline vs 32.09% increase, respectively).
The single study evaluating the efficacy of garlic as a galacatagogue recruited healthy mothers of exclusively breast-feeding infants.46 Milk intake was estimated based on the child's weight gain after feeding in 3 groups: a placebo group, a group receiving garlic on days 5-7, and a group receiving garlic on days 8-11 of treatment. The different timing of treatment with garlic was used to investigate whether recent exposure had an impact on infant milk intake. No significant difference was detected between any of the groups at the study's end (P>0.05).
Two studies investigated the use of capsules of malunggay leaves as a galacatagogue.39,44 The study by Espinosa-Kuo was blinded only to the participant and utilized capsules of 350 mg given twice daily for 8 days starting the third day postpartum and measured milk output (mL) from a manual pump.44 Yabes-Almirante and Lim provided mothers with 250-mg capsules twice daily for 4 months beginning within 6 hours of birth and measured serum prolactin levels (mIU/L) at 3 timepoints: within 6 hours of delivery before capsule administration and the first feeding, at 48 hours after delivery 30 min after infant suckling, and at 4 months postpartum 30 min after suckling. Yabes-Almirante and Lim also measured infant weight (kg) immediately after birth, at 1 week, 2 weeks, 1 month, and 4 months of age.39 Espinosa-Kuo described greater milk production in the group given malunggay capsules on each day of treatment.44 At day 3 postpartum, mean breast milk production was 96.4 ± 14.3 mL among women in the treatment group and 78.6 ± 9.8 mL among women in the control group. On day 10 postpartum, the mean volume of milk production among women in the treatment group was 395.9 ± 36.3 mL, while women in the control group produced an average of 150.8 ± 16.5 mL. No significant difference between treatment and control groups was detected on any day; however, exact P values were not provided for any time point.
DISCUSSION
Pharmaceutical Galactagogues
The results of this review suggest that domperidone may be efficacious in enhancing milk production in mothers experiencing some degree of lactation failure,28-30,32 or with less compelling evidence, in mothers who delivered via cesarean section.31 Only 2 domperidone studies28-30 provided breast-feeding counseling to all participating mothers before the start of the study. These studies28-30 were described as having low risk of bias in a metaanalysis published in 2012.47 In the remaining 2 studies included in this analysis, domperidone was administered within 2 days postpartum without provision of lactation counseling to resolve underlying issues.31,32
Systematic reviews of domperidone also indicate that the dopamine antagonist has shown effectiveness in increasing milk supply, although some controversy exists in the literature on the efficacy and safety of domperidone as a galactagogue.47,48 Domperidone has been extensively studied in clinical trials for gastric motility49 and has specifically been studied in infants as a treatment for gastroesophageal reflux disease.50-53 Previous research suggested that domperidone could increase the risk of sudden cardiac death or could be linked with increased risk of prolonged QT syndrome and arrhythmia;54 however, sudden death resulting from prolongation of QT intervals has never been reported with oral doses.49 A Health Canada advisory notes that sudden cardiac death is only associated with domperidone use in women >60 years and that these risks likely do not apply to women of childbearing age without heart conditions; therefore, extensive history should be considered before administration of domperidone.55
Although domperidone has been available worldwide since 1978, the US Food and Drug Administration (FDA) has never approved its use for any indication. In 2004, the FDA issued an alert, citing concerns over its use by breast-feeding mothers and instituted a block of any personal importation of domperidone.56 The alert cited cardiac side effects from the intravenous formulation of domperidone (without specific reference to published literature), although the intravenous formulation was removed from the market in the early 1990s and had been used almost exclusively for patients receiving chemotherapy.57 Domperidone does not appear to cross the blood-brain barrier as easily as metoclopramide, and very little of the drug appears to transfer into breast milk; a relative infant dose of approximately 0.012% at a 30-mg maternal intake daily and 0.009% at 60 mg daily is estimated.58 Additionally, domperidone does not appear to have adverse effects or cause tardive dyskinesia—a condition involving repetitive and involuntary body movements—when taken at such doses.9 Mild to moderate side effects include dry mouth, headache, and abdominal cramping, and one study suggests that these may be more prevalent as the dose of domperidone increases, although milk production may not be significantly greater at higher doses (60 vs 30 mg/d).58
Metoclopramide, another dopamine antagonist, is one of the only prescription medications widely accepted to impact milk supply and may also be prescribed off-label by health providers in the United States. Despite its popularity as a galactagogue, evidence from randomized, placebo-controlled clinical trials appears insufficient. The current review included clinical trials that collected baseline data and used a placebo group. Of the 6 identified studies on metoclopramide, 5 failed to show a significant difference in milk production or time to relactation between treatment groups.
Little research on metoclopramide as a galactagogue is available, despite the potentially serious and well-known side effects and off-label prescribing.9,24,59-62 Depression and tardive dyskinesia, along with cardiac arrest and severe neuropsychiatric side effects, have been documented following the use of metoclopramide, even in cases of short-term exposure in otherwise healthy individuals.63-67
Mild to moderate side effects of metoclopramide are more prevalent than those of domperidone in women using the drugs to increase lactation. A study comparing domperidone with metoclopramide for breast milk production found that 15 women reported side effects from metoclopramide, whereas 3 reported side effects from domperidone.68 Additionally, metoclopramide is known to cross the blood-brain barrier more easily and is secreted into breast milk at higher levels than domperidone.69 The number of women taking metoclopramide off-label to improve milk supply is unknown, and it is imperative that further research be conducted to establish therapeutic benefit and potential risks.
The studies included in this review suggest that domperidone may be more effective in increasing breast milk production than metoclopramide. While clinicians should continue to provide exhaustive counseling before the use of pharmaceutical galactagogues, many women experiencing difficulties with lactation are at a great risk of not breast-feeding and are mothers for whom other interventions have proven to be least effective.70 More evidence from high-quality studies is needed to determine effectiveness and safety of domperidone as a galacatagogue to increase confidence among clinicians when recommending a galactagogue if a mother wishes to induce lactation. Domperidone has been granted orphan drug status in the United States, obtained by Dr Thomas Hale, and more data will be available once approval for clinical trials is granted.10 A randomized controlled trial of the use of domperidone for increasing breast milk among mothers of preterm infants was completed in March 2016 in Canada and is a rare example of a pharmaceutical (or herbal therapy) being rigorously studied for lactation.71 Results are pending.
Herbal Galactagogues
Evidence from the studies of herbal preparations to increase lactation was mixed, as shown in Table 3. Only 3 of the popular herbs—shatavari, fenugreek, and malunggay—had more than 1 study identified for this review, and the results were statistically significant in only 1 of the studies for each herb. Additionally, inconsistencies in methodology, participant recruitment, dosage, and outcomes of interest make interpretation of evidence difficult.
Participants in the 2 studies on shatavari root extract had similar characteristics: infants were born at term, and mothers were experiencing some degree of lactation failure.40,41 However, lactation counseling was only reported in the study that failed to show a significant difference between groups, and the shatavari root extract was given with a combination of other herbs to the treatment group, making it impossible to isolate the effect of the herb under investigation.40 Information on lactation counseling was not reported in the other study on shatavari root extract.41 More evidence is needed to determine the utility of shatavari root extract for lactation. Future studies must isolate the herb under study and provide comparable access to lactation counseling to attribute significant differences in milk production to that herb. Based on rat models, shatavari root may increase lactation via an estrogenic effect on the mammary glands and increased prolactin production.72 While fatalities occurred in mice given long-term high doses (5,000 mg/kg body weight) of shatavari root extract,41 potential side effects in humans at moderate doses have not been documented.
Regarding fenugreek seed, a popular and widely available herb, mothers of healthy neonates treated with a fenugreek tea 3 times daily produced significantly more breast milk than mothers in the placebo group,43 while in another study, mothers of full-term neonates who were treated with 600-mg capsules of fenugreek seeds 3 times daily for 1 month did not show a significant difference in milk production compared with the placebo group.42 The proposed mechanism for the augmentation of breast milk through fenugreek supplementation is by its reported effect on the stimulation of sweat production (the mammary gland is a modified sweat gland).72 Many products available for purchase commercially contain fenugreek (ie, More Milk [Motherlove Herbal Company], fenugreek seeds [Nature's Way Products, LLC]); however, current evidence regarding the efficacy of fenugreek in increasing milk production is insufficient. Fenugreek was the herb most commonly used by women surveyed in Australia: 56% reported its use, 98.2% of whom used it to increase breast milk supply.22 Of lactation consultants surveyed, 15% in Switzerland and 99% in Canada reported that they had used fenugreek to increase lactation.27 Additionally, sales of fenugreek increased 137.7% in the United States between 2012 and 2013.25 Adverse events were not reported in the studies identified; however, fenugreek may cause side effects such as nausea, vomiting, and decreased glucose levels in the mother and diarrhea in the child.73
Healthy mothers who delivered infants at term were recruited in the 2 studies that investigated the efficacy of malunggay leaves; however, 1 study measured breast milk production (mL) after pumping for a minimum of 5 min for each breast,44 while the other measured serum prolactin and infant weight gain.39 Although more milk was produced by mothers receiving treatment than those receiving a placebo (mean milk production was 395.9 ± 36.3 mL in the treatment group compared to 150.8 ± 16.5 mL in the control group on the last day of treatment), the former study failed to show a significant difference between groups.44 In the latter study, women in the treatment group had significantly higher levels of prolactin in the 48 hours after delivery than women in the placebo group, and babies in the treatment group weighed significantly more than those in the placebo group at 4 months.39 These results merit more investigation, as malunggay leaves are commonly used among breast-feeding mothers in the Philippines to augment breast milk production, and a pooled analysis of 6 studies on the efficacy of Moringa oleifera (some of which were not identified for this review because of inclusion criteria) demonstrated a significant increase in milk produced 4-7 days after treatment.74 The change in serum prolactin levels was measured in 1 of the studies of malunggay leaves, and prolactin was significantly higher in the treatment group compared to the placebo group.39 In a 2015 study by Stohs and Hartman, no adverse events were reported in single doses up to 50 g or when a dose of 8 g/d was sustained for 40 days in human models or at doses up to 2,000 mg/kg body weight in rats.75
Of the studies identified, only one focused on silymarin. Di Pierro et al examined the effect of micronized silymarin on breast milk production in women experiencing borderline-normal milk production (700 mL/d).45 Silymarin comprises 65%-80% of milk thistle extract.76 Women in the treatment group produced 64.43% more milk than those in the placebo group, suggesting potential efficacy, although more evidence is needed to support this possibility.45 Milk thistle may cause an allergic reaction and have a laxative effect.73
No significant difference was found between treatment and placebo groups in a study on the efficacy of garlic in increasing breast milk production in healthy mothers who were exclusively breast-feeding their infants.46 A review of herbs used by mothers who are breast-feeding identified garlic as a galacatagogue and noted that the use of garlic may deter an infant from suckling if he or she finds the odor offensive.73
A 2012 review of pharmaceutical and herbal galactagogues surveyed the evidence for the pharmaceutical use of domperidone, metoclopramide, and oxytocin along with the efficacy of herbal galactagogues, including fenugreek, milk thistle, and shatavari.59 The review reached a similar conclusion to the current one, that domperidone may be efficacious after nonpharmacologic methods are exhausted, although safety information is limited and requires further investigation. The authors of that review did not draw any conclusions about the efficacy of herbal galactagogues because of limited evidence.
The most extensive compilation of evidence on herbals, including their use as galactagogues, is The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines; however, it is beyond the scope of our review.77
Popular Demand for Galactagogues
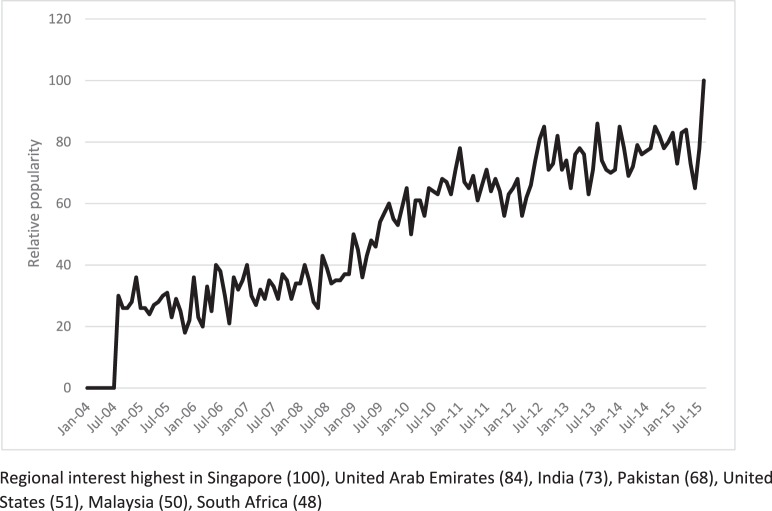
Herbal galactagogues are now widely available over the counter in the United States and other countries, and information is commonly sought on galactagogues and other means to increase or initiate lactation. Figure 3 presents an analysis of the relative popularity (number of searches at that time point divided by largest number of searches for that particular term in the time period) of Google searches for the term increase breast milk between January 1, 2004, and August 18, 2015, suggesting that the demand for information on galactagogues has been steadily increasing during the past decade. An internet search revealed >1.16 million distinct website references to breast-feeding and herbs and >100 herbal product descriptions containing the term lactation, breast milk, or milk for sale online to increase milk production (authors' search conducted August 11, 2015). The authors' search of the term increase breast milk at www.amazon.com on August 11, 2015, revealed that popular products (those with at least a 4-star rating and >100 reviews) frequently contained fenugreek, milk thistle, and goat's rue. Only herbs that have been studied in a scientific trial are included in this review.
Figure 3.
Google Trends analysis: relative popularity of the search term “increase breast milk” worldwide.
Exploratory work in Australia found that the majority of participants who reported using herbs for medicinal purposes during breast-feeding—approximately half of whom were using these supplements to augment breast milk production—believed there was a lack of informational resources regarding their use, and only 28.6% notified their doctors that they were using herbal supplements.22 Nearly three-quarters (71.6%) reported avoiding conventional medicines to address breast-feeding, citing concerns regarding safety to their breast-fed infants, and many expressed the desire for more evidence-based information from healthcare professionals regarding the use of herbal medications during breast-feeding.22,26 Demand for herbal galactagogues appears to be high; however, herbal products, including teas, tinctures, and capsules may not be regulated by government bodies and are not required to be tested in humans prior to sale. In the United States, the FDA does not analyze the content of dietary herbal supplements before they are marketed. While the manufacturer is prohibited from making false or misleading claims on the packaging, a disclaimer is often used to clear culpability, and action is not taken until after the product reaches market.78 Various herbal medicines that have very low amounts of active ingredients, as well as those that have resulted in adverse effects, have emerged during the past decade.79
Despite the dearth of evidence supporting the safety and effectiveness of herbal galactagogues, 69% of surveyed certified US lactation consultants reported that they had heard of herbal remedies for lactation, and 65% of those said that they recommended one or more of these methods to mothers.80 Adverse effects of herbal galactagogues are not well understood in human subjects; however, high dosages of some herbal galactagogues are often purportedly required to stimulate milk production. One of the most commonly used herbs, fenugreek, may be potentially taken in high doses, as women are often advised to use doses to “smell like maple syrup.”81 More high-quality evidence is needed to support the recommendation of herbal galactagogues by clinicians and lactation consultants. No published reports were identified of protocols for trials underway to assess the impact of common herbal galactagogues on lactation or breast-feeding.
STRENGTHS AND LIMITATIONS
Strengths of our review include a comprehensive search strategy with the use of multiple databases, well-defined inclusion and exclusion criteria to identify high-quality studies, and the addition of the Google Trends analysis to provide insight on consumer demand related to the use of galactagogues. Limitations of the study include review of only English language articles, which may have excluded relevant studies in other languages, and use of only large biomedical databases, which may have excluded studies not abstracted in those databases but pertinent to the topic. Additionally, a complete and thorough analysis of the quality of the studies included was beyond the scope of this review. We were also unable to provide an exhaustive review of herbs commonly used, but not commercially available, which may have been studied in other databases than those we searched.
CONCLUSION
Regarding pharmaceutical galactagogues, not enough information is available for clinicians and patients to make informed decisions on their use. Clinicians face complex decisions in counseling women experiencing difficulties with lactation or those who want to induce lactation. Considering the apparent demand for galactagogues and the low rates of sustained breast-feeding worldwide, this lack of reliable information is an important issue because clinicians and families may not be able to make safe choices when confronted with difficulties related to lactation. More information from well-controlled trials is needed to guide appropriate use of pharmaceutical and herbal galactagogues.
Regarding herbal galactagogues, the evidence is also inadequate to guide clinical recommendations. Future studies must employ robust study designs, recruit participants experiencing lactation failure, and provide lactation counseling to all participants. Evidence from future studies should also be used to inform policies on safety that can be enforced by government bodies, particularly for herbal supplements, as well as clear and informative standardized labeling to guide women in using supplements during lactation.
ACKNOWLEDGMENTS
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1. Horta BL, Victora CG. Long-Term Effects of Breastfeeding: A Systematic Review. Geneva, Switzerland: World Health Organization; 2013. http://apps.who.int/iris/bitstream/10665/79198/1/9789241505307_eng.pdf?ua=1. Accessed August 11, 2015. [Google Scholar]
- 2. Horta BL, Victora CG. Short-Term Effects of Breastfeeding: A Systematic Review on the Benefits of Breastfeeding on Diarrhoea and Pneumonia Mortality. Geneva, Switzerland: World Health Organization; 2013. http://apps.who.int/iris/bitstream/10665/95585/1/9789241506120_eng.pdf?ua=1. Accessed August 11, 2015. [Google Scholar]
- 3. World Health Organization. Protecting, Promoting and Supporting Breast-feeding: The Special Role of Maternity Services. A Joint WHO/UNICEF Statement. Geneva, Switzerland: World Health Organization; 1989. [Google Scholar]
- 4. American College of Obstetrics and Gynecology. Breastfeeding in Underserved Women: Increasing Initiation and Continuation of Breastfeeding. Committee Opinion No 570. Washington, DC: American College of Obstetrics and Gynecology; 2013. [DOI] [PubMed] [Google Scholar]
- 5. National Center for Chronic Disease Prevention and Health Promotion. Breastfeeding Report Card: United States/2014. Atlanta, GA: Centers for Disease Control and Prevention; 2014. http://www.cdc.gov/breastfeeding/pdf/2014breastfeedingreportcard.pdf. Accessed August 11, 2015. [Google Scholar]
- 6. World Health Organization. Essential Nutrition Actions: Improving Maternal, Newborn, Infant and Young Child Health and Nutrition. Geneva, Switzerland: World Health Organization; 2013. http://apps.who.int/iris/bitstream/10665/84409/1/9789241505550_eng.pdf. Accessed August 11, 2015. [PubMed] [Google Scholar]
- 7. Division of Child Health and Development. Evidence for the Ten Steps to Successful Breastfeeding. Geneva, Switzerland: World Health Organization; 1998. http://apps.who.int/iris/bitstream/10665/43633/1/9241591544_eng.pdf. Accessed August 11, 2015. [Google Scholar]
- 8. Neifert MR. Prevention of breastfeeding tragedies. Pediatr Clin North Am. 2001. April; 48 2: 273- 297. [DOI] [PubMed] [Google Scholar]
- 9. Nice FJ. Selection and use of galactogogues. Infant Child Adoles Nutr. 2015. August; 7: 192- 194. [Google Scholar]
- 10. Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003. September; 112 3 Pt 1: 607- 619. [DOI] [PubMed] [Google Scholar]
- 11. Chapman DJ, Perez-Escamilla R. Identification of risk factors for delayed onset of lactation. J Am Diet Assoc. 1999. April; 99 4: 450- 454 ; quiz 455-456. [DOI] [PubMed] [Google Scholar]
- 12. Arbour MW, Kessler JL. Mammary hypoplasia: not every breast can produce sufficient milk. J Midwifery Womens Health. 2013. Jul-Aug; 58 4: 457- 461. [DOI] [PubMed] [Google Scholar]
- 13. Duran MS, Spatz DL. A mother with glandular hypoplasia and a late preterm infant. J Hum Lact. 2011. November; 27 4: 394- 397. [DOI] [PubMed] [Google Scholar]
- 14. Mennella JA, Pepino MY, Teff KL. Acute alcohol consumption disrupts the hormonal milieu of lactating women. J Clin Endocrinol Metab. 2005. April; 90 4: 1979- 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mennella JA, Pepino MY. Breastfeeding and prolactin levels in lactating women with a family history of alcoholism. Pediatrics. 2010. May; 125 5: e1162- e1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rasmussen KM, Hilson JA, Kjolhede CL. Obesity may impair lactogenesis II. J Nutr. 2001. November; 131 11: 3009S- 3011S. [DOI] [PubMed] [Google Scholar]
- 17. Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr. 2010. September; 92 3: 574- 584. [DOI] [PubMed] [Google Scholar]
- 18. Michel MP, Gremmo-Féger G, Oger E, Sizun J. Pilot study of early breastfeeding difficulties of term newborns: incidence and risk factors [in French]. Arch Pediatr. 2007. May; 14 5: 454- 460. [DOI] [PubMed] [Google Scholar]
- 19. Davanzo R, Cannioto Z, Ronfani L, Monasta L, Demarini S. Breastfeeding and neonatal weight loss in healthy term infants. J Hum Lact. 2013. February; 29 1: 45- 53. [DOI] [PubMed] [Google Scholar]
- 20. Grzeskowiak LE, Lim SW, Thomas AE, Ritchie U, Gordon AL. Audit of domperidone use as a galactogogue at an Australian tertiary teaching hospital. J Hum Lact. 2013. February; 29 1: 32- 37. [DOI] [PubMed] [Google Scholar]
- 21. Grzeskowiak LE, Dalton JA, Fielder AL. Factors associated with domperidone use as a galactogogue at an Australian tertiary teaching hospital. J Hum Lact. 2015. May; 31 2: 249- 253. [DOI] [PubMed] [Google Scholar]
- 22. Sim TF, Sherriff J, Hattingh HL, Parsons R, Tee LB. The use of herbal medicines during breastfeeding: A population-based survey in Western Australia. BMC Complement Altern Med. 2013. November 13; 13: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #9: use of galactogogues in initiating or sugmenting the rate of maternal milk secretion. Breastfeed Med. 2011. February; 6 1: 41- 49. http://www.bfmed.org/Media/Files/Protocols/Protocol%209%20-%20English%201st%20Rev.%20Jan%202011.pdf. Accessed August 12, 2015. [DOI] [PubMed] [Google Scholar]
- 24. Gabay MP. Galactogogues: medications that induce lactation. J Hum Lact. 2002. August; 18 3: 274- 279. [DOI] [PubMed] [Google Scholar]
- 25. Lindstrom A, Ooyen C, Lynch ME, Blumenthal M, Kawa K. Sales of herbal dietary supplements increase by 7.9% in 2013, marking a decade of rising sales: turmeric supplements climb to top ranking in natural channel. HerbalGram. 2014. ;(103):52-56. [Google Scholar]
- 26. Sim TF, Hattingh HL, Sherriff J, Tee LB. Perspectives and attitudes of breastfeeding women using herbal galactagogues during breastfeeding: a qualitative study. BMC Complement Altern Med. 2014. July 2; 14: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winterfeld U, Meyer Y, Panchaud A, Elnarrson A. Management of deficient lactation in Switzerland and Canada: a survey of midwives' current practices. Breastfeed Med. 2012. August; 7: 317- 318. [DOI] [PubMed] [Google Scholar]
- 28. da Silva OP, Knoppert DC, Angelini MM, Forret PA. Effect of domperidone on milk production in mothers of premature newborns: a randomized, double-blind, placebo-controlled trial. CMAJ. 2001. January 9; 164 1: 17- 21. [PMC free article] [PubMed] [Google Scholar]
- 29. Campbell-Yeo ML, Allen AC, Joseph KS, Ledwidge JM, Allen VM, Dooley KC. Study protocol: a double blind placebo controlled trial examining the effect of domperidone on the composition of breast milk [NCT00308334]. BMC Pregnancy Childbirth. 2006; 6: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campbell-Yeo ML, Allen AC, Joseph KS, et al. Effect of domperidone on the composition of preterm human breast milk. Pediatrics. 2010. January; 125 1: e107- e114. [DOI] [PubMed] [Google Scholar]
- 31. Jantarasaengaram S, Sreewapa P. Effects of domperidone on augmentation of lactation following cesarean delivery at full term. Int J Gynaecol Obstet, 2012. Mar:116(3)240-243. [DOI] [PubMed] [Google Scholar]
- 32. Petraglia F, De Leo V, Sardelli S, Pieroni ML, D'Antona M, Genazzani AR. Domperidone in defective and insufficient lactation. Eur J Obstet Gynecol Reprod Biol. 1985; 19 5: 281- 287. [DOI] [PubMed] [Google Scholar]
- 33. de Gezelle H, Ooghe W, Thiery M, Dhont M. Metoclopramide and breast milk. Eur J Obstet Gynecol Reprod Biol. 1983. April; 15 1: 31- 36. [DOI] [PubMed] [Google Scholar]
- 34. Hansen WF, McAndrew S, Harris K, Zimmerman MB. Metoclopramide effect on breastfeeding the preterm infant: a randomized trial. Obstet Gynecol. 2005. February; 105 2: 383- 389. [DOI] [PubMed] [Google Scholar]
- 35. Fife S, Gill P, Hopkins M, Angelo C, Boswell S, Nelson KM. Metoclopramide to augment lactation, does it work? A randomized trial. J Matern Fetal Neonatal Med. 2011. November; 24 11: 1317- 1320. [DOI] [PubMed] [Google Scholar]
- 36. Seema, Patwari AK, Satyanarayana L. Relactation: an effective intervention to promote exclusive breastfeeding. J Trop Pediatr. 1997. August; 43 4: 213- 216. [DOI] [PubMed] [Google Scholar]
- 37. Sakha K, Behbahan AG. Training for perfect breastfeeding or metoclopramide: which one can promote lactation in nursing mothers? Breastfeed Med. 2008. June; 3 2: 120- 123. [DOI] [PubMed] [Google Scholar]
- 38. Lewis PJ, Devenish C, Kahn C. Controlled trial of metoclopramide in the initiation of breast feeding. Br J Clin Pharmacol. 1980. February; 9 2: 217- 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yabes-Almirante C, Lim CHTN. Effectiveness of Natalac as a galactagogue. J Pak Med Assoc. 1996; 71 3: 265- 272. [Google Scholar]
- 40. Sharma S, Ramji S, Kumari S, Bapna JS. Randomized controlled trial of Asparagus racemosus (Shatavari) as a lactogogue in lactational inadequacy. Indian Pediatr. 1996. August; 33 8: 675- 677. [PubMed] [Google Scholar]
- 41. Gupta M, Shaw B. A double-blind randomized clinical trial for evaluation of galactogogue activity of Asparagus racemosus Willd. Iran J Pharm Res. 2011. Winter; 10 1: 167- 172. [PMC free article] [PubMed] [Google Scholar]
- 42. Damanik R, Wahlqvist ML, Wattanapenpaiboon N. Lactagogue effects of Torbangun, a Bataknese traditional cuisine. Asia Pac J Clin Nutr. 2006; 15 2: 267- 274. [PubMed] [Google Scholar]
- 43. Turkyilmaz C, Onal E, Hirfanoglu IM, et al. The effect of galactagogue herbal tea on breast milk production and short-term catch-up of birth weight in the first week of life. J Altern Complement Med. 2011. February; 17 2: 139- 142. [DOI] [PubMed] [Google Scholar]
- 44. Espinosa-Kuo CL. A randomized-controlled trial on the use of malunggay (Moringa oleifera) for augmentation of the volume of breastmilk among mothers of term infants. Filipino Fam Physician. 2005; 43 1: 26- 33. [Google Scholar]
- 45. Di Pierro F, Callegari A, Carotenuto D, Tapla MM. Clinical efficacy, safety and tolerability of BIO-C (micronized Silymarin) as a galactagogue. Acta Biomed. 2008. December; 79 3: 205- 210. [PubMed] [Google Scholar]
- 46. Mennella JA, Beauchamp GK. The effects of repeated exposure to garlic-flavored milk on the nursling's behavior. Pediatr Res. 1993. December; 34 6: 805- 908. [DOI] [PubMed] [Google Scholar]
- 47. Donovan TJ, Buchanan K. Medications for increasing milk supply in mothers expressing breastmilk for their preterm hospitalised infants. Cochrane Database Syst Rev. 2012. Mar 14;3:CD005544. doi: 10.1002/14651858.CD005544.pub2. [DOI] [PubMed] [Google Scholar]
- 48. Osadchy A, Moretti ME, Koren G. Effect of domperidone on insufficient lactation in puerperal women: a systematic review and meta-analysis of randomized controlled trials. Obstet Gynecol Int. 2012; 2012: 642893 doi: 10.1155/2012/642893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ahmad N, Keith-Ferris J, Gooden E, Abell T. Making a case for domperidone in the treatment of gastrointestinal motility disorders. Curr Opin Pharmacol. 2006. December; 6 6: 571- 576. [DOI] [PubMed] [Google Scholar]
- 50. Carroccio A, Iacono G, Montalto G, Cavataio F, Soresi M, Notarbartolo M. Domperidone plus magnesium hydroxide and aluminum hydroxide: a valid therapy in children with gastroesophageal reflux. A double-blind randomized study versus placebo. Scand J Gastroenterol. 1994. April; 29 4: 300- 304. [DOI] [PubMed] [Google Scholar]
- 51. Bines JE, Quinlan JE, Treves S, Kleinman RE, Winter HS. Efficacy of domperidone in infants and children with gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 1992. May; 14 4: 400- 405. [DOI] [PubMed] [Google Scholar]
- 52. De Loore I, Van Ravensteyn H, Ameryckx L. Domperidone drops in the symptomatic treatment of chronic paediatric vomiting and regurgitation. A comparison with metoclopramide. Postgrad Med J. 1979; 55 suppl 1: 40- 42. [PubMed] [Google Scholar]
- 53. Cresi F, Marinaccio C, Russo MC, Miniero R, Silvestro L. Short-term effect of domperidone on gastroesophageal reflux in newborns assessed by combined intraluminal impedance and pH monitoring. J Perinatol. 2008. nov; 28 11: 766- 770. [DOI] [PubMed] [Google Scholar]
- 54. Paul C, Zenut M, Dorut A, et al. Use of domperidone as a galactagogue drug: a systematic review of the benefit-risk ratio. J Hum Lact. 2015. February; 31 1: 57- 63. [DOI] [PubMed] [Google Scholar]
- 55. Bozzo P, Koren G, Ito S. Health Canada advisory on domperidone: should I avoid prescribing domperidone to women to increase milk production? Can Fam Physician. 2012. September; 58 9: 952- 953. [PMC free article] [PubMed] [Google Scholar]
- 56. US Food and Drug Administration. FDA Talk Paper: FDA warns against women using unapproved drug, domperidone, to increase milk production . June 7, 2004. http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm173886.htm. Accessed April 27, 2016. [Google Scholar]
- 57. Reddymasu SC, Soykan I, McCallum RW. Domperidone: review of pharmacology and clinical applications in gastroenterology. Am J Gastroenterol. 2007; 102 9: 2036- 2045. [DOI] [PubMed] [Google Scholar]
- 58. Wan EW, Davey K, Page-Sharp M, et al. Dose-effect study of domperidone as a galactagogue in preterm mothers with insufficient milk supply, and its transfer into milk. Br J Clin Pharmacol. 2008. August; 66 2: 283- 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Forinash AB, Yancey AM, Barnes KN, Myles TD. The use of galactogogues in the breastfeeding mother. Ann Pharmacother. 2012. October; 46 10: 1392- 1404. [DOI] [PubMed] [Google Scholar]
- 60. Betzold CM. Galactagogues. J Midwifery Womens Health. 2004. Mar-Apr; 49 2: 151- 154. [DOI] [PubMed] [Google Scholar]
- 61. Marasco L. Inside track. Increasing your milk supply with galactogogues. J Hum Lact. 2008; 24 4: 455- 456. [DOI] [PubMed] [Google Scholar]
- 62. Royal Pharmaceutical Society. A new mother is having trouble producing breast milk. What options are available to help? Clinical Pharmacist. 2010. January; 2: 260. [Google Scholar]
- 63. Shaffer D, Butterfield M, Pamer C, Mackey AC. Tardive dyskinesia risks and metoclopramide use before and after U.S. market withdrawal of cisapride. J Am Pharm Assoc. 2004. Nov-Dec; 44 6: 661- 665. [DOI] [PubMed] [Google Scholar]
- 64. Dahl E, Diskin AL. Long-lasting adverse effects after short-term low-dose treatment with metoclopramide for vomiting. Int Marit Health. 2014; 65 1: 16- 19. [DOI] [PubMed] [Google Scholar]
- 65. Rumore MM, Lee SE, Wang S, Farmer B. Metoclopramide-induced cardiac arrest. Clin Pract. 2011. November 2; 1 4: e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Anfinson TJ. Akathisia, panic, agoraphobia, and major depression following brief exposure to metoclopramide. Psychopharmacol Bull. 2002. Winter; 36 1: 82- 93. [PubMed] [Google Scholar]
- 67. Surawski RJ, Quinn DK. Metoclopramide and homicidal ideation: a case report and literature review. Psychosomatics. 2011; 52 5: 403- 409. [DOI] [PubMed] [Google Scholar]
- 68. Ingram J, Taylor H, Churchill C, Pike A, Greenwood R. Metoclopramide or domperidone for increasing maternal breast milk output: a randomised controlled trial. Arch Dis Child Fetal Neonatal Educ. 2012; 97 4: F241- F245. [DOI] [PubMed] [Google Scholar]
- 69. Zuppa AA, Sindico P, Orchi C, Carducci C, Cardiello V, Romagnoli C. Safety and efficacy of galactogogues: substances that induce, maintain and increase breast milk production. J Pharm Pharm Sci. 2010; 13 2: 162- 174. [DOI] [PubMed] [Google Scholar]
- 70. Rasmussen KM, Dieterich CM, Zelek ST, Altabet JT, Kjolhede CL. Interventions to increase the duration of breastfeeding in obese mothers: the Bassett Improving Breastfeeding Study. Breastfeed Med. 2011. April; 6 2: 69- 75. [DOI] [PubMed] [Google Scholar]
- 71. Asztalos EV, Campbell-Yeo M, daSilva OP, Kiss A, Knoppert DC, Ito S. Enhancing breast milk production with domperidone in mothers of preterm neonates (EMPOWER trial). BMC Pregnancy Childbirth. 2012. August 31; 12: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mortel M, Mehta SD. Systematic review of the efficacy of herbal galactogogues. J Hum Lact. 2013; 29 2: 154- 162. [DOI] [PubMed] [Google Scholar]
- 73. Nice FJ. Common herbs and foods used as galactogogues. Infant Child Adolesc Nutr. 2011. June; 3 3: 129- 132. [Google Scholar]
- 74. King J, Raguindin PF, Dans LF. Moringa oleifera (Malunggay) as a galactagogue for breastfeeding mothers: a systematic review and meta-analysis of randomized controlled trials. Philipp J Pediatr. 2013. December; 61 2: 34- 42. [Google Scholar]
- 75. Stohs SJ, Hartman MJ. Review of the safety and efficacy of Moringa oleifera. Phytother Res. 2015. June; 29 6: 796- 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007. June; 6 2: 110- 119. [DOI] [PubMed] [Google Scholar]
- 77. American Botanical Council. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines: Part One — Introduction. Austin, TX: American Botanical Council; 1999. http://cms.herbalgram.org/commissione/intro/comm_e_int.html. Accessed April 27, 2016. [Google Scholar]
- 78. Food and Drug Administration. Dietary Supplement Products & Ingredients. http://www.fda.gov/Food/DietarySupplements/ProductsIngredients/default.htm. Accessed August 21, 2016. [Google Scholar]
- 79. Posadzki P, Watson LK, Ernst E. Adverse effects of herbal medicines: an overview of systematic reviews. Clin Med (Lond). 2013. February; 13 1: 7- 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schaffir J, Czapla C. Survey of lactation instructors on folk traditions in breastfeeding. Breastfeed Med. 2012. August; 7: 230- 233. [DOI] [PubMed] [Google Scholar]
- 81. Jensen R. Fenugreek: overlooked but not forgotten. UCLA Lact Alumni Newsletter. 1992 1: 2- 3. Breastfeeding Online. http://www.breastfeedingonline.com/fenugreekoverlooked.shtml#sthash.c0c9BUGE.dpbs. Accessed April 27, 2016. [Google Scholar]