Abstract
Background
The pendulum test is a standard clinical test for quantifying the severity of spasticity. In the test, an electrogoniometer is typically used to measure the knee angular motion. The device is costly and difficult to set up such that the pendulum test is normally time consuming.
Objective
The goal of this study is to determine whether a Nintendo Wii remote can replace the electrogroniometer for reliable assessment of the angular motion of the knee in the pendulum test.
Methods
The pendulum test was performed in three control participants and 13 hemiplegic stroke patients using both a Wii remote and an electrogoniometer. The correlation coefficient and the Bland–Altman difference plot were used to compare the results obtained from the two devices. The Wilcoxon signed-rank test was used to compare the difference between hemiplegia-affected and nonaffected sides in the hemiplegic stroke patients.
Results
There was a fair to strong correlation between measurements from the Wii remote and the electrogoniometer (0.513 < R2 < 0.800). Small but consistent differences between the Wii remote and electrogoniometer were identified from the Bland–Altman difference plot. Within the hemiplegic stroke patients, both devices successfully distinguished the hemiplegia-affected (spastic) side from the nonaffected (nonspastic) side (both with p < .0001*). In addition, the intraclass correlation coefficient, standard error of measurement, and minimum detectable differences were highly consistent for both devices.
Conclusion
Our findings suggest that the Wii remote may serve as a convenient and cost-efficient tool for the assessment of spasticity.
Keywords: Pendulum test, Spasticity, Wii remote, Stroke
1. Introduction
Spasticity is a complex phenomenon in which excessive muscle activity is present. Lance defined spasticity as “amotor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) and increased tendon jerks resulting from disinhibition of the stretch reflex, as one component of the upper motor neuron lesion” [1]. The North American Task Force for Childhood Motor Disorders described spasticity as “avelocity-dependent increase in hypertonia with a catch when a threshold is exceeded” [2]. Subsequently, the SPASM consortium (A European Thematic Network to Develop Standardised Measures of Spasticity) suggested that spasticity should be redefined as “disordered sensory-motor control, resulting from an upper motor neuron lesion, presenting as intermittent or sustained involuntary activation of muscles” [3]. Spasticity can be observed in patients with diverse sources of upper motor neuron lesions such as stroke [4], multiple sclerosis [5], spinal cord injury [6], and cerebral palsy [7], and it can cause severe pain, disability, and impaired quality of life [8].
Subjective clinical assessments of spasticity include the Tardieu scale, the Ashworth scale [9], and the modified Ashworth scale (MAS) [10]. Although these tests have the advantage of clinical feasibility, the interpretation of the results relies on the operator’s experience and judgement, which may vary substantially between different clinicians and lead to low test reliability. To address the concern, several objective approaches based on assessments of the electromyography (EMG) of muscles or electrodiagnostic parameters have been developed. For instance, the H reflex in electrodiagnosis is analogous to the mechanically induced spinal stretch reflex and can be used to quantify spinal alpha motor neuron excitability that increases in spasticity [11]. EMG-based methods have been used to assess spasticity in certain circumstances. However, there is no well established standard for EMG-based evaluation of spasticity. Other objective tests such as the pendulum test and robot-assisted spasticity measurement are focused on describing the mechanical features of limbs responding to movement or stretching [12]. The pendulum test, which describes the knee extensor’s response to stretching, is an alternative method for measuring spasticity at the knee. The pendulum test depicts the movement of patient’s a leg following a free drop under gravity from a horizontal position. Wartenberg was the first to propose its use in order to describe the stiffness and damping characteristics of limb swing movement [13]. Several sensing devices, including electrogoniometry [14], videography [15], and magnetic sensing devices [16], have been used to monitor the oscillating movements after the leg is dropped. Nevertheless, these tools are not often used in clinical practice because the required devices are costly and the setup procedures are time-consuming. The Nintendo Wii, developed as a video game console, is a handheld pointing device with a gyroscope sensor for detecting three-dimensional movements. The Wii is inexpensive and easily available on the market. When appropriate device setting and signal processing are adopted, the Wii controller can be used as a high-performance movement detector (Fig. 1A). Therefore, we propose to use the Wii remote as a convenient tool for measuring spasticity in the pendulum test.
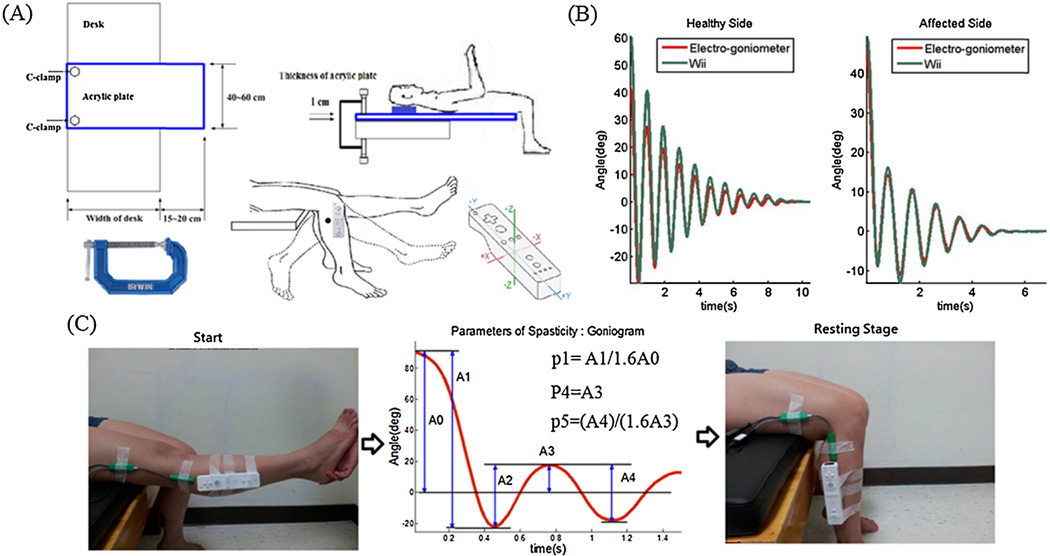
Fig. 1.
(A) Experimental setup for the pendulum test with a Wii remote. The Wii remote, which is well-known for its motion-sensing capability, is a multifunctional Bluetooth-based wireless device. It uses a three-axis accelerometer sensor and internal three-axis gyroscope to measure motion in all three directions. (B) The angular signals of the electrogoniometer and Wii remote for both sides during a typical pendulum test. (C) Parameters of the pendulum test.
The present study tested the reliability of the Wii remote in assessing the angular motion of the knee during the pendulum test, and compared the data obtained from the Wii remote with those obtained from a standard electrogoniometer. Three healthy control participants and 13 patients with hemiplegic stroke underwent the pendulum test in which the angular motion of the knee was monitored with a Wii remote controller and electrogoniometer to record simultaneously. We tested whether the results from the Wii remote are consistent with those from the electrogoniometer.
2. Methods
2.1. Background information
For the kinematic recording of passive movements, we used two independent measurement tools. We provide information about the pendulum test and the two devices in the supplementary materials (“Appendix 2.1.1–2.1.3”) [17–26]. The study protocol is described in the following subsections.
2.2. Participants
To test whether the spasticity measurements recorded using the Wii remote can distinguish the affected and nonaffected sides of hemiplegia patients, 13 hemiplegic stroke patients were included in the study. Each patient had spasticity at the knee, with an MAS score between 1+ and 3. To widen the range of severity in spasticity and ensure consistency between the Wii and electrogoniometer measurements in healthy adults, we also included three healthy controls. Table 1 presents the clinical information of both participant groups. Healthy participants were recruited from the community. They had no known diseases and were not taking any medications. The stroke patients with hemiplegia were recruited from the database of Taipei Medical University Hospital. The diagnosis of stroke was based on brain magnetic resonance imaging and computerized tomography. None of these stroke patients had intrathecal baclofen pump therapy or had received antispastic medications in the 3 months before the current study. Patients with an impaired communication ability, significantly impaired cognition (Mini-Mental Status Examination score <24), previous trauma or prior surgery on the affected knee joint, active knee arthritis or pain, a recent botulinum toxin injection to the quadriceps or hamstring muscles, significant contracture (defined as the loss of more than half of the passive range of knee motion), or deformities over the knee joints were excluded. The study was approved by the Institutional Review Board of Taipei Medical University Hospital, and each patient provided informed consent.
Table 1.
Clinical information.
| Patient no. | Age | Sex | Height (cm) | Weight (kg) | Affected side | Stroke onset | Site | Type | MAS |
|---|---|---|---|---|---|---|---|---|---|
| Stroke | |||||||||
| 1 | 71 | M | 170 | 69 | L | >5Y | MCA | H | 3 |
| 2 | 66 | M | 171 | 67 | R | 10M | BG | I | 2 |
| 3 | 44 | M | 171 | 80 | R | 6M | BG | H | 1 + |
| 4 | 70 | M | 163 | 78 | L | 3Y5M | MCA | I | 2 |
| 5 | 52 | M | 165 | 74 | R | 3Y3M | BG | H | 2 |
| 6 | 68 | M | 171 | 68 | R | 2Y1M | BG | H | 2 |
| 7 | 58 | F | 155 | 60 | R | >5Y | BG | H | 2 |
| 8 | 60 | F | 140 | 43 | R | 2Y5M | BG | I | 2 |
| 9 | 61 | F | 157 | 56 | R | >5Y | Periventricular white matter |
I | 1+ |
| 10 | 56 | F | 156 | 56 | R | 3Y4M | MCA | I | 3 |
| 11 | 59 | F | 162 | 64 | L | 1Y2M | ACA | I | 2 |
| 12 | 64 | M | 173 | 81 | R | >5Y | MCA | I | 2 |
| 13 | 69 | F | 164 | 65 | R | 2Y2M | BG | H | 3 |
| Control | |||||||||
| 14 | 28 | F | 162 | 54 | |||||
| 15 | 31 | M | 169 | 66 | |||||
| 16 | 32 | M | 175 | 73 |
L: left; R: right; MCA: middle cerebral artery; BG: basal ganglion; H: hemorrhage; I: ischemia; MAS: modified Ashworth scale of the knee extensor.
2.3. Protocols
Fig. 1A shows the experimental setup, and Fig. 1C depicts the position of a subject’s leg at the start and end of the experiment. The pendulum test was repeated on each of the knees for ten times in 10 patients with hemiplegia stroke, and five times in the other six participants. The Wii remote controller was attached to each subject’s leg with two Velcro bands. The location of the Wii remote was 3 cm below the fibular head, and the long axis of the remote was aligned parallel to the line from the fibular head to the lateral malleolus. Simultaneously, two electrogoniometer sensors (Pro-Comp Infiniti, Thought Technology Ltd., Canada) were used to determine the knee angle change during the pendulum test. The upper sensor was attached to the lateral femoral epicondyle with its axis paralleling the line from the lateral femoral epicondyle to the gluteal tuberosity of the femur. The lower sensor was attached to the lateral fibular head with its axis paralleling the line from the fibular head to the lateral malleolus. The location of the Wii remote was allowed to change because the derived measures were dimensionless except for the first maximum of the oscillation, and the number of swings is not dependent on the location. We established the interface for recordings using a software (source code written in C installed a computer).
2.4. Data analysis
Several steps were taken to pre-process the raw data in order to obtain a representative analytical source. For the data collected by the electrogoniometer, a first-order Savitzky–Golay finite impulse response (FIR) smoothing filter was adopted to smooth noisy signals, and high-frequency noise was eliminated using a 20th-order FIR low-pass filter with a cutoff frequency of 5 Hz.
For the Wii remote, only the gyroscope measurements were used. The voltage reference was assumed to be half of the analog-to-digital converter range (8192 units or 1.35 V in reality), e.g., by using 2.27 mV/deg/s, 8192 is 595 deg/s (1.35 V/2.27 mV). To obtain correct values in unit of deg/s, the measurements must be divided by ~13.768 units/deg/s (=8192/595). Using a 20th-order FIR low-pass filter with a cutoff frequency of 4 Hz, high-frequency noise was eliminated. All processes were performed using MATLAB (R2009a).
2.5. Statistical analysis
The analysis was designed to determine the ability to distinguish between affected and nonaffected sides with an electrogoniometer and Wii remote. We first calculated the mean and standard deviations from all trials for each participant and each side individually. We then removed the obvious outliers (beyond three standard deviations) and the adjusted mean values were obtained. The correlation coefficient [27] and Bland–Altman difference plot were used to test the consistency between the measures obtained from the Wii remote and the electrogoniometer. To test whether the Wii remote was effective in recognizing spastic hemiplegia, the Wilcoxon signed-rank test was used to examine the significance in differentiating between the affected and nonaffected sides. A p value of less than 0.05 was considered statistically significant. We also examined the distribution of the normalized relaxation index for the different groups of participants by using the two devices, separately. To assess the consistency and reproducibility of the quantitative measurements, the intraclass correlation coefficient (ICC) was applied to determine how strongly units in the same group resembled each other. The standard error of measurement (SEM) was also calculated to estimate the intraclass measurement error. The smallest detectable difference (SDD), which indicates the smallest difference between two independently obtained measures that are larger than the measurement error and can be considered “real,” was calculated to examine the reliability of the spasticity measurements obtained from the electrogoniometer and Wii remote. All analyses were conducted using JMP Pro (Version 11.0.0, SAS Institute Inc., USA).
3. Results
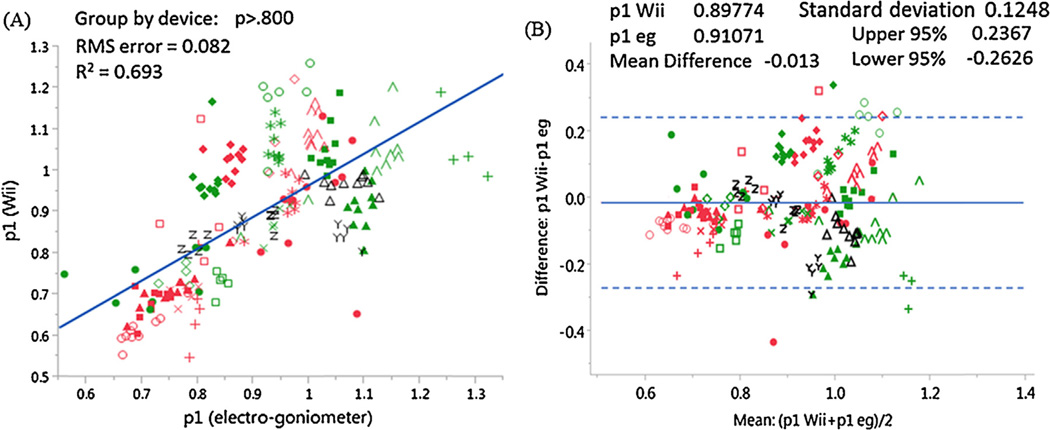
Fig. 1B presents the pendulum test measurements. In this study, we focused on the normalized relaxation index of the pendulum test for its clinical significance. The correlation coefficients between the Wii and electrogoniometer results for all tested measurements are summarized in Table 2 and Fig. 2A. The reliability of the Wii measurements was supported by the small biases of the Wii results as compared to the electrogoniometer results that were revealed by the Bland–Altman difference plot (Fig. 2B and Table 2).
Table 2.
Correlation coefficients and statistical parameters obtained from the Bland–Altman difference plot and Wilcoxon signed-rank test. The correlations between Wii-based and electrogoniometer-based parameters (i.e., for p1, p2, p4, and p5) confirm that the Wii remote can replace the electrogoniometer with an acceptable degree of confidence. Note that the time length must be selected wisely to guarantee a high correlation for p2. For the four Wii remote measurements, three of them (p1, p2, and p5) were reliable with a steady bias without heterogeneous variance. Nearly all data were within 95% confidence intervals. To test the side differences in the patients with spastic hemiplegia, a paired, two-sided signed-rank test was performed. Two of the four parameters (p1 and p5) showed highly significant side differences (p < .0001) with the Wii remote.
| Measure | Square of correlation coefficient (R2) |
Bland–Altman difference plot | Paired, two-sided signed-rank test | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | 95% confidence interval (lower limit of agreement) |
95% confidence interval (upper limit of agreement) |
Electrogoniometer | Wii | ||
| Normalized relaxation index (p1) | .693 | −.013 | .125 | −.263 | .237 | p <.0001* | p <.0001* |
| Number of swings (p2) | .589 | .646 | 3.173 | −5.699 | 6.992 | p = .0113* | p = .5319 |
| First maximum of the oscillation (p4) | .800 | 18.790 | 23.114 | −27.439 | 65.019 | p <.0001* | p = .4085 |
| Relaxation index at the half swing (p5) | .513 | −.029 | .722 | −1.472 | 1.414 | p = .5860 | p <.0001* |
| Outliers on average ≤.1 | |||||||
Fig. 2.
(A) Correlation coefficients and (B) Bland–Altman difference plots for the critical spasticity measure (normalized relaxation index) obtained from the Wii remote and the electrogoniometer. Different colored markers represent the varieties of groups and participants (red: affected side; green: nonaffected side; black: control). In general, the p1 value for nonaffected sides and controls is higher than for affected sides, and the intrinsic difference between the participants can be observed. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
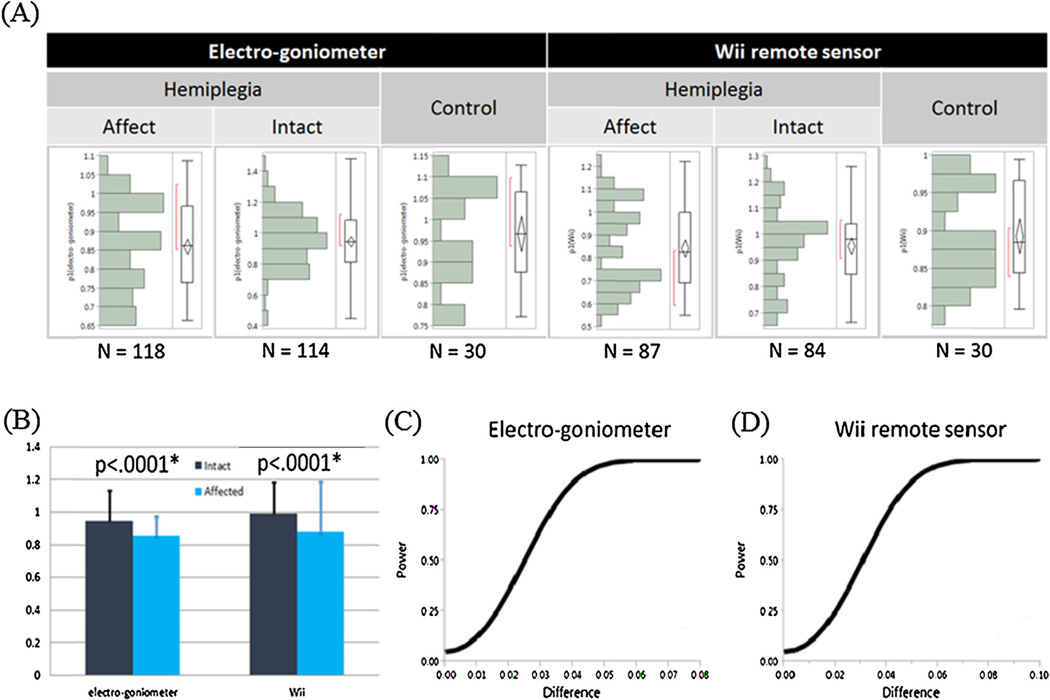
Fig. 3A shows the distribution of parameter 1 (p1) for both devices (electrogoniometer and Wii), sides (affected and nonaffected), and groups (hemiplegia and control). In general, p1 of the nonaffected side followed a normal distribution, whereas the distribution in the affected side deviated from the normal distribution with seemingly two peaks in the distribution. The side difference in the distribution was particularly pronounced in the Wii results. Using a paired, two-sided signed-rank test, we found that both Wii-based and electrogoniometer-based measures (i.e., Normalized relaxation index, Number of swings, First maximum of the oscillation and Relaxation index at the half swing) were able to differentiate the affected and the nonaffected sides in patients with spastic hemiplegia (Fig. 3B and Table 2).
Fig. 3.
(A) Distribution of the normalized relaxation (p1) index for different groups of participants and sides for both devices. (B) Both the devices distinguished between affected and nonaffected sides (p < .0001*). (C and D) The smallest detectable difference between the Wii-derived p1 and the electrogonoimeter-derived p1 is relatively small (<.05; we can declare significance when power >.8 and α = .05) for both devices.
We also examined the reliability of the normalized relaxation index. Table S1 show the results of the ICC and SEM for all patients. Table S2 and Fig. 3C and D provide the SDD results for both devices.
4. Discussion
In this study we designed a Wii-based system to assess the pendulum kinematics of the leg and to quantify spasticity involving the knee. As expected, the Wii remote described pendulum kinematics approximately as accurate as the electrogoniometer did. We found adequate agreement between the measures from both devices during the pendulum test (Fig. 2 and Table 2). Specifically, the correlations between the electrogoniometer and Wii-based spasticity measures were fair to strong, with the squares of the correlation coefficients of the parameters all surpassing 0.5 [30]. Calibration between the electrogoniometer and Wii gyroscope may be unnecessary if the measured parameters are expressed as a ratio. In addition to the high correlation coefficients, a Bland–Altman difference plot also confirmed the agreement for each comparison, the two assays were not far apart. In summary, we found that the Wii-based measurements had no heterogeneous variance with fixed bias as compared to the electrogoniometer-based measurements.
To examine the clinical feasibility of the Wii remote measurements, both the electrogoniometer and Wii remote were found to have significant powers in distinguishing spastic hemiplegia for two of the four parameters. This was particularly the case of the normalized relaxation index (p < .0001), which was valid for both electrogoniometer and Wii remote.
The reliability in quantifying spasticity was also examined. More than two thirds of the participants showed an ICC higher than 0.7. The high agreement among the trials indicated that the differences of the normalized relaxation index (p1) were mainly caused by interparticipant variability. However, intraparticipant differences might have been caused by muscle looseness from the increasing number of swing motions and by the different thigh muscle thicknesses of the participants, which could have lead to different relative motions between the two devices. Taking the normalized relaxation index (p1) as an example, we also examined the SEM (<.095) and found its value to be smaller than a 10th of the value of healthy subjects (i.e., p1 = 1). The smallest detectable difference was also relatively small (<.05; we can declare significance when power >.8 and α = .05) for both devices, which gave us confidence in applying the Wii-based measure to distinguish the hemiplegia-affected sides.
Notably, small differences were observed in the swing angle, especially with the starting angle because of differences in the unpredicted gain in voltage transformation, thigh muscle thickness, and mounted machine weight. These limitations are acceptable because three of the four parameters were not influenced; hence, we can use p1, p2, and p5 adaptively because of their fractional and directional nature. The influences on p4 can be overlooked because the angular attenuation caused by muscle spasticity is much more obvious. Other problems can be solved as described in Section 2.4. Considering its economic potential, the Wii remote has been proposed for several applications in rehabilitative medicine. However, using the Wii remote to assist with plasticity measurements has rarely been discussed. This may be because the device was originally designed for video games. Furthermore, movement artifacts and AC noise mixing during recording also complicate artifacts the application. To address the concerns, we employed the Savitzky–Golay smoothing method and the FIR low-pass filter to eliminate varieties of artifacts and noise where the cutoff frequency could be confirmed using the Gabor spectrum.
Although several parameters can be obtained by observing the time-angle slope during the pendulum test, the association between these parameters and clinical outcomes remains unclear. The angle of the first swing excursion was described by Fowler et al. [31] as the “most sensitive outcome measure” in people with cerebral palsy [32]. Therefore, we focused on this measure instead of other parameters obtained from the pendulum test, such as the angle of the first reversal with reference to the resting angle and the average relaxation index, because the clinical significance of these parameters is still not established.
The present study has the following limitations. First, three healthy subjects appear to be too few to test the group difference (i.e., as compared to the hemiplegic group). Second, we only used the MAS for the clinical evaluation of spasticity. Although the MAS has a generally adequate intrarater reliability and is widely used in clinical settings [28,29], this measure does not consider stretching velocity and thus may miss some information regarding spastic characteristics. The Tardieu or modified Tardieu scale may be superior in describing the velocity-dependent behavior of spasticity. For increased homogeneity of the study group, we included only patients with a MAS score for knee extensor spasticity of more than 1+. Third, we did not analyze the correlation between parameters obtained from the pendulum test and clinical features. Further studies are warranted to explore more about the clinical relevance of these parameters and to elucidate the feasibility of their clinical applications.
5. Conclusion
The parameters of the pendulum test obtained using the Wii-based system agreed closely with those from the electrogoniometer. The Wii system is able to distinguishe the spastic hemiplegia side from non-spastic side. By examining the reliability of the Wii remote, we found that the Wii-based system provides an alternative and feasible tool for an objective assessment of spasticity.
Supplementary Material
Acknowledgments
This research was sponsored by the Ministry of Science and Technology, Taiwan (Taiwan, R.O.C.; Grants NSC 104-3115-E-008-001, 103-2321-B-008-003, 103-2221-E-008-006-MY3), joint foundation of Cathay General Hospital and National Central University (Grants CNJRF-101CGH-NCU-A4 and VGHUST103-G1-3-3), Center for Dynamical Biomarkers and Translational Medicine at National Central University (Grant NSC 102-2911-I-008-001), and grants from NIH (R00HL102241, P01AG009975, R01AG048108).
We thank the group members of Taipei Medical University Hospital for providing medical care for the study participants, and the group members of the Center for Dynamical Biomarkers and Translational Medicine for their support of the study, as well as Melissa Patxot in the Division of Sleep Medicine from Harvard Medical School for proofreading service.
Footnotes
Conflict of interest statement
The authors declare that they have no competing interests, and they were fully involved in the study and preparation of the manuscript and that the material within has not been and will not be submitted for publication elsewhere.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.gaitpost.2015.10.025.
References
- 1.Lance JW. Symposium synopsis; Spasticity: disordered motor control; 1980. pp. 485–494. 1980. [Google Scholar]
- 2.Terence DS, Mauricio RD, Deborah GS, Mark H, Jonathan WM Task Force on Childhood Motor Disorders. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111:89–97. doi: 10.1542/peds.111.1.e89. [DOI] [PubMed] [Google Scholar]
- 3.Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, et al. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil. 2005;27:2–6. doi: 10.1080/09638280400014576. [DOI] [PubMed] [Google Scholar]
- 4.Rosales RL, Chua-Yap AS. Evidence-based systematic review on the efficacy and safety of botulinum toxin-A therapy in post-stroke spasticity. J Neural Transm. 2008;115:617–623. doi: 10.1007/s00702-007-0869-3. [DOI] [PubMed] [Google Scholar]
- 5.Hobart JC, Riazi A, Thompson AJ, Styles IM, Ingram W, Vickery PJ, et al. Getting the measure of spasticity in multiple sclerosis: the Multiple Sclerosis Spasticity Scale (MSSS-88) Brain. 2005;129:224–234. doi: 10.1093/brain/awh675. [DOI] [PubMed] [Google Scholar]
- 6.Loubser PG, Narayan RK, Sandin KJ, Donovan WH, Russell KD. Continuous infusion of intrathecal baclofen: long-term effects on spasticity in spinal cord injury. Spinal Cord. 1991;29:48–64. doi: 10.1038/sc.1991.7. [DOI] [PubMed] [Google Scholar]
- 7.Lauer RT, Stackhouse CA, Shewokis PA, Smith BT, Tucker CA, McCarthy J. A time–frequency based electromyographic analysis technique for use in cerebral palsy. Gait Posture. 2006;26:420–427. doi: 10.1016/j.gaitpost.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Thibaut A, Chatelle C, Ziegler E, lie Bruno MA, Laureys S, Gosseries O. Spasticity after stroke: physiology, assessment and treatment. Brain Inj. 2013;27:1093–1105. doi: 10.3109/02699052.2013.804202. [DOI] [PubMed] [Google Scholar]
- 9.Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540. [PubMed] [Google Scholar]
- 10.Campanini I, Merlo A, Cavazzuti L. What’s the risk of using the Modified Ashworth Scale (MAS) to assess spasticity at the ankle? Gait Posture. 2011;33:S18–S19. [Google Scholar]
- 11.Yates C, Garrison K, Reese NB, Charlesworth A, Garcia-Rill E. Novel mechanism for hyperreflexia and spasticity. Prog Brain Res. 2011;188:167–180. doi: 10.1016/B978-0-444-53825-3.00016-4. [Chapter 11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmartz AC, Meyer-Heim AD, Müller R, Bolliger M. Measurement of muscle stiffness using robotic assisted gait orthosis in children with cerebral palsy: a proof of concept. Disabil Rehabil Assist Technol. 2011;6:29–37. doi: 10.3109/17483107.2010.509884. [DOI] [PubMed] [Google Scholar]
- 13.Wartenberg R. Pendulousness of the legs as a diagnostic test. Neurology. 1951;1:18. doi: 10.1212/wnl.1.1.18. [DOI] [PubMed] [Google Scholar]
- 14.Jamshidi M, Smith AW. Clinical measurement of spasticity using the pendulum test: comparison of electrogoniometric and videotape analyses. Arch Phys Med Rehabil. 1996;77:1129–1132. doi: 10.1016/s0003-9993(96)90134-3. [DOI] [PubMed] [Google Scholar]
- 15.Larsen KL, Maanum G, Frøslie KF, Jahnsen R. Ambulant adults with spastic cerebral palsy: the validity of lower limb joint angle measurements from sagittal video recordings. Gait Posture. 2011;35:186–191. doi: 10.1016/j.gaitpost.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Fee JW, Miller F. The leg drop pendulum test performed under general anesthesia in spastic cerebral palsy. Dev Med Child Neurol. 2004;46:273–281. doi: 10.1111/j.1469-8749.2004.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 17.Bajd T, Vodovnik L. Pendulum testing of spasticity. J Biomed Eng. 1984;6:9–16. doi: 10.1016/0141-5425(84)90003-7. [DOI] [PubMed] [Google Scholar]
- 18.Doheny EP, Walsh C, Foran T, Greene BR, Fan CW, Cunningham C, et al. Falls classification using tri-axial accelerometers during the five-times-sit-to-stand test. Gait Posture. 2013;38:1021–1025. doi: 10.1016/j.gaitpost.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Coley B, Najafi B, Paraschiv-Ionescu A, Aminian K. Stair climbing detection during daily physical activity using a miniature gyroscope. Gait Posture. 2007;22:287–294. doi: 10.1016/j.gaitpost.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Findlow A, Goulermas JY, Nester C, Howard D, Kenney LPJ. Predicting lower limb joint kinematics using wearable motion sensors. Gait Posture. 2007;28:120–126. doi: 10.1016/j.gaitpost.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Tripoliti EE, Tzallas AT, Tsipouras MG, Rigas G. Automatic detection of freezing of gait events in patients with Parkinson’s disease. Comput Methods Programs Biomed. 2013;110:12–26. doi: 10.1016/j.cmpb.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Schou T, Gardner HJ. A Wii remote, a game engine, five sensor bars and a virtual reality theatre; Proceedings of the 19th Australasian conference on computer-human interaction: entertaining user interfaces; 2007. pp. 231–234. 2007. [Google Scholar]
- 23.Lau H, Tong K. The reliability of using accelerometer and gyroscope for gait event identification on persons with dropped foot. Gait Posture. 2007;27:248–257. doi: 10.1016/j.gaitpost.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 24.McDowell BC, Hewitt V, Nurse A, Weston T, Baker R. The variability of goniometric measurements in ambulatory children with spastic cerebral palsy. Gait Posture. 2000;12:114–121. doi: 10.1016/s0966-6362(00)00068-0. [DOI] [PubMed] [Google Scholar]
- 25.Vodovnik L, Bowman BR, Bajd T. Dynamics of spastic knee joint. Med Biol Eng Comput. 1984;22:63–69. doi: 10.1007/BF02443747. [DOI] [PubMed] [Google Scholar]
- 26.Karpovich PV, George PK. Electrogoniometer – a new device for study of joints in action. Fed Proc. 1959;18:79. [Google Scholar]
- 27.Richard B, Jeffrey KS. Reliability and validity of pendulum test measures of spasticity obtained with the Polhemus tracking system from patients with chronic stroke. J Neuroeng Rehabil. 2009;6:1–7. doi: 10.1186/1743-0003-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marjan B, Paulette VV, Simon PM. Reliability of measurements obtained with the modified Ashworth scale in the lower extremities of people with stroke. Phys Ther. 2002;82:25–34. doi: 10.1093/ptj/82.1.25. [DOI] [PubMed] [Google Scholar]
- 29.Kaya T, Goksel KA, Gunaydin R, Koc A, Altundal EU. Inter-rater reliability of the Modified Ashworth Scale and modified Modified Ashworth Scale in assessing poststroke elbow flexor spasticity. Int J Rehabil Res. 2011;34:59–64. doi: 10.1097/MRR.0b013e32833d6cdf. [DOI] [PubMed] [Google Scholar]
- 30.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 31.Fowler V, Canning CG, Carr JH, Shepherd RB. Muscle length effect on the pendulum test. Arch Phys Med Rehabil. 1998;79:169–171. doi: 10.1016/s0003-9993(98)90294-5. [DOI] [PubMed] [Google Scholar]
- 32.Fowler EG, Nwigwe AI, Ho TW. Sensitivity of the pendulum test for assessing spasticity in persons with cerebral palsy. Dev Med Child Neurol. 2000;42:182–189. doi: 10.1017/s0012162200000323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.