Abstract
Here we examine the structure of the various types of spine synapses throughout the animal kingdom. Based on available evidence, we suggest that there are two major categories of spine synapses: invaginating and non-invaginating, with distributions that vary among different groups of animals. In the simplest living animals with definitive nerve cells and synapses, the cnidarians and ctenophores, most chemical synapses do not form spine synapses. But some cnidarians have invaginating spine synapses, especially in photoreceptor terminals of motile cnidarians with highly complex visual organs, and also in some mainly sessile cnidarians with rapid prey capture reflexes. This association of invaginating spine synapses with complex sensory inputs is retained in the evolution of higher animals in photoreceptor terminals and some mechanoreceptor synapses. In contrast to invaginating spine synapse, non-invaginating spine synapses have been described only in animals with bilateral symmetry, heads and brains, associated with greater complexity in neural connections. This is apparent already in the simplest bilaterians, the flatworms, which can have well-developed non-invaginating spine synapses in some cases. Non-invaginating spine synapses diversify in higher animal groups. We also discuss the functional advantages of having synapses on spines and more specifically, on invaginating spines. And finally we discuss pathologies associated with spine synapses, concentrating on those systems and diseases where invaginating spine synapses are involved.
Keywords: Invaginating, Postsynaptic, Crest synapse, Gemmule, Excrescence, Photoreceptor, Dendrite
Introduction
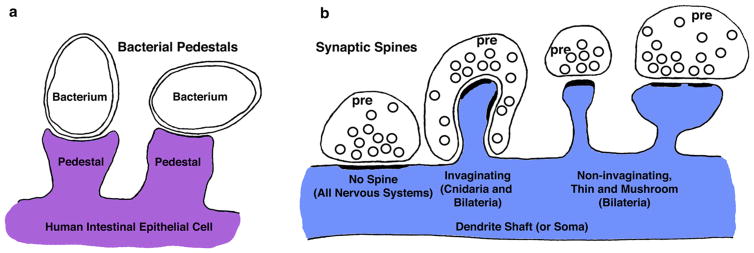
A synaptic spine can be defined simply as a chemical synapse formed at the end of a specialized protrusion/protuberance on the shaft of a neuron process, most commonly a dendrite. Such a relatively basic cell process can be formed by animal cells under different circumstances. For example, this can be seen in the formation of the bacterial pedestal (Fig. 1). In mammalian cells, pathogenic bacteria readily induce the formation of an actin-based spine-like structure, the pedestal, which the bacteria use for adhesion to the cell surface (Goosney et al. 1999; Campellone and Leong 2003). So a spine synapse is a combination of two things, i.e., the function of a chemical synapse (1) is modified by placing it at the end of a cell protuberance (2). The basic structure of synaptic spines was established early in evolution and appears to be roughly the same in all animal groups, but they have been studied best in mammals. Typical spines have a head and narrower neck region and project from the sides of dendrites (Gray 1959; Coss and Perkel 1985; Peters et al. 1991; Harris and Kater 1994; Sorra and Harris 2000; Harris and Weinberg 2012; Frotscher et al. 2014) (Fig. 1); spines also may project from a neuron cell soma (Peters et al. 1991). They broadly come in three basic shapes: (1) large, mushroom spines with enlarged head regions, (2) short, stubby spines without a clearly defined neck, and (3) thin spines with a relatively slender head and neck (there are also cup-shaped and branched spines; Hering and Sheng 2001; Bourne and Harris 2008; Harris and Weinberg 2012; Petralia et al. 2014). In addition to the actin core (Dent et al. 2011; Racz and Weinberg 2013; Chazeau and Giannone 2016), synaptic spines contain a few other typical components that can only be described briefly in this review. The postsynaptic portion of the spine has a post-synaptic density (PSD) containing the scaffolding and regulatory proteins associated with the neurotransmitter receptors and other membrane proteins of the postsynaptic membrane (PSM) (for more information, see for example, Zheng et al. 2011; Emes and Grant 2012; Harris and Weinberg 2012; Sala and Segal 2014; Chen et al. 2015). Typically, spines also contain some portions of endoplasmic reticulum and endosomes/endosomal vesicles and occasionally singular ribosomes and polyribosomes (Spacek 1985; Peters et al. 1991; Cooney et al. 2002; Sorra and Harris 2000; Petralia et al. 2001; Harris and Weinberg 2012; Horak et al. 2014; Lichnerova et al. 2015). Micro-tubules may enter spines under certain circumstances and may be important for neuronal plasticity (Hoogenraad and Bradke 2009; Hoogenraad and Akhmanova 2010; Dent et al. 2011). Mitochondria generally are excluded from most kinds of spines, but are present in some specialized spines (Peters et al. 1991; Sorra and Harris 2000; Harris and Weinberg 2012).
Fig. 1.
a Animal cells can be induced readily to form an actin-filled projection. In this case, pathogenic bacteria have induced intestinal epithelial cells to form actin-filled bacterial pedestals for attachment of the bacteria. b A typical synaptic spine is basically a chemical synapse projected from the end of an actin-filled projection. All animals with nervous systems have chemical synapses that are formed with a presynaptic terminal (pre; synaptic vesicles are illustrated as black circles) directly on a dendrite shaft or other postsynaptic structure such as a soma, but without an intervening spine. Spine synapses include invaginating ones that are found in simple animals without brains, the Cnidaria, as well as in several examples from higher animals with brains (Bilateria). Non-invaginating spine synapses are common in many bilaterians; typical kinds include thin spines and mushroom spines. See text for details. Note: that all postsynaptic structures in all of the figures are colored blue (Color figure online)
However, many postsynaptic processes are difficult to classify as spines versus dendrite branches. In some of these cases, it is difficult to distinguish a profile of a spine in an electron micrograph from a cross or oblique section through a thin dendrite or other neurite; postsynaptic profiles that are filled with many microtubules (cut in cross/oblique section) can be considered definitive dendrite shafts, but often this is not evident in published micrographs. This is especially true for little studied animals or neural circuits. In other cases, the postsynaptic processes are the smallest terminal branches of dendrites and can appear spine-like in micrographs, e.g., in the vertebrate retinal ribbon synapse or the terminal dendritic claws of cerebellar granule cells (Shepherd 2004; Sterling and Demb 2004). Other dendritic protuberances such as the thorny excrescences of the CA3 region of the hippocampus are very large, branching and filled with many organelles (Johnston and Amaral 2004); they are considered to be complex, branched spines, but are they actual spines or highly modified dendritic branches. These varied spine-like structures defy easy classification as definitive spines or definitive dendrite shafts and probably there is some continuum between them; they are not prototypes to spines, but are fully functional postsynaptic processes that function much like idealized spines.
In this review we will examine the diversity of morphology of both definitive synaptic spines and a variety of synaptic spine-like processes, considering their origins in the simplest animals and their variety among various invertebrates and vertebrates (Table 1). And we will suggest that spine synapses can be divided into two functional types—invaginating and non-invaginating, with the former appearing first in the evolution of the animal nervous system, and the latter appearing with the first brains (Fig. 1). We will concentrate on these points; other excellent reviews already provide more detailed coverage on general synapse structure and molecular biology (Newpher and Ehlers 2009; Harris and Weinberg 2012; Bailey et al. 2015), spine actin dynamics and spinogenesis (Dent et al. 2011; Bosch et al. 2014; Bellot et al. 2014; Sala and Segal 2014; Chazeau and Giannone 2016), and general evolution of synapses (Emes and Grant 2012).
Table 1.
Summary of the major groups of animals described in this review, and their invaginating and non-invaginating synaptic spines
| Group | Invaginating Spines | Non-invaginating Spines |
|---|---|---|
| Porifera (sponges; no definitive neurons or synapses) | Possible spine-like invaginating processes1 | None described |
| Ctenophora (comb jellies) | None described | Possible spine-like process on Colloblast2 |
| Cnidaria (jellyfish, sea anemones, corals, hydroids) | Well-developed in photoreceptor synapses of cubozoan jellyfish3
|
None described |
| Flatworms (Platyhelminthes; Acoelomorpha) | Occasionally seen5 | Well-developed6 |
| Nematodes (roundworms) | None described | None described, but have postsynaptic spine-like muscle arms at neuromuscular synapses7 |
| Chaetognatha (arrow worms) | None described | None described |
| Rotifera (rotifers) | Possible example8 | None described |
| Phoronida (horseshoe worms) | None described | None described |
| Bryozoa (moss animals) | None described | None described |
| Annelida (leeches, earthworms, various marine worms) | None definitive | In leeches, spines on processes of large motor neurons9
|
| Mollusca (gastropods like snails and sea hares, bivalves like clams, cephalopods like squid and octopi) | In photoreceptor terminals of squid and octopi11 | In the stellate ganglion of squid, associated with giant axons for rapid escape jetting14 |
| Arthropoda (horseshoe crabs, spiders, crustaceans like crabs and lobsters, insects) | In photoreceptor terminals of wolf spiders17 and lobsters18 | Common in insect brain; good examples include Kenyon cell dendritic spines of the honeybee19 and those in a group of visual interneurons of Drosophila20 |
| Onychophora (velvet worms) | None described | Possible spines23 |
| Echinodermata (starfish, sea urchins, sea cucumbers) | One illustrated from a sea cucumber24 | None described |
| Hemichordata (acorn worms, pterobranchs) | None described | None described |
| Invertebrate chordates (sea squirts or ascidians, amphioxus or lancelets) | At the base of coronal organ hair cells of a colonial ascidian25 | In the larvae of the amphioxus (lancelet)26 and adult sea squirt27 |
| Vertebrates (jawless fish including hagfish and lampreys, sharks and rays, bony fish, amphibians like frogs and salamanders, reptiles like lizards and turtles, birds, and mammals like rats, mice, rabbits and monkeys) | In photoreceptor terminals of all vertebrate groups; synaptic structure evolves from simple invaginating postsynaptic processes in some hagfish, to complexes of invaginating postsynaptic processes in other vertebrates28 | Widespread on many kinds of neurons in all classes of vertebrates |
Includes various postsynaptic spine-like protuberances as described in the text
References: Major references are included here. These and additional references are discussed in the appropriate sections of the text
Holmberg (1970, 1971); Holmberg and Ohman (1976); Haverkamp et al. (2000); Sterling and Matthews (2005)
Invaginating Spine Synapses in Early Animal Evolution
Were the First Synaptic Spines Invaginating Projections?
Porifera
A definitive nervous system and synapses are found in only two of the four simplest metazoan/multicellular animal groups, the Ctenophora or comb jellies and the Cnidaria, which includes jellyfish, sea anemones, corals, and hydroids. The other two groups of simple metazoans, the Placozoa and Porifera (sponges), can exhibit some simple coordinated motions/behaviors, but lack any clear evidence of definitive neurons and chemical synapses (Mackie et al. 1983; Nickel 2004; Ellwanger et al. 2007; Jorgensen 2014; Smith et al. 2014, 2015; Leys 2015; Ryan and Chiodin 2015). Yet both groups have some cells with elongate processes that resemble neurons, at least superficially (Jorgensen 2014; Smith et al. 2014; Leys 2015). Some cells in the mesenchyme of the sponge, Tethya lyncurium have “bouton”-like structures resembling presynaptic terminals at the end of some of the elongate cell processes, and also have spine-like structures that can project from the sides of the elongate cell processes. Both the bouton-like and spine-like structures invaginate into the sides of other cells (Pavans de Ceccatty 1966). Invaginating projections are common in the nervous system of most kinds of animals, including many kinds that are directly associated with synapses, but in most cases, they are not definitive synaptic spines, simply because they do not form a direct chemical synapse with a presynaptic process; and except for this one example in sponges, they will not be described in this review (we have reviewed these structures in detail previously; Petralia et al. 2015). While many of these invaginating projections are rather small structures, in some cases, the entire synaptic spine can be an invaginating projection into the presynaptic terminal. In this sponge, the spine-like invaginating structures project from a main shaft that is filled with parallel filaments about the size of actin filaments. In the micrograph shown in the paper (Fig. 28 in Pavans de Ceccatty 1966; see also illustrations in Petralia et al. 2015), a few of these filaments appear to extend up into the spine-like structure, which also contains some irregular vesiculate organelles. The cytoplasm of the opposing cell (a scleroblast) contains a variety of vesicles and vesiculate organelles near the invaginating spine-like structure, with at least one small vesicle appearing to contact the opposing membrane (i.e., that is across the “cleft”), and a mitochondrion also is adjacent to this contact point. We cannot rule out that such spine-like structures have only a mechanical function in sponges, but if they do represent some kind of early evolved synaptic spine precursor, it is curious that the first ones would be invaginating structures. Invaginating projections may have a number of different functions, not directly related to definitive chemical neurotransmission (Petralia et al. 2015). Notably they form an efficient means to isolate the transfer of a chemical signal from one cell to another, i.e., there is less chance of spillover of the chemical messenger if it is confined to an invagination.
Ctenophora and Cnidaria
Synapses are well developed in the two groups of simple metazoans with nervous systems, the Ctenophora and Cnidaria. These groups show a wide variety of synaptic structure and arrangement (Hernandez-Nicaise 1973; Westfall 1996); synapses in both groups include those that are one-way (asymmetrical polarity), two-way (symmetrical polarity), and reciprocal (adjacent, opposite synapses). Note that one-way synapses, of course, became the preferred type in all higher animal groups, with reciprocal synapses fairly widespread also; two-way synapses may be rare in higher animal groups (possible two-way synapses have been described in some crustacea; Hama 1961; Hamori and Horridge 1966). Some recent studies have suggested that the Ctenophora and Cnidaria have evolved separately, and thus have separate origins for their nervous systems (Moroz et al. 2014; Moroz and Kohn 2016), but this is still controversial (Pisani et al. 2015; Arendt et al. 2016). Indeed, ctenophores may lack many of the common neurotransmitters found at the synapses of Cnidaria and higher animals, such as acetylcholine, serotonin, dopamine, and norepinephrine, but they do utilize glutamate and glycine as do other animals (Moroz et al. 2014; Alberstein et al. 2015; Moroz and Kohn 2016). Ctenophores show an unusual synaptic ultrastructure called a presynaptic triad, with a row of vesicles lining the presynaptic membrane, followed by a flattened sac (cistern) of endoplasmic reticulum (ER; connected to the rough ER of the neuron), and one or more mitochondria (Hernandez-Nicaise 1973). However, this is not entirely a unique structure; the cnidarian jellyfish, Cyanea capillata (Anderson and Grunert 1988), has two-way synapses that have a similar triadic structure, thus resembling those of Ctenophora (Hernandez-Nicaise 1973).
Definitive spine synapses do not seem to occur in the Ctenophora. However, synapses do appear to form on a spine-like structure at the base of colloblasts (Franc 1978). Colloblasts are specialized adhesive cells on the surface of tentacles and are used for prey capture; they are pear-shaped with an expanded peripheral part and a slender, deep basal portion ending in a cone-shaped root structure. Colloblasts are similar in many structural and functional ways to the nematocytes/cnidoblasts of cnidarians (Franc 1978). The synapse onto the colloblast is described for four species from different genera (Franc 1978) although illustrated only for one of them, Pleurobrachia rhodopsis. The synapse forms on a spine-like expansion (not invaginating) of the basal membrane near the root structure. The micrograph (figure 14 in Franc 1978) of this expansion shows little evidence of cytoplasmic structures, so possibly the enlarged size of this structure is somewhat artifactual. Also, while Franc (1978) implies that this synaptic spine-like structure is present in the four different species studied, Benwitz (1978) illustrates this synaptic contact in Pleurobrachia pileus and shows only a small protuberance rather that an enlarged expansion of the postsynaptic membrane.
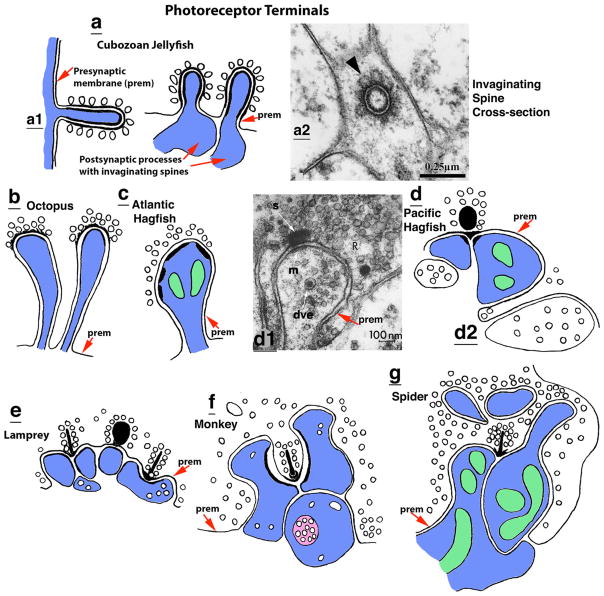
Synapses in cnidarians are well developed and include a variety of structures as noted above (Westfall 1996), and these appear to utilize a wide array of neurotransmitters including serotonin, dopamine, norepinephrine, GABA, glutamate, acetylcholine, and neuropeptides such as RFamide and vasopressin (Koizumi et al. 2004; Westfall 2004; Oliver et al. 2008; Marlow et al. 2009; Kelava et al. 2015; Arendt et al. 2016). In spite of the wide variety of synapse types, there do not appear to be any conventional spine synapses. However, Holtmann and Thurm (2001) show a micrograph of a postsynaptic afferent ending that appears to be a spine invaginating into a “concentric” sensory hair cell of a spherical end-knob of a tentacle of the hydroid (hydra-like), Coryne tubulosa (Holtmann and Thurm 2001). In addition, in this species, the stinging cells or nematocytes (another type of sensory hair cell; Oliver et al. 2008) have a basal invagination called a “basal tunnel” with a bundle of 10–20 neurites that may be in synaptic contact with the nematocyte (Holtmann and Thurm 2001). Holtmann and Thurm (2001) note that the latter structure somewhat resembles the invaginating synaptic complex of vertebrate photoreceptors (see later in this review); however, the “presynaptic” nematocyte here contains only a single large vesicle, and the neurites appear to be en passant contacts and not spines. More definitive invaginating synaptic spines project from photoreceptor cells of the upper and lower lensed eyes of several species of cubozoans (cubomedusae; Yamasu and Yoshida 1976; Gray et al. 2009) (Fig. 2a). These eyes are developed to an incredible extent, resembling the complex eyes of higher invertebrates, with cornea, lens and three-layered retina (vitreous, pigmented, and nervous layers). The region containing these invaginating spine synapses bears a number of common neurotransmitters including RFamide, glutamate, serotonin, and GABA (Martin 2002). Overall spine diameter ranges from 0.08 to 0.23 μm with an average of 0.154 μm (Gray et al. 2009). In long section, the one illustrated in Yamasu and Yoshida (1976) resembles a typical spine with enlarged head and short neck and is about 0.25 μm long. In this study, the postsynaptic spines originate from neurites and invaginate into sensory cells (presumably the photoreceptor cells); both pre- and post-synaptic membranes are thickened and the presynaptic vesicles are about 80 nm in diameter. In contrast, illustrated examples cut length-wise in Gray et al. (2009) are more elongate with straight sides and a slightly enlarged head, and are about 0.5 μm long (the longest is 629 nm). In the latter study, pre- and postsynaptic cells were not often identified, but putative photoreceptor cells could be either presynaptic (invaginated) or postsynaptic (invaginating) or both for adjacent putative photoreceptor cells, and neurites could be presynaptic to the spine. As many as three invaginating spines are found in a single process although most have only one visible in sections. As for Yamasu and Yoshida (1976), both pre- and postsynaptic membranes of invaginating spine synapses described by Gray et al. (2009) are thickened. Some non-invaginating postsynaptic processes also are present, but are not described in detail by Gray et al. Finally, at least one other cnidarian may have invaginating spine synapses (see Fig. 6 in Singla 1978).
Fig. 2.
Invaginating spine synapses typically form with the photoreceptor (retinal) terminals of animal eyes. a Invaginating postsynaptic processes in photoreceptors of cubozoan jellyfish. a1 In the example on the left, the invagination is between the sides (lateral membranes) of two putative photoreceptor cells, while the example on the right illustrates two postsynaptic spines invaginating into the base of a photoreceptor cell. a2. This is a cross section through the invaginating spine. Note the two dense rings representing the pre- and postsynaptic membranes, and the single row of large presynaptic vesicles (arrowhead; Gray et al. 2009). b In the octopus, several postsynaptic spines will invaginate into a large photoreceptor terminal (Dilly et al. 1963; Case et al. 1972). c The Atlantic hagfish, Myxine glutinosa, possesses the simplest kind of invaginating synapses among vertebrate photoreceptor synapses. The large spine-like processes have mitochondria and several active zones with PSDs, but seem to lack presynaptic dense bodies (Holmberg 1970; Holmberg and Ohman 1976). d Other kinds of hagfish, such as the Pacific hagfish, have presynaptic vesicles surrounding spherical synaptic bodies (two of the variations in synapses are shown in d1 and d2; Holmberg, 1971; Holmberg and Ohman 1976). s. presynaptic body; m. postsynaptic membrane; dve, dense-cored vesicle; R, photoreceptor terminal filled with synaptic vesicles (letters have been superimposed over the letters of the original micrograph, for clarity). e Lampreys (Lampetra fluviatilis) are the simplest fish to show the characteristic, vertebrate elongate, ribbon synapse, typically contacting two postsynaptic processes (but probably from one to three); occasionally, a more plate-like ribbon profile (middle one; asterisk) is seen in sections (Holmberg and Ohman 1976). f Invaginating postsynaptic processes (two from horizontal cells and one from a bipolar cell) at a cone cell ribbon synapse in the retina of the macaque monkey, Macaca fascicularis (Haverkamp et al. 2000). g The invaginating synapse in the photoreceptor of the wolf spider resembles those of vertebrates, including a rod or ribbon-like structure in the presynaptic terminal (Trujillo-Cenoz and Melamed 1967). The postsynaptic processes also extend some thinner processes deeper into the presynaptic terminal and these are surrounded closely by presynaptic vesicles; but it is not certain whether these thinner processes (spinules) form definitive synapses or not (see Petralia et al. 2015 for a detailed discussion on various kinds of invaginating projections that are not described in this review). (mitochondria are colored green, and a multivesicular body is colored pink; prem = the presynaptic membrane that is invaginated by the postsynaptic process). Drawings in all figures are original and based on micrographs and illustrations from works published by other authors, and described in the text. The micrograph in a2 is figure 3B from Gray et al. (2009; Biol. Bull. 217:35–49), reprinted with permission from the Marine Biological Laboratory, Woods Hole, MA (and from Dr. R.A. Satterlie); that in d2 is a reprint of Figure 11 from Holmberg (1970), with permission from Springer Publishing Company (Color figure online)
Are Invaginating Spine Synapses Found in Those Simple Metazoans with Relatively More Active Responses and Complex Behaviors?
“Life is a beautiful magnificent thing, even to a jellyfish” (Sir Charles Spencer “Charlie” Chaplin, from Limelight, 1952).
The discussion above shows that only a few examples of spine synapses appear to be present among the many kinds of simple metazoans. Why do spine synapses form in only these select cases? What do they have in common? These cases seem to involve relatively rapid responses and complex behaviors, at least in comparison to most responses and behaviors of simple metazoans.
First, we noted that the sponge, Tethya lyncurium, has spine-like invaginating processes growing off of elongate, neurite-like processes (Pavans de Ceccatty 1966). Sponges generally are regarded as sessile, quiescent animals. However, those in the genus Tethya are unusually active. They show rhythmic body contractions (spreading at 12.5 μm/s; Nickel 2004), respond to stimuli, and can even walk on “feet” (podia)! In the wild, Tethya sponges can move 5–8 cm per week, often either moving toward or moving away from other Tethya sponges in the vicinity (Fishelson 1981). Contractions in Tethya can be induced by a number of neuroactive substances and especially GABA and to a lesser extent, glutamate; these probably activate a metabotropic glutamate/GABA-like receptor known to occur in sponges (Ellwanger and Nickel 2006; Ellwanger et al. 2007).
In the Ctenophora, Franc (1978) noted a possible spine-like synaptic structure (although not invaginating) at the base of the colloblast of four kinds of ctenophores. Col-loblasts are utilized as part of the mechanism for prey capture in ctenophores, so this might be a case where rapid neural control of cell function is beneficial.
The two kinds of Cnidaria, cubomedusae and hydroids, in which invaginating spine synapses have been described so far, seem at first to be very different. The cubomedusae have invaginating spine synapses (Fig. 2a) associated with the receptors of their highly complex eyes that resemble those of higher animals. These eyes may be involved in many advanced behaviors such as avoiding obstacles, active prey capture (even of fish), and complex mating behaviors (Coates 2003; Martin 2004; Nilsson et al. 2005; Parkefelt et al. 2005). In contrast to the studies of cubomedusae, the other study where we have noted an invaginated, spine-like synapse concerns an afferent neurite terminal on a sensory hair cell on tentacles of a hydroid, Coryne tubulosa (Holtmann and Thurm 2001). Hydroid polyp forms lack the eyes found in medusa (jellyfish) forms and have comparatively simple nervous systems; most are sessile, passive feeders. In contrast, a couple of kinds of polyps including Coryne show active prey capture behavior; when the polyp senses prey it bends itself toward the prey with a quick jerk of the body (Tardent and Schmid 1972; Miglietta et al. 2000).
Do Invaginating Spine Synapses in Higher Animals Also Support Relatively More Active and Rapid Sensory Responses?
The spine synapses in the first animals may have evolved in response to development of more active and rapid responses to sensory stimuli. Interestingly, these first spines invaginate into the presynaptic process, with the most definitive examples associated with the most highly complex sensory structure known in simple animals—the lensed eyes of cubozoan cnidarians. Are there examples of invaginating spine synapses associated with advanced vision and other such fast sensory/response systems in higher animals? Indeed, there are examples in a number of higher taxa of animals, especially in some mollusks and vertebrates.
Mollusca
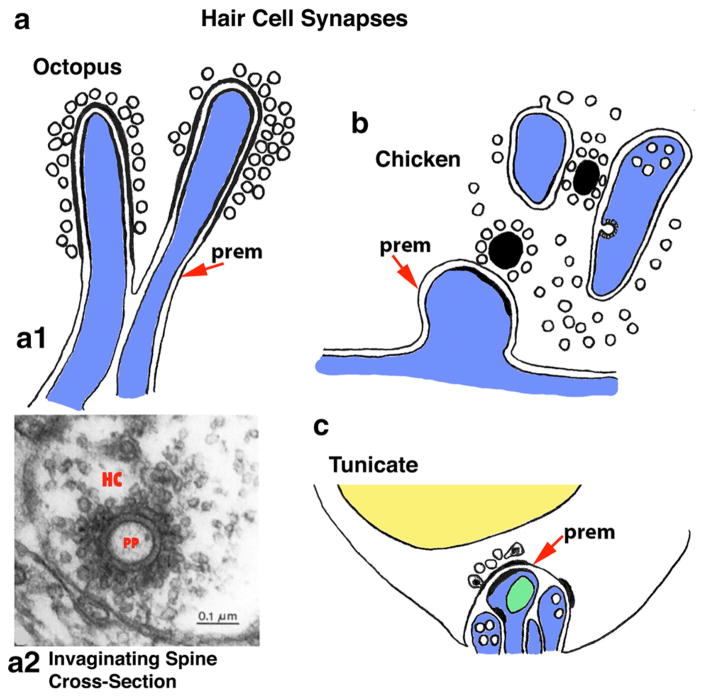
In mollusks, examples of invaginating spine synapses are associated with the gill-withdrawal reflex, a rapid reaction to danger, of the sea hare, Aplysia californica (a gastropod), and for the octopi and squid (cephalopods), both in association with balance and hearing and with their highly complex eyes and vision. The invaginating spine synapses associated with the gill-withdrawal reflex of Aplysia (Bailey and Thompson 1979; Bailey et al. 1979) are roughly similar in size or slightly larger, and similar in structure to those that we described above in cnidarians. Both regular, flat synaptic contacts, which may be spines in many cases, and the invaginating spine (indented) contacts are formed with the mechanoreceptor sensory neuron terminals; the invaginating ones have about twice the number of presynaptic vesicles. The authors suggest that the invaginating spine synapses are more effective and are formed in association with learning plasticity in the animal’s defensive gill-withdrawal reflex. In the macula of the octopus (Octopus vulgaris) statocyst (organ of balance and hearing; Budelmann and Thies 1977; Colmers 1977; Budelmann et al. 1987; Kaifu et al. 2008), postsynaptic spines invaginate into the receptor hair cell base up to 1.5 μm (maximum diameter of 0.3 μm; Budelmann and Thies 1977) (Fig. 3a). These spines can be relatively straight or have an enlarged head. The best developed invaginating spine synapse may be in the photoreceptor terminals from the eyes of squid (Cohen 1973) and octopi (Dilly et al. 1963; Case et al. 1972). In particular, the large, carrot-shaped photoreceptor terminals in octopi may contain several invaginating spines (Fig. 2b).
Fig. 3.
Invaginating spine synapses often form at the base of mechanoreceptor hair cells of sensory organs that sense sounds, gravity, and motion. a Invaginating spines in the synapses with hair cells of the macula of the statocyst of the octopus, involved in balance and hearing (Budelmann and Thies 1977). a2 is a cross section of one of the invaginating spines shown in a1; note the two dark rings surrounding the postsynaptic process (pp), representing the pre- and postsynaptic membranes, and the surrounding cluster of presynaptic vesicles in the hair cell (HC) (red letters have been superimposed over the black letters of the original micrograph, for clarity). b Invaginating postsynaptic processes in the hair cells of the inner ear of the chicken. The synaptic bodies are round or oval; the bottom structure is a definitive spine synapse, while the upper two structures are deeply invaginating processes that may also act as definitive synapses, but the synaptic contacts are not distinctive in this example (Tanaka and Smith 1978). c Hair cells of the coronal organ of the colonial ascidian or tunicate, a simple chordate (vertebrates also are chordates). Note how several processes form together in an invaginating pocket at the base of the hair cell (reminiscent of the complex of processes at the base of mammalian rods and cones of the retina). The larger postsynaptic afferent process with a mitochondrion (in green) forms opposite a few presynaptic vesicles including a couple with dense cores. Other processes in this complex include a couple of presynaptic efferent terminals forming synapses with the postsynaptic afferent process and hair cell base, as illustrated (Burighel et al. 2003). (mitochondrion is green, and nucleus of hair cell is yellow; prem = the presynaptic membrane that is invaginated by the postsynaptic process). The micrograph in a2 is a reprint of Figure 2D from Budelmann and Thies (1977) with permission from Springer Publishing Company (Color figure online)
Chordata/Vertebrata
There are remarkably close parallels between the invaginating spine synapses associated with balance/hearing and vision in mollusks and vertebrates; in both groups of animals, the invaginating synapses enter either the base of the mechanoreceptor hair cell (balance/hearing) or the photoreceptor cell’s enlarged presynaptic terminal (vision). The striking difference between these synapses in mollusks versus vertebrates is the presence of a presynaptic ribbon structure that anchors and organizes the synaptic vesicles in vertebrate balance/hearing and vision receptor cell presynaptic terminals; ribbon synapses have not been described in the mollusk versions of these synapses. Invaginating spines forming synapses with synaptic ribbon structures (usually they form more oval synaptic body structures rather than elongate ribbons) are found in the hair cells of some vertebrates. They can be common during postnatal development of mouse cochlear inner hair cell synapses (Sobkowicz et al. 2003). Similarly, invaginating spine synapses can form during a mid-synaptogenesis developmental stage of inner ear hair cells in the embryonic chicken (Whitehead and Morest 1985). They also appear in hair cell synapses in the inner ear of the adult chicken (Tanaka and Smith 1978) (Fig. 3b), although other studies using different breeds (and ages) of chickens illustrate indenting/partially invaginating postsynaptic processes of afferent fibers but do not describe fully invaginating ones (Hirokawa 1978; Fischer 1992). Takasaka and Smith (1971) note that afferent processes “occasionally make small invaginations” into the hair cells of the adult pigeon, although they do not illustrate this phenomenon; however, they do show partial invagination of a presynaptic efferent terminal into a hair cell. Images in Hamilton (1968) show invaginating postsynaptic processes in some vestibular hair cells of the adult rat, but it is not clear whether these are really involved directly in a synaptic connection (Petralia et al. 2015). The formation of invaginating spines in the hair cell synapses of the mouse ear in early postnatal development, as described above (Sobkowicz et al. 2003) is paralleled by a similar formation of invaginating spine synapses in the next synapse in the hearing neural pathway. Thus, in early postnatal development of the cat, giant endbulbs of Held, formed by peripheral auditory fibers in the anteroventral cochlear nucleus, are invaginated by spines from the postsynaptic spherical bushy neurons; these invaginations are large at birth and lost gradually over the first three weeks of postnatal development, leaving only flat or slightly indented synaptic contacts (Ryugo et al. 2006; Baker et al. 2010).
Evidence of the evolution of the invaginating spines in hair cells may be found in the ascidians or urochordates (sea squirts), which are a group of invertebrate chordates and probably close to the ancestors of vertebrates (Burighel et al. 2003). The colonial ascidian, Botryllus schlosseri, bears a “coronal organ” that contains hair cells and appears to be homologous to the vertebrate acoustic-lateralis system (i.e., hearing, balance and lateral line sense in vertebrates). Afferent terminals, typically in groups of two or more (and sometimes with presumed efferent terminals) invaginate into a pocket at the base of the hair cell (Fig. 3c). The presynaptic side has clear and dense-cored synaptic vesicles, but no synaptic ribbon structure (Burighel et al. 2003). It is curious that at least one cnidarian, as described earlier, has a group of neurites in a hair cell “basal tunnel” that looks at least superficially like the basal hair cell pocket described here; and similarly, both of these look superficially like the basal neurite-filled pocket in many vertebrate photoreceptor cells, described below. This suggests some kind of common functional organization for neurotransmission in these sensory cells.
In contrast to those of vertebrate hair cells, postsynaptic processes in the photoreceptor (rods for sensitivity and cones for color vision) terminal synapses of vertebrates often invaginate into the enlarged terminals in the outer plexiform layer of the retina. The invaginating processes in cones are the ends of dendrites of horizontal and bipolar neurons and form tetrads or triads of postsynaptic processes around the ribbon synapse (Sterling and Matthews 2005). These invaginating processes typically have few if any organelles except some vesicles and flocculent material (Blanks et al. 1974; Fisher and Boycott 1974; Kolb 1977; Dacheux and Raviola 1982), and thus appear to be spine-like although most authors do not call them spines. Sterling and Mathews (2005) do call the horizontal cell processes in both rod and cone terminals, “spines.” Note that rod terminal horizontal processes originate from the horizontal cell axon, identified by the neuron’s general structure; however, this axon does not have action potentials and its terminal arborization may behave in some ways as an independent structure (Nelson et al. 1975; Sterling and Demb 2004; the complexities of these interactions are beyond the scope of this review). As we noted for mollusks, the degree of invagination of processes into photoreceptor terminals could be associated with synaptic plasticity. In the cone cell terminals of the turtle, Pseudemys scripta elegans, the invaginating processes are extensive in dark-adapted turtles and greatly reduced after light exposure; the degree of invagination also is temperature dependent (Schaeffer and Raviola 1976). A similar phenomenon has been studied in more detail for fish retinal spinules; these elaborate, thin processes are smaller than true synaptic spines and extend from horizontal cell dendrite processes in the cone cell terminals of fish; a large number of studies have looked at their plasticity in response to light and to a wide variety of neuroactive substances (reviewed in Petralia et al. 2015). Finally, note that in addition to invaginating synapses in photoreceptor terminals of the outer plexiform layer of the retina, synapses in the inner plexiform layer of the retina can have invaginating spines or spine-like postsynaptic processes (Dubin 1970; Wässle et al. 1995).
The early evolution of the vertebrate retinal ribbon synapse with its invaginating postsynaptic processes is not well understood. Structural and functional studies of the retinal synapses in the most structurally primitive living vertebrates, the cyclostomes or agnathans (hagfish and lampreys), may provide clues to this evolution. The simplest eyes and corresponding retinal structures are found in the hagfish (and probably larval lampreys), while the eyes and retinal structure of adult lampreys are more similar to the general pattern of other vertebrates, as described in the previous paragraph (Lamb et al. 2007, 2008). The exact phylogenetic relationships between hagfish and lampreys is controversial, with some authors suggesting that hagfish are more primitive (Lamb et al. 2007, 2008) and others considering hagfish structural simplifications to be the result of degeneracy (Heimberg et al. 2010), but this problem is beyond the scope of this review. The retinal photoreceptor synapses of the Atlantic hagfish, Myxine glutinosa, are the simplest, lacking any kind of presynaptic ribbon or similar dense structure; postsynaptic processes are large invaginating structures, with either few or with many vesicles and often with mitochondria (Holmberg 1970, 1971; Holmberg and Ohman 1976) (Fig. 2c). Other kinds of hagfish have similar invaginating postsynaptic processes but also have distinctive round, synaptic bodies surrounded by the presynaptic vesicles (Holmberg 1971; Holmberg and Ohman 1976; Lamb et al. 2007) (Fig. 2d). Finally, lamprey photoreceptor synapses (Fig. 2e) mainly resemble those of jawed vertebrates (Fig. 2f) with long synaptic ribbons (rod-like in profile in micrographs) apposing small, postsynaptic processes (typically two); however, occasional synapses have plate-shaped ribbons resembling the round synaptic bodies found in hagfish (Holmberg and Ohman 1976; Lamb et al. 2007). Hagfish probably lack vision although they can respond to light, and this accounts for their rather simple retinal synaptic structure; their eyes are believed to act as nonvisual receptors similar to the pineal organ of other vertebrates. Interestingly, the pineal and related organs of vertebrates also have definitive ribbon synapses (Vigh et al. 2002); typically, the synaptic contacts are flat, but in some cases, there are distinctive invaginations of the dendritic postsynaptic processes (Oksche and von Harnack 1963; Kelly and Smith 1964). Another structure associated with photoreception is the suprachiasmatic nucleus (Güldner 1976); it receives input from the retina, controls circadian rhythms, and regulates melatonin secretion by the pineal (Benarroch 2008). It has some interesting “spine-like protrusions” that invaginate into presynaptic terminals; these terminals have round and flat vesicles and form symmetric synapses with the protrusions; and there are 5–10 dense projections (60–80 nm) on the presynaptic membrane. Thus, it appears that the evolutionary design of photoreceptor synapses of vertebrates includes consistently both ribbon synapses and associated invaginating postsynaptic dendritic endings or spines. The unique hagfish photoreceptor synapse, with invaginating processes but lacking definitive ribbons, may represent an intermediate evolutionary stage between ancestral invertebrate and vertebrate designs, but it may also be just a consequence of degeneracy in vertebrate evolution, as noted above.
Arthropoda
The high complexity of the advanced visual systems seen in mollusks and vertebrates also is present in arthropods; this includes both simple eyes and compound eyes that can have thousands of separate photoreceptor units (ommatidia). However, these eyes have relatively few examples of invaginating spine synapses. The photoreceptor synapse structure of arthropod eyes is remarkably similar to that of vertebrate eyes, typically with presynaptic vesicles surrounding a synaptic bar or ribbon and two or three postsynaptic processes (Trujillo-Cenoz 1965). In the wolf spider, Lycosa erythrognata or L. thorelli, the bar has a rod-like profile in sections (Fig. 2g), similar to the synaptic ribbon of vertebrate retinal photoreceptors (Fig. 2f), while it is T-bar shaped in flies (Trujillo-Cenoz 1965; Trujillo-Cenoz and Melamed 1967). Among spiders, wolf spiders are active hunters with particularly good vision; similarly, most of the familiar kinds of flies have excellent vision. In fact, flies and mammals have a very similar pattern of circuitry for motion vision (Borst and Helmstaedter 2015). A variety of processes can invaginate into the photoreceptor terminal of arthropods. Perhaps the most well known are the capitate projections in fly eyes, but these are projections from glial cells (Trujillo-Cenoz 1965; Petralia et al. 2015). There also can be various non-synaptic invaginating projections derived from the postsynaptic processes; most of these probably are not true synaptic spines because they appear to lack a definitive active zone of neurotransmitter release (Trujillo-Cenoz 1965; Trujillo-Cenoz and Melamed 1967; Hafner 1974). An analogy to these are the fish retinal spinules mentioned above. True invaginated spines may be found between one of two kinds of postsynaptic processes formed with the large photoreceptor (retinula) terminals in the optic lamina of the lobster, Homarus vulgaris (Hamori and Horridge 1966). These terminals form typical ribbon synapses with postsynaptic ganglion cell axons, which also invaginate processes into the retinula terminals. While the latter may not be true synaptic spines, true spines appear to be associated with a second kind of postsynaptic process—the transverse fibers. The synapses formed between transverse fibers and retinula terminals contain presynaptic vesicles but lack ribbons, and some of these synapses are on elongate postsynaptic spines that can invaginate more than a micrometer into the retinula terminals.
The Predominance of the Non-Invaginating Dendritic Spine Synapse in Bilaterian Animals
Non-Invaginating Spine Synapses are Found in the First Brains
“Why, anybody can have a brain. That’s a very mediocre commodity. Every pusillanimous creature that crawls on the Earth or slinks through slimy seas has a brain.” (L. Frank Baum, from The Wonderful Wizard of Oz, 1900).
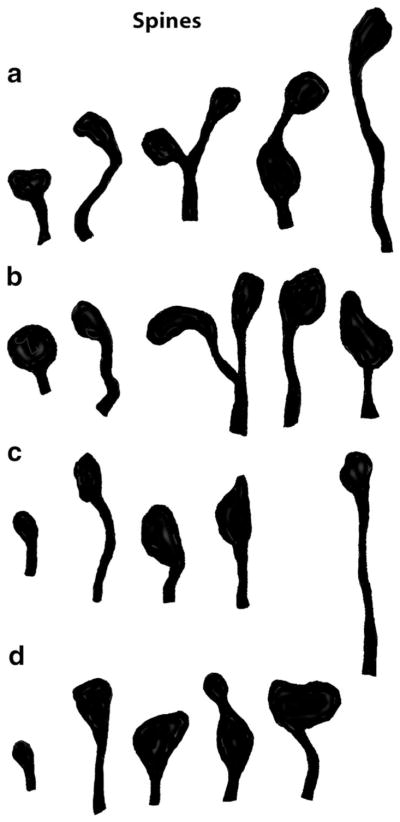
The nervous systems of flatworms, the first animals to evolve bilateral symmetry and heads, and with it, a true brain, are already similar to the vertebrate brain in many ways, and show a similar range of classical neurotransmitters and neuropeptides (Keenan et al. 1981; Halton and Gustafsson 1996; Buttarelli et al. 2008). The well-organized, compact brains of free-living flatworms (Turbellaria) contain from 50 to 550 neuron somas, while the brains of parasitic flatworms such as flukes and tapeworms tend to be less compacted (Halton and Gustafsson 1996). Golgi staining of the brain of the free-living flat-worm, Notoplana acticola, reveals several types of neurons, with multipolar ones most common (Keenan et al. 1981). At least 3 kinds of neurons have processes with spines; they are best developed on the stubby processes of the fusiform neuron with an average length of just under 2 μm. Coss and Perkel (1985) used Golgi staining to compare spine synapses in flatworms to those in honeybees and vertebrates, showing that the range of spine shapes are rather similar among these various animals (Fig. 4). Studies of flatworm synapses at the ultrastructural level do not specifically identify spines, but show that synapses in general are abundant and vary in form; vesicles include various combinations of small clear ones and larger dense-cored vesicles (Fig. 5). There sometimes is a presynaptic paramembranous density roughly resembling the T-bar shaped presynaptic densities seen in many arthropods (types of “ribbon” synapses, as discussed above for arthropods and vertebrates), and there may be one to three postsynaptic processes (Reuter 1981; Halton and Gustafsson 1996; Bedini and Lanfranchi 1998; Mäntylä et al. 1998). Occasionally postsynaptic processes can invaginate into the presynaptic terminal, but these invaginating synapses do not seem to be common (Morita and Best 1966; Petralia et al. 2015). Typically, it is difficult to identify synaptic spines definitively from individual electron micrographs, because it can be difficult to tell a spine profile from that of a cross section of the shaft of a dendrite or other kind of neurite. But in Fig. 5, the lack of labeling for microtubules in the spine-like postsynaptic processes, compared to abundant labeling in adjacent neurites, suggests that these postsynaptic processes are spines.
Fig. 4.
Synaptic spines are generally similar in shape in various animals. These silhouettes of spines are based on drawings of Golgi stained neuronal spiny processes from different animals (Coss and Perkel 1985). The longest spines are under 4 μm (all drawn to scale). a Polyclad flatworm. b Honeybee. c Jewel fish. d Mongolian Gerbil
Fig. 5.
Electron microscope immunogold localization of tubulin in the brain of a planarian flatworm. In the top half of the micrograph, there is a bundle of neuron processes with 10 nm immunogold-labeled microtubules; below this is a cluster of synapses. The postsynaptic processes of these synapses lack labeling for tubulin and are spine-like (s). Most of these synapses have two postsynaptic processes. Presynaptic bar or ribbon structures are evident (asterisk). Scale bars is 100 nm. (E7 primary monoclonal antibody to beta tubulin, from Developmental Studies Hybridoma Bank [E7 was deposited to the DSHB by Klymkowsky, Michael (DSHB Hybridoma Product E7)]; specificity of this antibody has been demonstrated previously in a wide range of species (Chu and Klymkowsky 1989, plus numerous references in DSHB); probably Dugesia tigrina, the brown planarian, obtained from Carolina Biological Supplies Company; see Petralia et al. 2010 for general methods, and Petralia et al. 2015 for images from this material showing short invaginating processes in neurons; unpublished data of RSP and Y-XW)
Interestingly, the basic plan of synapses in flatworms, including the presynaptic “ribbon”-like structure and more than one postsynaptic process, is found even in the simplest of flatworms, the acoels (Bedini and Lanfranchi 1991; Bery et al. 2010). Some postsynaptic processes shown in micrographs look spine-like (Ferrero 1973; Achatz and Martinez 2012), although they have not been identified as spines. The acoel flatworms may represent the simplest know bilateral metazoans and thus closest in evolution to simpler metazoans such as cnidarians and ctenophores; also as in the latter, some synapses in acoel flatworms and another simple group of flatworms, are two-way with synaptic vesicles on both sides of the synaptic cleft (Reuter and Palmberg 1990; Bedini and Lanfranchi 1991). It should be noted though that the simplest bilaterians, relatives of acoel flatworms in the genus Xenoturbella, lack a brain (Raikova et al. 2000; Cannon et al. 2016). They do have chemical synapses, but none of the images resemble spine synapses (Raikova et al. 2000).
Non-Invaginating Spine Synapses are a Common Component of the Nervous Systems of Higher Animals
The extent of synaptic spine formation in flatworms is still not well understood, but it is clear that neurotransmission via synaptic spines is a major component of the nervous system of higher animals; and most examples are non-invaginating spines. Coss and Perkel (1985) compare (their figure 10) tracings of Golgi stained spiny dendrites in a flatworm, honeybee, fish and gerbil, and they note the similarity in overall spine length, and in the variation of spine stem (neck) length and maximum head width (Fig. 4). All of the major groups of higher animals except the nematodes have some examples of definitive spines. Most of the few reports of synapses in relatively minor groups show no definitive evidence of spine synapses, including in hemichordates (Dilly et al. 1970; Dilly 1972), chaetognaths (Rehkämper and Welsch 1985); phoronids (Pardos et al. 1991), and bryozoans (Gordon 1974); the latter study notes that synapses appear to be “en passage” (en passant). However, in a description of synapses in the brain of the rotifer, Trichocerca rattus, Clément (1977) shows a micrograph of a synaptic contact in which the postsynaptic neurite appears to extend an invaginating spine into the presynaptic terminal, but it is not described further.
Nematoda
Nematodes, or roundworms, have a relatively simple body structure and nervous system. The latter has been studied most thoroughly in Caenorhabditis elegans, and contains a specific number of neurons—302 (Albertson and Thomson 1976; White et al. 1976, 1986; Hall and Russell 1991; Toth et al. 2012). Notably, White et al. (1986) examined most of the synaptic connections in the worm using electron microscopy of serial sections, and found about 5000 chemical synapses, 2000 neuromuscular junctions, and 600 gap junctions. Chemical synapses are en passant between parallel neuronal processes; one presynaptic process may form a synapse with one, two, or occasionally three post-synaptic processes. A few of the ultrastructure profiles of postsynaptic processes do resemble spines. However, reconstructions of the whole neuronal processes in this and other studies show few if any simple branches from the primary process, and no definite spines (Albertson and Thomson 1976; White et al. 1976, 1986; Hall and Russell 1991; Toth et al. 2012).
Annelida
The Annelida or segmented worms, including earthworms, leeches, and a large variety of marine worms, have a higher body complexity than flatworms, and are roughly on a similar level of design as mollusks and arthropods, especially with complex coelomic, circulatory, and nervous systems. Synapses of the segmental ganglia of the medicinal leech, Hirudo medicinalis, have been studied in detail, using injections of Procion yellow (Purves and McMahan 1972) or horseradish peroxidase (Muller and McMahan 1976), and suggest a continuation of basic synaptic structure from flatworms to arthropods. Typically, the presynaptic terminal has a small, indistinct paramembranous density roughly similar to the better developed ones in many flatworms; compare these to the more distinctive T-bar and rod/ribbon-shaped structures in the synapses of arthropods. Also, like many flatworm and arthropod synapses, there often are two postsynaptic processes; these processes include spines projecting from the shafts of the processes of the large motor neurons. When the postsynaptic process can be identified as a spine, structure is variable—spines can be broad and short or slender and elongate. Most seemed to lack internal structures other than some flocculent material.
It is likely that other major groups of annelids have spine synapses. For example, Wells et al. (1972) show a micrograph of a synapse from the nerve cord of the bristle worm, Myxicola infundibulum, a marine polychaete, in which the postsynaptic process looks very much like a vertebrate spine head. In the earthworm, Lumbricus terrestris, Günther and Schürmann (1973) show examples of synapses on spine-like processes in the nerve cord; some of these show a distinct appearance of head and neck like a spine, although the authors describe them as “feinsten Dendritenverzweigungen” (finest dendrite branches). In the nerve cord of the earthworm, Helodrilus caliginosus, synapses are formed between axons in which the postsynaptic process is a small, spine-like structure, about the size of a small vertebrate synaptic spine head (de Robertis and Bennett 1955). The authors describe this as “…a finger-like process invaginating and infolding the presynaptic element.”
Mollusca
We noted in the previous section that synapses associated with sensory neurons in the sea hare, Aplysia (a marine gastropod), can have spine synapses that make either flat contacts or that invaginate deep into the terminal (Bailey et al. 1979). Kotsyuba and Kotsyuba (2002) describe spine synapses in two marine clams (bivalves) and even mention the presence of invaginating spine synapses; however, their micrographs are not very definitive for these structures.
Postsynaptic spines may be found in the stellate ganglion of the squid (Hama 1962; Castejón and Villegas 1964). These typically are about 1–2 μm long and have a short neck and round head, and they can have some vesicles and smooth endoplasmic reticulum but no other organelles. Those described in Castejón and Villegas (1964) form a synapse with a presynaptic terminal containing a round or oval mass of “homogeneous dense substance” surrounded by the synaptic vesicles. This structure is believed to be equivalent to the presynaptic dense bodies and ribbons that we have described in the previous section in the other higher animal groups. While these postsynaptic spines are not invaginating, note that other synapses in the stellate ganglion have invaginating presynaptic terminals (also with a synaptic body made of the “homogeneous dense substance”). In the review of Petralia et al. (2015), invaginating presynaptic terminals are described briefly. Interestingly, these synapses in the squid stellate ganglion are associated with giant axons that are adaptations for the rapid escape jetting behavior of squid (Hartline and Colman 2007). As we have discussed in the previous section, common structural modifications of synapses associated with rapid response behavior include synaptic bodies and invaginated synapses, although in this case the invaginating process is the presynaptic terminal instead of the postsynaptic spine.
Arthropoda
Probably the best studied spine synapses among invertebrates are in the insect brain. There are numerous studies in various insects including flies (Hausen et al. 1980; Nässel and Strausfeld 1982; Fischbach and Dittrich 1989; Meinertzhagen and O’Neil 1991; Scott et al. 2003; Yasuyama et al. 2003; Leiss et al. 2009a, b), honeybees (Coss et al. 1980; Brandon and Coss 1982; Farris et al. 2001), ants (Stieb et al. 2010), and crickets (Frambach et al. 2004). Spines have been studied mainly with various light microscope techniques such as the rapid Golgi method, showing that many neurons have large, branching dendritic arborizations that are studded with abundant spines (Fig. 4). A good example is the dendritic arborization of the Kenyon cell, the chief interneuron of the calyces of the mushroom bodies (corpora pedunculata), an important brain center for sensory integration and memory formation. Kenyon cell dendritic spines in the honeybee, Apis mellifera, are about 3 μm long, with a slender neck and a roughly oval head (Coss et al. 1980; Brandon and Coss 1982; Coss and Perkel 1985). Size and shape of these spines are affected by experience, aging and learning (Coss et al. 1980; Brandon and Coss 1982; Farris et al. 2001). The spines of Kenyon cells appear to be enriched in actin and CaMKII, both major components of vertebrate spines (Pasch et al. 2011); an abundance of actin as well as a general lack or paucity of tubulin may be the typical pattern of Kenyon cell dendritic spines, as in vertebrates (Frambach et al. 2004; Leiss et al. 2009a; Stieb et al. 2010).
Among the best characterized spines of the insect brain are those found on a group of visual interneurons in Drosophila called lobula plate tangential cells. As for typical vertebrate spines, these spines are filled with actin but lack tubulin (Scott et al. 2003; Leiss et al. 2009b). Spine density is sensitive to the action of the small GTPases, Rac1 and Cdc42, shown to regulate the actin cytoskeleton of spines in vertebrates (Scott et al. 2003; Leiss et al. 2009b). These spines appear to support active excitatory cholinergic neurotransmission; they label with the presynaptic marker, Bruchpilot, and have acetylcholine receptors (Leiss et al. 2009b). They are about 1–2 μm long and their shapes include stubby, thin, mushroom, and branched; the last kind can have up to three heads (Leiss et al. 2009b). The first three categories correspond roughly to similar categories of vertebrate spines (Peters and Kaiserman-Abramof 1970), although the mushroom spines of Drosophila probably do not attain the very large, wide head size seen in vertebrates. Electron microscopic images of spines in Leiss et al. (2009b) are shown only in low magnification; spines lack mitochondria and other large organelles and form synaptic contacts containing presynaptic T-bars and synaptic vesicles. T-bar synapses are found on both the spine head and neck regions. Other studies have examined fly brain spine synapses with electron microscopy, but again only at relatively low magnification, showing few details (Hausen et al. 1980; Nässel and Strausfeld 1982;
Yasuyama et al. 2003). Also many of the postsynaptic processes at the T-bar synapses of photoreceptor terminals in the optic lobes in flies, seen with electron microscopy, are assumed to be spines; these generally contain only a few vesicles, small reticular cisternae, and occasionally fine filaments and flocculent material (Meinertzhagen and O’Neil 1991). These postsynaptic processes often can be very spine-like and may be described as collaterals or side branches; some also have a distinct postsynaptic/subsynaptic cisterna of ER (Musca domestica; Boschek 1971). The latter author shows one interesting micrograph (his figure 26) with double small postsynaptic processes, and both are apposing two presynaptic photoreceptor terminals with T-bars; both postsynaptic processes have a pair of subsynaptic ER cisterns, i.e., opposite the two terminals. Postsynaptic spines also are shown in studies of the retinal lamina of a dragonfly (Procion yellow filling; Laughlin 1973) and grasshopper (ultrastructure; Shaw 1978).
Postsynaptic spines also are described in the brains of other arthropods, and in an earlier section we described invaginating spine synapses in the photoreceptor terminals of crustaceans and spiders. Spine synapses are found in the hemiellipsoid bodies of the land hermit crab, Coenobita clypeatus (a crustacean); these structures may be functionally similar to and possibly homologous with the mushroom bodies (corpora pedunculata) of insects (Brown and Wolff 2012; Wolff et al. 2012). Ultrastructural studies show that these spines can receive convergent inputs on both their spine head and neck (Brown and Wolff, 2012). Postsynaptic spines have been described on the axons of monopolar neurons of the optic lamina of the crayfish, Pacifastacus leniusculus (crustacean), using Golgi methods for both light and electron microscopy (Nässel 1977; Nässel and Waterman 1977). These spines include members of the postsynaptic triad of processes forming a synaptic contact with retinular (photoreceptor) cell terminals, which we described for arthropods in our earlier section on invaginating spines. In the crayfish retinular terminal synapse, central and lateral postsynaptic spines are derived from different classes of monopolar neurons. Lateral spines can be partly invaginating and in some cases, also invaginate a smaller process that the authors call a “knob” deep into the terminal, but it is not clear whether the knobs form true active zones. The spider, Cupiennius salei, has spine synapses, evident with the Golgi method, in brain neuropil associated with the circuitry of its principal and secondary eyes (Strausfeld and Barth 1993; Strausfeld et al. 1993). Also using the Golgi method, Fahrenbach (1979) describes and illustrates “spines” on axonal processes in the corpora pedunculata of the horseshoe crab, Limulus polyphemus (a marine chelicerate, i.e., the group that includes also arachnids such as spiders), but apparently these are presynaptic structures. The author also describes and illustrates dendritic claw terminals of the Kenyon cell dendrites; these are similar in appearance to the dendritic claws of granule cells of the mammalian cerebellar cortex (described in a later section here). In the sea spider (Pycnogonida: another chelicerate group), Achelia langi, a single presynaptic terminal can contact five small postsynaptic processes. While some of the postsynaptic processes can look rather spine-like in profile, three-dimensional serial reconstructions of postsynaptic processes indicate that synapses occur mainly along the processes, and no definitive postsynaptic spines are described (Lehmann et al. 2014).
Onychophora
The “velvet worms” show many characteristics of both annelids and arthropods (Strausfeld et al. 2006); they are worm-shaped but with many paired segmental appendages with claws. And their large brain is similar in many ways to the arthropod brain (Strausfeld et al. 2006). Using the Golgi method, Strausfeld et al. (2006) describe “numerous swellings and spines” on parallel fibers of the heterolateral lobes of the mushroom bodies, but apparently these are presynaptic structures. With electron microscopy, some of the postsynaptic processes shown in figures look spine-like with little internal structure, but others have mitochondria or other organelles. Interestingly, it is common to find a postsynaptic, flat ER cistern (Schürmann 1978; Strausfeld et al. 2006; Peña-Contreras et al. 2007), as we noted earlier for some synapses in flies. In some unusual synapses, the postsynaptic process looks flattened and plate-like, with the PSD along the side (Strausfeld et al. 2006); mitochondria may be present on the edges but are excluded from the center of the thinnest “plates.” They somewhat resemble half of a crest synapse (described later in this review).
Chordata
Spine synapses, of course, are well known in the vertebrates, and their variety will be described in the next section. Vertebrates make up the largest group of the phylum Chordata, which also includes some small invertebrate groups. The cerebral ganglion of the adult sea squirt, Ciona intestinalis (a urochordate or ascidian), contains some spine synapses (Dilly 1969). The postsynaptic spines include short, stubby forms and ones that invaginate into the presynaptic terminal. Spines contain a few large vesicles about 100 nm or so in diameter, and are similar in size range to the more abundant presynaptic vesicles. Dendritic spines also appear to be common in the nervous system of the larvae of the amphioxus or lancelet, Branchiostoma floridae (Lacalli 2002; Lacalli and Kelly 2003). Interestingly, these larvae also have some unusual spine-like axonal processes that can invaginate into neuron somas and form specialized, symmetric “juxta-reticular” junctions with an endoplasmic reticular cisterna lining each side of the junction; it is not known whether these form some kind of specialized synapse (Lacalli 2002). A possible similar structure is found occasionally in photoreceptor synapses of the pineal organs of frogs (Kelly and Smith 1964). Note that Branchiostoma likely has some pineal photoreceptors in its brain (Vigh et al. 2002), but it is not known whether their synapses have “juxta-reticular” junctions.
Interestingly, subsurface cisternae are found sometimes associated with vertebrate synapses. These include examples of postsynaptic subsynaptic cisternae (Rosenbluth 1962; Fuchs 2014); those associated with cholinergic efferent synapses on mammalian cochlear hair cells help regulate postsynaptic calcium signaling (Fuchs 2014). There also are subsurface cisternae opposite “free postsynaptic-like densities” (FPSDs) in the apposing cell processes (Spacek 1982). The latter includes examples of a spine with a distinctive FPSD resembling the PSD of a typical asymmetric, excitatory synaptic spine, and apposing a subsurface cistern in a neuron cell body. Compare these examples to those of synapse-associated cisternae that we have described earlier in this review for the ctenophores, insects, and onychophores.
Echinodermata
Relatively little is know about the evolutionary relationships of spine synapses in chordates with those of other animals. The only other large group of animals that is known to be closely related to chordates is the echinoderms; together they form the major groups of the deuterostomes (animals with mouths not derived from the blastopore that is formed in early stages of embryogenesis). Unfortunately, living echinoderms show little centralization of their nervous system and none have a definitive brain, even though recent studies indicate that well-developed sensory organs are present in some kinds, most notably the unique compound eyes of starfish (Garm and Nilsson 2014). Furthermore, their mesodermal skeletal elements and tough and often spiny skin may make it more difficult to study their nerve structure. Only a few synapses have been described in various echinoderm groups including the brittle stars (Cobb and Stubbs 1982) and sea urchins (Weber and Grosmann 1977; Peters and Campbell 1987), with the best examples of synapses described in sea cucumbers (Mashanov et al. 2006, 2008), including an apparent invaginating spine (Mashanov et al. 2006). Presumably the synaptic structures, including spines, in the deuterostomes harken back to the earliest evolution of these synapses in cnidarians and later the first bilaterian metazoans, as happened also for the other major group of animals, the protostomes (mouth derived from the blastopore; annelids, arthropods, mollusks, etc.).
Muscle Arms
Finally, mention should be made of a few cases where muscle cells project spine-like structures that act as post-synaptic processes. These include neuromuscular synapses of nematodes (Rosenbluth 1965; White et al. 1986), the invertebrate chordate group that includes the amphioxus, and some echinoderms (Flood 1966). In these cases, it is the muscle fiber that sends elongate postsynaptic processes to form synapses in the nervous system. The postsynaptic processes from the muscle cells are called ventral root fibers in Amphioxus (actually Branchiostoma lanceolatum) and contain a granular or fibrillar matrix with glycogen granules (Flood 1966). The terminal expansions also can have a few vesicles. The myoneural junction of the parasitic nematode, Ascaris lumbricoides, is even more synapse-like (Rosenbluth 1965). The presynaptic terminal contains giant mitochondria and numerous presynaptic vesicles; it is separated from the postsynaptic processes called muscle arms, by an ~ 50 nm synaptic cleft. Muscle arms contain “fine filaments and scattered vesicles.” Interestingly, Rosenbluth (1965) notes that he found one slender muscle process that invaginated into a nerve fiber and formed a tight junction with it. White et al. (1986), studying the nematode, Caenorhabditis elegans, show some micrographs in which the postsynaptic muscle process appears very spine-like. Also, in Amphioxus, Flood (1966) found a single example of a postsynaptic muscle process that protruded into the spinal cord but its association with the presynaptic structures was obscured. Thus, these types of muscle-derived postsynaptic processes do not seem to form invaginations normally, although they do seem to have some structural analogies to postsynaptic spines.
Variations in Vertebrate Spine Synapses
We already have discussed varieties of invaginating spine synapses of vertebrate hearing and vision circuitry in the earlier section on adaptations for rapid responses. There are other examples of invaginating spine synapses in a variety of regions of the vertebrate nervous system, and we shall mention a few of them in this section. But the more general, non-invaginating spine synapse type, with a flat or only partially indented contact with the presynaptic terminal, is common and characteristic of many parts of the vertebrate nervous system (Fig. 6).
Fig. 6.
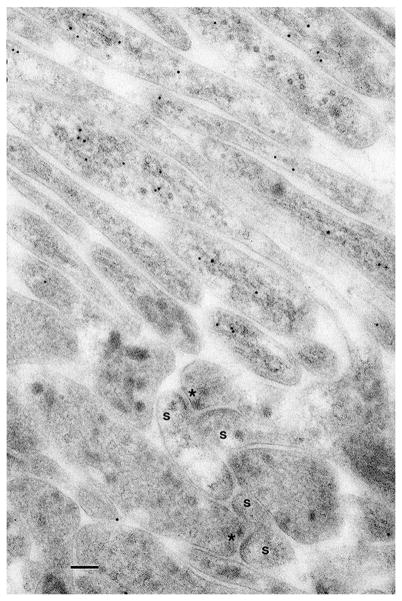
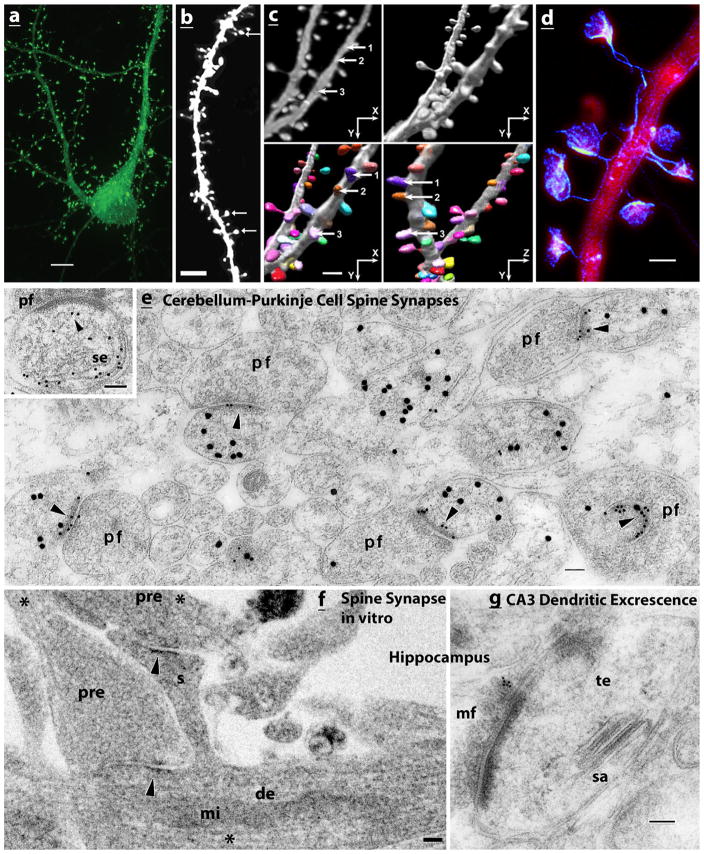
Examples of mammalian spine synapses utilizing light (ad) and electron (e–g) microscopy. a–d. Light microscopy of spines labeled using a variety of methods demonstrates how spines of various sizes and shapes can cover much of the surface of a dendrite. a A neuron from a rat hippocampal neuron culture, expressing GFP-SAP102. Note the prominent labeling of synaptic spines on the dendrites as well as on the soma. b Dendritic spines on a basal dendrite of a projection neuron from the cerebral cortex of a 3-month-old wild-type mouse from a model of Down Syndrome. The neuron is expressing GFP (construct controlled by a tamoxifen-inducible Cre); arrows indicate mushroom-type spines. c Spiny dendrites from a layer II/II pyramidal neuron of the frontal cortex of a 9-month-old mouse, loaded with 5 % Lucifer Yellow. 2D and 3D renderings (top, left and right) are used in an automated 3D detection and shape classification method; the two bottom pictures show the 3D rendering rotated at different angles to discern the wide variety of spine shapes; spines are numbered (1,2,3) and colored differently, allowing the viewer to follow the individual spines. d A neuron from a rat hippocampal neuron culture, revealing the shapes of spines by their actin content. Expressed mCherry is visualized with standard confocal microscopy (red), while expressed LifeAct-Venus, which binds to actin, is visualized using STED superresolution (blue). Scale bars in a, b, c, and d are 5, 3.6, 1.76, and 1 μm, respectively. Micrographs were modified from previously published ones (a, Zheng et al. 2011; b, Haas et al. 2013 (open access); c, Rodriquez et al. 2008 (open access); d, Chevy et al. 2015 (Journal of Neuroscience cover photo; with permission of the authors and the journal)). e–g. Electron microscopy. e Cerebellar Purkinje cells (rat) have typical synaptic spines. The postsynaptic spine heads of five spine synapses, formed with parallel fiber terminals (pf), are labeled with 30-nm gold for GABA neurotransmitter (Purkinje neurons are GABAergic through all cell components), and their postsynaptic densities (arrowheads) are labeled with 10-nm gold for delta glutamate receptor. This rat is 10 days postnatal, and at this age, invaginating spines are fairly common at parallel fiber synapses (lower right synapse). Some tubulovesicular organelles are evident in all of the spine heads, with the best example in the upper right spine. The inset from a mouse (~3 weeks old) demonstrates that this includes a well-developed smooth endoplasmic reticulum (SER; se) with abundant IP3 receptors (10-nm gold); in this case, the SER tubule runs along the spine and curls back up to approach (arrowhead) the postsynaptic density. f An interesting example comparing a spine (s) synapse and dendrite shaft synapse on a dendrite (de) of a neuron from a rat hippocampal culture. Both are probably excitatory since they appear to have round vesicles in the presynaptic terminal (pre) and appear to be asymmetric, with a thickened postsynaptic density (arrowheads). Note that the dendrite shaft and the presynaptic terminals contain microtubules (asterisks), which are absent from the spine; the shaft also has a mitochondrion (mi). g A typical thorny excrescence (te), partly invaginated in a mossy fiber (mf) terminal in the CA3 region of the adult rat hippocampus. The spine apparatus (sa) is distinct (the section was labeled with 10 nm gold for sonic hedgehog). Scale bars are 100 nm. Micrographs were modified from previously published ones (e, Zhao et al. 1998; inset in e, Petralia et al. 2001; f, unpublished data; g, Petralia et al. 2011 (open access)) (Color figure online)
Spine Apparatus
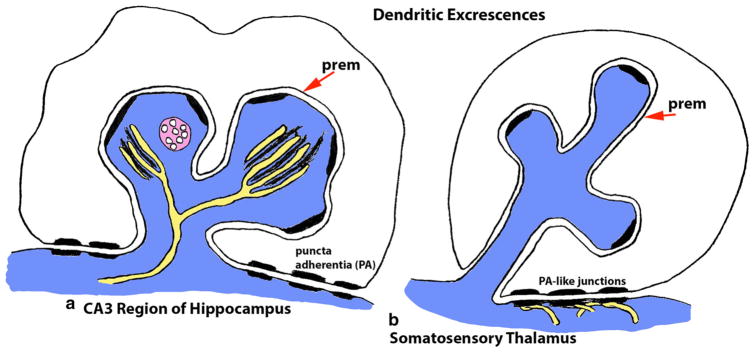
The first really distinctive innovation in spine morphology evolution may be the spine apparatus, found in some mammalian synaptic spines. In the larger spines of mammals, the smooth endoplasmic reticulum (SER) may become elaborated into a stack of tubules or flattened cisternae or cisterns (Figs. 6, 7). In many cases, it forms a spine apparatus (SA), which consists of a series of membranous sacks or cisterns separated by plates of dense material (Gray 1959; Gray and Guillery 1963; Spacek 1985; Segal et al. 2010). The spine apparatus is best known in the mammalian hippocampus and neocortex. In the CA1 stratum radiatum of the hippocampus of adult rats, Spacek and Harris (1997) found a mature spine apparatus in 82 % of large, mushroom-shaped spines; most had three cisterns but one spine had eight, with the number of cisterns directly proportional to spine size. A definitive SA may occur only in telencephalic structures including the cerebral cortex, olfactory bulb, hippocampus, and striatum (Hamlyn 1962; Gray and Guillery 1963; Jones and Powell 1969, 1970; Spacek 1985; Steward and Reeves 1988; Smith et al. 1994). It is not found in cerebellar Purkinje cell spines; these spines do have well-developed SER tubules but lack the dense plates seen between cisterns of a definitive SA (Fig. 6) (Gray 1961; Spacek 1985; Petralia et al. 2001). The SA can be continuous with presumed SER extending from the dendrite shaft (Spacek 1985; Steward and Reeves 1988; Harris and Weinberg 2012). An SA also may be found in some dendrites in the forebrain (Jones and Powell 1969). Thus, SA-like structures with 2–3 cisternae are found in dendrites in the dog cerebral cortex and in cell processes and spines of the spinal cord (Gray and Guillery 1963). Colonnier and Guillery (1964) report that the SA is found in some spines in the ventral lateral geniculate thalamic nucleus of the monkey, but no micrographs are included. In contrast, Spacek and Lieberman (1974) do not find an SA in neurons of the rat somatosensory thalamus. In addition to SAs in spines and dendrites, an SA-like structure is found in the axon initial segment in the forebrain (Palay et al. 1968; Orth et al. 2007).
Fig. 7.
Two examples of invaginating dendritic excrescences. a Thorny excrescences from postsynaptic pyramidal neuron apical dendrites invaginate into mossy terminals in the CA3 region of the hippocampus. These are complex structures, with multiple active zones and typically containing a spine apparatus (yellow) and other structures such as multivesicular bodies (pink). Note also the puncta adherentia (PA) that bind the mossy terminal edges to the main dendrite shaft. b Dendritic excrescences of neurons of the somatosensory thalamus invaginate into large terminals, forming a glomerulus wrapped with glial processes (glia not shown; presynaptic organelles also not shown for clarity). Note that these excrescences are simpler, with fewer internal organelles compared to those in Fig. 7a. Like Fig. 7a, these terminals also make puncta adherentia-like junctions with the dendrite shaft; in this case, these structures show a very distinctive association on the postsynaptic side with a complex of SER tubules (yellow; Spacek and Lieberman, 1974). (prem = the presynaptic membrane that is invaginated by the postsynaptic process) (Color figure online)
The evolutionary origin of the SA is not known. Bedini and Lanfranchi (1998) illustrate a “spine apparatus” at a synapse in a flatworm, but the structure in the micrograph is not distinct. It appears to have one or two irregular cisterns and a dense band of material. The structure may be similar to the simple SA-like structure in the cat and rat spinal cord described above (Gray and Guillery 1963). A number of studies have noted the absence of an SA in fish and reptiles (reviewed in Hamlyn 1962; lamprey, Stefanelli and Caravita 1970; turtle, Nomokonova and Ozirskaya 1984; lizard, Boycott et al. 1961); Henselmans and Wouterlood (1994) note that an SA is found in some spines in the striatal complex of the lizard, Gekko gecko, but they do not provide micrographs of the SA. Lieberman (1971) shows a micrograph of an SER complex in a Purkinje spine of the frog, Rana termporaria, that may be more elaborate than the SER in mammalian Purkinje spines; the micrograph also shows how the SER in the spine appears to be continuous with SER in the dendrite shaft. But like that in mammalian Purkinje spines, this structure appears to lack the dense plates.
The SA may play a role in calcium regulation, possibly associated with LTP and spatial learning (Jedlicka et al. 2008; Vlachos et al. 2009; Korkotian et al. 2014). The SA appears to help regulate calcium levels in the spine, probably via ryanodine receptors that act as calcium channels in the membranes of the SA cisternae (Sharp et al. 1993; Vlachos et al. 2009; Segal and Korkotian 2014; Grigoryan and Segal 2016). Calcium regulation is a key aspect of spine plasticity. For example, glutamate binding to NMDA receptors causes entry of calcium into the spine that then acts as a second messenger for plasticity associated with learning and memory (Vlachos et al. 2009; Horak et al. 2014; Maggio and Vlachos 2014; Sala and Segal 2014; Lichnerova et al. 2015). A similar calcium-regulating function may occur in the complex SER of Purkinje cell spines in the cerebellum. But in this case, it involves IP3 receptors on the spine SER instead of ryanodine receptors (Petralia et al. 2001; Goto and Mikoshiba 2011; Segal and Korkotian 2014; Okubo et al. 2015) (Fig. 6). Thus, there are at least two kinds of calcium-regulating mechanisms, one mediated by an SA with ryanodine receptors in the fore-brain, and the other by SER with IP3 receptors in Purkinje cells. The SA is associated with a specific actin and actinin binding protein called synaptopodin (Deller et al. 2000, 2007; Vlachos et al. 2009; Segal et al. 2010; Korkotian et al. 2014); in fact, distribution of synaptopodin in the brain matches that of the SA, confined to the main parts of the telencephalon (Mundel et al. 1997).
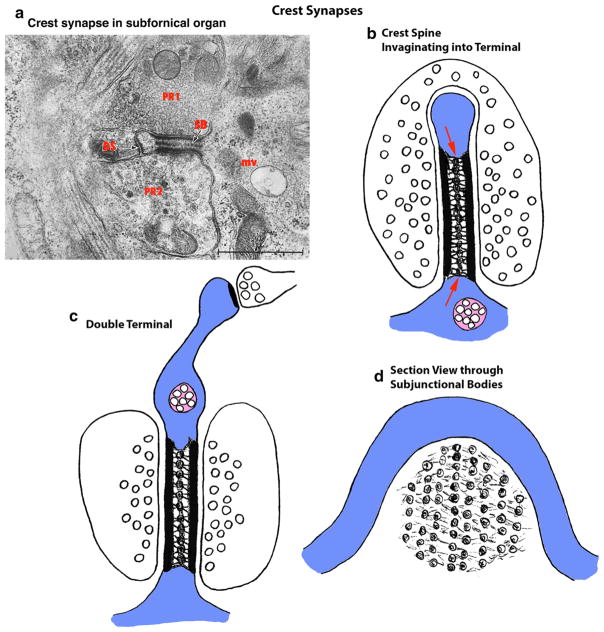
Hippocampus and Cerebral Cortex (Neocortex)
The large, principal neurons of the hippocampus and cerebral cortex typically have large, branching dendrites that are studded with many postsynaptic spines that don’t usually invaginate into the presynaptic terminal (Fig. 6). These spines include thin, mushroom, stubby and branched forms (Harris et al. 1992; Spacek and Harris 1997; Sorra and Harris 2000). To recap, they are filled with actin but lack microtubules, and they can contain SER and vesicles, and especially in the larger spines, sometimes other structures including a spine apparatus (1/15 mature dendritic spines in the rat hippocampus), polyribosomes, RER, coated vesicles, endosomes, multivesicular bodies, and puncta adherentia. In the rat hippocampus, the typical spines can be contrasted with the special, large thorny excrescences of the mossy terminals of the CA3 region and hilus (described in the next paragraph). Typical spines in the cerebral cortex (rat and cat) are similar to those in the hippocampus (Gray 1959; Jones and Powell, 1969; Peters and Kaiserman-Abramof 1970; but as expected, there are a few exceptions: for example, including spines with mitochondria (Jones and Powell 1970), a few microtubules in large spines (Peters and Kaiserman-Abramof 1970), and maybe occasional invaginating spines (Jones and Powell 1969). Peters and Kaiserman-Abramof (1970) also note that the largest spines may have up to three separate spine apparatuses. Some GABAergic, spiny interneurons in the hippocampus are innervated by multiple excitatory terminals (Gulyas et al. 1992; Acsády et al. 1998). These spines are up to 5 μm long and thin, and usually do not have a distinct head region (Fig. 8a); they typically bear 4–6 synapses with asymmetric densities. In lower vertebrates, there are few descriptions of the spines in areas believed to be homologous to the mammalian hippocampus and cerebral cortex. Nomokonova and Ozirskaya (1984) note that spines in the turtle hippocampus are frequent but contain only “granular or vesicular matter” and never have a spine apparatus. They also note invaginating spine synapses. In the chick, there are typical synaptic spines in the cerebral cortical region called the intermediate and medial extent of the hyperstriatum ventral; Horn et al. (1985) found significant changes in the length of the postsynaptic density in two different learning experiments.
Fig. 8.
Spines with multiple presynaptic terminals. a An example of a spine from one kind of GABAergic, inhibitory spiny interneuron; note that it bears several excitatory terminals, each with asymmetric densities and round vesicles (Gulyas et al. 1992; Acsády et al. 1998). b Gemmules are specialized, reciprocal terminals of granule cells in the olfactory bulb. Note how it is postsynaptic at the top, right synapse with a mitral/tufted cell process (right side of drawing); here there is an excitatory synapse (e) with asymmetric density and round, presynaptic vesicles in the mitral/tufted cell process. The gemmule is presynaptic on the bottom, right, filled with pleomorphic vesicles and forming a symmetric synapse (inhibitory; i) with the mitral/tufted cell process. Occasionally, other small terminals with asymmetric densities and round vesicles form synapses on one end (presumably excitatory; e; top synapse in drawing; Price and Powell, 1970a, b). c Some spines receive synapses from presynaptic terminals containing different neurotransmitters. Typically, in these cases, an excitatory, glutamatergic terminal (e) forms on the top of the spine head (asymmetric density, round vesicles) and one or two other terminals with other neurotransmitters such as GABA or dopamine form on the side of the spine head or on the neck (typically symmetric; Kubota et al. 2007). (mitochondria are colored green; the granule cell gemmule is shown in light blue because it is reciprocal) (Color figure online)
Hippocampal Thorny Excrescences and Purkinje Spines
Some major examples of invaginating spine synapses do occur in the brain of vertebrates and involve some of the most complex synaptic structures in the brain, such as the thorny excrescences in hippocampal mossy fiber synapses (Figs. 6, 7a), and cerebellar Purkinje cell spine synapses in some cases (Fig. 6). Both kinds of synapses represent part of the evolution of complex behaviors in vertebrates. In mammals, the thorny excrescences are in the mossy fiber circuit between the dentate gyrus and the CA3 region; these modified spines project from the proximal regions of the main CA3 pyramidal dendrite (thorny excrescences also are found at mossy terminals on some neurons in the hilus). Thorny excrescences are large and complex spines or spine-like structures, often highly branched; they can contain mitochondria, multivesicular bodies, ribosomes, and occasional microtubules–structures that generally are excluded from spines, and they often have a spine apparatus, a structure typically found also only in some large spines (Hamlyn 1962; Frotscher et al. 1991; Chicurel and Harris 1992; Petralia and Wenthold 1992; Johnston and Amaral 2004; Petralia et al. 2015; Wiera and Mozrzymas 2015). Induction of long-term potentiation in slice cultures can increase the formation of invaginating processes from the excrescences into the mossy terminal; typically, these form new synaptic active zones on the sides of the new “finger-like extensions” (Zhao et al. 2012; also reviewed in Frotscher et al. 2014, Wiera and Mozrzymas 2015). This circuit may be key to the higher abilities of mammals for pattern separation of episodic memory (Treves et al. 2008; Schmidt et al. 2012). While the evolution of this synapse is not well understood, very similar synapses are found in the dorsomedial cortex of lizards (Martinez Guijarro et al. 1984; Lopez-Garcia and Martinez-Guijarro 1988; Treves et al. 2008). As in mammals, these large specialized spines are often invaginated into the large presynaptic terminal, and they can have more than one active zone/postsynaptic density, multivesicular bodies and abundant smooth endoplasmic reticulum. However, other components found in the mammalian ones, the spine apparatus and occasional mitochondria, have not been described in the lizard spines. Probably there is a direct evolution of these invaginating spines and their circuitry from early amniote vertebrates (i.e., reptile-like) to mammals; the associated structures in birds are more controversial (Treves et al. 2008).
Unlike the case for thorny excrescences of the mammalian hippocampus, the Purkinje cell and its spine synapses have been fairly distinctive throughout vertebrate evolution, and these are central to the function of the cerebellum for “…performance of smooth and accurate goal-directed movements, making postural adjustments to maintain balance and also learning new motor skills.” (Cerminara et al. 2015). Purkinje spines include those that form synapses with parallel and climbing fiber glutamatergic inputs, and sometimes are invaginating in adult mammals (more commonly in early postnatal development; Mugnaini 1972; Palay and Chan-Palay 1974; Altman and Bayer 1997; Zhao et al. 1998; Petralia et al. 2015). The presence of definitive Purkinje cells in lampreys and other jawless fish is controversial (Lannoo and Hawkes 1997; Bell et al. 2008), while Purkinje cell spine synapses in teleosts (bony, jawed fish) are very similar to those in mammals. As in mammals, they include both parallel and climbing fiber synapses; many of the spines are at least deeply indented into the presynaptic terminal and some are fully invaginating into the terminal (Meek and Nieuwenhuys 1991; Castejón and Apkarian 1993). SER is often prevalent in the spines of fish as in mammals. Castejón and Apkarian (1993) indicate that Purkinje spines synapsing with parallel fibers have an enlarged postsynaptic density, appearing to extend as diffuse material through much of the spine head, but perhaps this is due to some effect of the preparation methods. Possibly related to this, delayed perfusion fixation of mice can result in the appearance of spherical to patchy dense structures beneath the postsynaptic density, which also shows increased curvature (Tao-Cheng et al. 2007). In addition, in mammals, the delta 2 glutamate receptor is very abundant only in Purkinje spines (Fig. 6) (and in spines of a related cell type in the dorsal cochlear nucleus; Petralia et al. 1996; Zhao et al. 1998), and light microscope studies indicate that the distribution of this receptor is the same in the fish cerebellum (Mikami et al. 2004; Bell et al. 2008). Sharks also have definitive Purkinje spines, but invaginating spines are not described (Alvarez-Otero et al. 1993).
Gemmules
Spines of the granule cells of the olfactory bulb tend to be atypical, and cover both their small, deep dendrites and large peripheral dendrite (there is no true axon; Rall et al. 1966; Price and Powell 1970a, b) (Fig. 8b). The spines are identified by their lack of microtubules, but often contain numerous structures including small vesicles, dense-cored vesicles, SER, spine apparatus, ribosomes, multivesicular bodies, and mitochondria. Possibly more than half of all spines contain mitochondria; often they are mitochondria that begin in the main process shaft and continue into the spine. The most unusual spines are the “gemmules” on the distal portion of the peripheral dendrite. These spines contain reciprocal synapses so that they function both as presynaptic and postsynaptic structures; for this reason, Price and Powell (1970a, b) call the peripheral dendrite a peripheral process instead. Reciprocal synapses on neuronal processes are actually found in a number of regions of the vertebrate brain and are fairly common in various invertebrate groups as noted earlier. The gemmules are typically spine-like and similar to the other spines on the granule cell, but sometimes they have microtubules in their neck (stalk); a few are short and neckless and occasionally a reciprocal synapse is formed on the main peripheral process shaft. Reciprocal synapses are formed between gemmules and the dendrites and somas of mitral and tufted cells; gemmules also can be postsynaptic to excitatory synaptic terminals from axons. Typically the gemmule reciprocal synapse consists of two adjacent parts: first there is an asymmetric synapse with a group of large, round presynaptic vesicles in the mitral/tufted cell process and a thickened PSD in the gemmule; second, there is a symmetric synapse with many flattened or pleomorphic presynaptic vesicles in the gemmule (Rall et al. 1966; Price and Powell 1970a, b). The asymmetric part is excitatory, glutamatergic, and the symmetric portion is inhibitory, GABAergic (Rall and Shepherd 1968; Chen et al. 2000; Shepherd et al. 2007). This reciprocal synaptic pattern appears to function similarly in lower vertebrates including reptiles (turtle, Pseudemis scripta elegans; Jahr and Nicoll 1982) and amphibians (tiger salamander, Ambystoma tigrinum; Wellis and Kauer 1994). Goldfish (Carassius auratus) also have similar reciprocal synapses between granule cell gemmules and mitral cell dendrites; these gemmules lack microtubules and have abundant vesicles, but the examples shown do not have other organelles (Ichikawa 1976). In the bonnethead shark, Sphyrna tiburo, reciprocal synapses form between presumptive granule cell dendrites and mitral cell dendrites and somas (Dryer and Graziadei 1996). In the micrographs, the granule cell dendrites, presumably gemmules, resemble those illustrated by Ichikawa for the goldfish, but those of this shark have mitochondria.
Dendritic Claws, Protrusions and Appendages
It should be obvious from the examples discussed so far that some kinds of dendritic processes cannot be defined strictly as dendrite shaft versus spine. Good examples are a number of dendritic structures associated with glomeruli from various parts of the nervous system. A glomerulus is an intimate association among neuronal processes from several kinds of neurons, and often its edge is defined by a glial wrapping (Spacek and Lieberman 1974). One of the best known examples is the cerebellar granular layer glomerulus (Mugnaini 1972; Palay and Chan-Palay 1974; Llinás et al. 2004). There is a large glutamatergic mossy terminal at the center that forms excitatory synapses on interdigitating dendrites from up to 20 granule cells. There also are inhibitory terminals from Golgi cells and the glomerulus is encapsulated by glial lamellae. Typically, each granule cell dendrite ends in a “…digitate spray of short, varicose fibers that curl about the mossy fiber rosette.” (Palay and Chan-Palay 1974). These terminal processes have been called dendritic “claws” (Llinás et al. 2004); they typically contain a central, elongate mitochondrion associated with SER. The dendrite processes interconnect via puncta adherentia. Fast excitatory synaptic transmission mediated by postsynaptic AMPA-type glutamate receptors appears to be enhanced via spillover of glutamate among the synaptic active zones of the mossy terminal (DiGregorio et al. 2002). Interestingly, both the asymmetric synapses and the puncta adherentia contain NMDA-type glutamate receptors and associated scaffolding proteins, suggesting that NMDA receptors also may be involved in neurotransmission via glutamate spillover (Petralia et al. 2002; Petralia 2012). Granule cell dendrite structure in the glomerulus of the chick is similar to that in mammals, e.g., with similar puncta adherentia; but often the synaptic active zones join together into an invaginating crest synapse with subjunctional plaque, as described in the next section (Mugnaini 1972).
The synaptic glomeruli in the dorsal lateral geniculate (thalamic) nucleus of the cat (Famiglietti and Peters 1972) and monkey (Macaca mulatta; Hamori et al. 1974) contain a number of unusual, spine-like dendritic processes called “protrusions” and “appendages,” that surround the central optic (retinal) axon terminal. The glomerulus also contains some smaller branching axon terminals called “claw-like,” not to be confused with the dendritic claws described above in the cerebellar glomeruli. One common arrangement within the glomerulus is a “triad” composed of the optic axon terminal that forms synapses onto (i.e., is presynaptic to) two dendritic components: one is a postsynaptic dendritic protrusion or thorn (described as being from a principal cell in monkeys and a spinous Class II cell dendrite in cats); the other is a dendritic appendage (described as being from an interneuron in monkeys and a smooth Class III cell dendrite in cats). In monkeys (Hamori et al. 1974), the dendritic protrusions make distinct asymmetric synapses with the retinal terminals. These protrusions contain mitochondria and SER, numerous “filaments,” and form puncta adherentia with the retinal terminal and with adjacent principal cell dendrites; they also form spine-like secondary processes in contact with the retinal terminal. The dendritic appendages of interneurons in the monkey contain mitochondria and SER, and may contain ribosomes and RER. But they are distinguished mostly by their numerous flattened to pleomorphic vesicles, and are presynaptic (and presumably inhibitory) to the principal cell dendritic protrusion in the same triad. At least in the cat (Famiglietti and Peters 1972), the dendritic appendage appears to be a large multi-lobed structure extending from a long, slender stalk from the parent dendrite shaft; these lobes may enter more than one glomerulus.
In the glomeruli of the rat somatosensory thalamus, dendritic excrescences, similar overall to the hippocampal CA3 thorny excrescences, invaginate deeply into large terminals that are enwrapped in glial processes (Spacek and Lieberman 1974) (Fig. 7b). Like those in the CA3 region described in an earlier section, these excrescences can be simple or compound/branched, but they contain fewer organelles; for example, there is no spine apparatus (as noted above, the spine apparatus may be limited to telencephalic structures). This synapse also is similar to the CA3 one due to the presence of multiple puncta adherentia-like structures, each forming a double density between the terminal and dendrite, and that bind the large invaginated presynaptic terminal to the parent dendrite of the excrescences (Spacek and Lieberman 1974; Chicurel and Harris 1992). This pattern of excrescence-like invaginations into a large terminal with multiple active zones and puncta adherentia also is seen in the endbulb of Held/spherical bushy cell synapse in the early postnatal development of the cat, as described earlier in this review (Ryugo et al. 2006). However, the puncta adherentia-like structures in the somatosensory thalamus glomeruli may be unusual—on the dendritic side, the densities of these structures are shown to be interconnected with a distinctive complex of SER tubules.
Finally, we will examine the spine-like processes on the dendrites of the giant neurons of the red nucleus of the cat (Wilson et al. 1987). The dendrites have two very different zones: their proximal and distal portions, each with different spine-like processes and associated with different afferent inputs; this is similar to the CA3 pyramidal neurons of the hippocampus, where the proximal parts of the dendrites have the thorny excrescences that we have described, and the more distal parts have more typical spine synapses. Fine, filopodia-like spines, 0.1–0.25 μm wide and about 1 μm long, are found on the soma and proximal portions of the dendrites; these appear to have much of their sides in active synaptic contact and sometimes are invaginated into the presynaptic terminal. The more distal parts of the dendrites bear spines and spine-like appendages of various shapes and sizes, as seen with light microscopy. One large kind is up to about 10 μm long with a stalk and large head region, made of several extensions from the stalk, containing mitochondria and SER, and spreading several micrometers in diameter to form a glomerulus. There are fine dendritic processes (the finest about 0.1–0.2 μm in diameter) that extend from the dendritic stalk and surround and often invaginate into a central presynaptic terminal, which originates in some cases from the sensorimotor cortex; other presynaptic terminals also are part of the glomerulus.
Crest Synapses
Crest synapses (Fig. 9) are unique structures that have been described in a wide variety of scattered locations in the nervous system. In mammals, they are found in the sub-fornical organ (Akert et al. 1967a, b), habenula and interpeduncular nuclei (Milhaud and Pappas 1966; Murray et al. 1979; Hamill and Lenn 1983; Lenn et al. 1985), locus coeruleus (Mizuno and Nakamura 1972; Yamashita et al. 1997), globus pallidus (Cano et al. 1989); amygdala (figure 6 in Muller et al. 2016), suprachiasmatic nucleus (Güldner 1976), inferior olive (De Zeeuw et al. 1994), dorsal raphe nucleus (Descarries et al. 1982; Kapadia et al. 1985), medial preoptic area (Prince and Jones-Witters 1974), dorsal horn of the spinal cord (Aronin et al. 1981), and superior cervical ganglion (Kasa et al. 1991); and in birds, they have been described in the cerebellar cortex (Mugnaini 1972) and ciliary ganglion (Takahashi 1967; De Stefano et al. 1993). In many cases they form an unusual structure that looks in profile in micrographs like an invaginating spine; or if not invaginating into the presynaptic terminal, its spine-like shape is formed by the contours of presynaptic terminals (usually two but up to three). In either case, the synaptic active zones are formed on the sides of the spine-like structure rather than the distal tip. The shape of the synapse as seen in micrograph profiles is misleading as it actually has a disk shape like that of the typical active zone of a synapse, except that the crest synapse disk is sticking up, perpendicular to the parent dendrite or soma surface. The two synaptic active zones usually appear to be strongly asymmetric (thick PSDs), with the parallel postsynaptic membranes about 130 nm apart, as described in the subfornical organ (Akert et al. 1967a, b) and locus coeruleus (Yamashita et al. 1997); the width of the active zone is roughly half a micrometer in diameter, with the whole crest synapse structure roughly 1 μm. Centered between the two postsynaptic membranes is a subjunctional plaque of dense material with obscure perpendicular filaments connecting the plaque with the two PSDs. There is a regular array of subjunctional (subsynaptic or postsynaptic) bodies aligned in the central plaque; each is about 20–30 nm in diameter and arranged about 50 nm apart (Akert et al. 1967a, b). These subjunctional bodies are round and dense, but do not appear to have a definitive limiting membrane, although a high-magnification image (figure 7 in Akert et al. 1967a) seems to show a partial limiting membrane of some sort. There may be up to 25 subjunctional bodies in a row (Akert et al. 1967b), although most images show less than half that many and often only 3–5 in a row (Akert et al. 1967a). Serial sections of one example revealed 8 rows of up to 12 bodies, with a total of about 80 in the whole crest synapse (Yamashita et al. 1997). In another series illustrated in Akert et al. (their figure 11; 1967a), there are 7 rows of up to 13 bodies.
Fig. 9.
Crest synapses. a Micrograph showing a crest synapse from the subfornical organ (reprint of figure 4A from Akert et al. 1967a; reprinted by permission from Springer Publishing Company). Note the characteristic crest spine with central subjunctional bodies (SB). Other crest synapse structures vary, and in this example, there are two postsynaptic multivesicular bodies (mv; a dark and a light one), and a desmosome-like junction (DS) at the apical bulge of the crest; the presynaptic terminals in this case (pr1 and pr2) contain morphologically different vesicles (red letters have been superimposed over the black letters of the original micrograph, for clarity). Note also that this example is rotated ninety degrees counterclockwise from the drawings in (b–d). b Crest synapse spine invaginating into a single terminal. Red arrows indicate the central plaque of subjunctional bodies between the two PSDs. c Crest synapse spine with a presynaptic terminal on each side of the crest. This example also shows how occasionally a normal spine synapse can project from the top of the crest. d A typical crest synapse that is cut directly through the plane of the central plaque of the subjunctional bodies between the two PSDs, showing the array of subjunctional bodies. The crest synapse often is associated with multivesicular bodies (pink), either in the dendrite near the base of the crest or in the thickened top of the crest spine (Akert et al. 1967a, b; Yamashita et al. 1997) (Color figure online)
Those crest synapses with one presynaptic terminal form double active zones (i.e., the two sides of the crest) so that the crest invaginates into the presynaptic terminal. If there are two presynaptic terminals, it is called a “twin synapse.” This may be a clue to the function of some crest synapses. Thus, the two axons forming the twin synapses of crest synapses in the interpeduncular nucleus of the rat appear to originate from right and left nerve tracts, respectively (habenula input via the fasciculi retroflexus; Murray et al. 1979; Hamill and Lenn 1983). Similarly, some crest synapses in the rabbit inferior olive may receive their two inputs from the two sides of the brain (De Zeeuw et al. 1994). But many crest synapses appear to receive input from two different kinds of sources. Thus, the majority of the synapses in the inferior olive are GABAergic; labeling for GABA is in either one or both of the presynaptic terminals in a two-terminal crest synapse (De Zeeuw et al. 1994). Yamashita et al. (1997) found in the locus coeruleus that about 10 % of the terminals on a crest synapse are noradrenergic. In the monkey dorsal horn of the spinal cord, Leuenkephalin labeling can be found either in the dendrite and crest or in one of the terminals (Aronin et al. 1981). Terminals with either round or pleomorphic vesicles can be part of crest synapses. In both the globus pallidus (Cano et al. 1989) and subfornical organ (Akert et al. 1967a), the crest can form synapses with two different kinds of presynaptic terminals with distinctly different matrix material and vesicles (e.g., one having many more dense-cored vesicles along with clear vesicles). On the other hand, cholinergic innervation at crest synapses in the interpeduncular nucleus (Lenn et al. 1985) and superior cervical ganglion forms both terminals on a crest (Kasa et al. 1991). In the monkey (Macaca fascicularis) dorsal raphe nucleus, which contains the largest number of serotonergic neurons in the brainstem, the postsynaptic structures of the crest synapse can be either labeled or unlabeled for serotonin, while the presynaptic terminals are not labeled (Kapadia et al. 1985). In contrast, Descarries et al. (1982) do not report any labeling for serotonin in the crest synapses of the rat dorsal raphe nucleus.
The function of crest synapses is not clear. Crest synapses are fairly common in the locus coeruleus of the monkey, Macaca fuscata (Japanese macaque), and most of them originate from dendrites of noradrenergic neurons (Yamashita et al. 1997). The locus coeruleus is the main source of noradrenergic input to the cerebral cortex, and so is better developed in primates, along with their larger cerebral cortex compared to many other mammals. In relation to this, the authors note that rodents or cats have only rare crest synapses in the locus coeruleus, in comparison to monkeys; they argue that a more complex locus coeruleus needs better control, and crest synapses may mediate this.
Crest synapses can have a variety of shapes and arrangements (Akert et al. 1967a; Murray et al. 1979). Often there is a multivesicular body in the dendrite, near the base of the crest (Akert et al. 1967a, b; Hamill and Lenn 1983) (Fig. 9). Typically, the extended portion of the crest synapse, distal to the active zones, is enlarged and may have small vesicles, or larger endosomal vesicles or multivesicular bodies (Milhaud and Pappas 1966; Akert et al. 1967a; Yamashita et al. 1997). In some cases, there may be an additional regular postsynaptic spine structure that continues from the extended portion, forming a synapse with another presynaptic terminal, or a possible adherens-type junction in a dendro-dendritic junction between spines (Akert et al. 1967a; Yamashita et al. 1997). There also can be Y-shaped crests forming synapses with up to three terminals (Akert et al. 1967a; Murray et al. 1979). In addition, Murray et al. (1979) describe “crested dendrites,” which bear a series of crested dendritic processes often in a row on one side of the dendrite shaft, and forming synapses with up to five axon terminals; some of these terminals appear to be invaginated among the dendritic processes.
Moshkov et al. (2013) use the term “crest” to describe structures on the goldfish Mauthner neuron that actually appear to be regular invaginating spine synapses. Among invertebrates, a structure described as a “crest synapse” in the flatworm, Stenostomum sp. (Moraczewski et al. 1977), actually looks considerably different from any examples of vertebrate crest synapse, but it might be homologous and perhaps ancestral to those in vertebrates. It looks like a double crest synapse with a narrow cleft in between, and instead of a row of subjunctional bodies, there is an obscure elongate, cisterna-like structure. We also have noted earlier in the review that flattened, plate-like synapses have been described in the Onychophora, but these are one-sided only (Strausfeld et al. 2006).
Variations in Neurotransmitter Types at Spine Synapses
Most spine synapses in the vertebrate nervous system appear to be excitatory, glutamatergic (Fig. 6), based on very numerous studies including many described throughout this review. In most cases, spines either have only a synapse with a glutamatergic terminal, or they have both a glutamatergic terminal and one containing another neuro-transmitter. This other terminal usually can be identified by its symmetric density, and it forms on the other side of the spine head or on the neck of the spine (Fig. 8c). Determination of presynaptic vesicle shape generally has been problematic. A classic study by Tatsuoka and Reese (1989), based on a simple model of terminal synapses formed directly on a cell body (anteroventral cochlear nucleus), demonstrated ultrastructural variations in vesicle shape in synapses depending on transmitter type and preparation methods: glutamatergic = round, cholinergic = small round, GABAergic and glycinergic = oval/flattened; the associated densities for these synapses are asymmetric (i.e., thick PSD) for glutamatergic and symmetric for the others. In another example noted in an earlier section, Güldner (1976) describes “spine-like protrusions” in the rat suprachiasmatic nucleus that invaginate into the presynaptic terminal and have active zones with symmetric densities; the shape of the vesicles in these terminals vary from round to flattened and this varies with the preparation method. While there is substantial evidence that most glutamatergic terminals on spines have mainly round vesicles using standard fixation protocols, structures inside the terminal often are obscured by peroxidase-related chemical reaction products used for labeling (e.g., Papadopoulos et al. 1989; Dehay et al. 1991; Petralia and Wenthold 1998), or fixation may not be optimal in protocols needed for immunogold localization (Petralia and Wenthold 1998, 1999; Zhang et al. 2016). So here we will describe spine structure related only to the position of terminals for different neurotransmitters and the nature of the density (symmetric or asymmetric).
GABAergic synapses on spines have been studied best in the cerebral cortex. Typically, they form symmetric synapses on the sides of spine heads and necks (mice, Knott et al. 2002; rats, Kubota et al. 2007) or just the head (cats, Dehay et al. 1991) and usually, these spines also have an asymmetric synapse. Kubota et al. (2007) show examples of these spines with: (1) labeling for VGLUT2 (vesicular glutamate transporter 2; mainly for thalamocortical axons) in the asymmetric synapse terminal and two unlabeled symmetric terminal synapses; (2) VGLUT2 in the asymmetric terminal plus GABA in the symmetric terminal; (3) GABA receptor in the symmetric terminal; and (4) GABA receptor in the symmetric terminal and AMPA-type glutamate receptor in the asymmetric terminal. These authors estimate that this double innervation of spines occurs for about 3 % of cerebral cortical excitatory synapses. Of these, at least all of those with VGLUT2-positive excitatory, asymmetric terminals may have GABAergic inhibitory, symmetric terminals; other symmetric terminals in double innervations may contain GABA or some other neuro-transmitter. This GABA innervation of glutamatergic spines serves to “…compartmentalize GABAergic inhibition, limiting both action potential and synaptically evoked calcium influx and regulating NMDAR receptor-dependent synaptic integration.” (Chiu et al. 2013). In the hippocampus, Megias et al. (2001) show that some GABAergic, symmetric synapses form on spines in the CA1 stratum lacunosum-moleculare. Also, it is interesting that axons from the ventral tegmental area form synaptic terminals in the lateral habenula that release both glutamate and GABA at separate asymmetric and symmetric active zones that express glutamate (on dendrite shafts and spines) and GABA (on shafts) receptors, respectively (Root et al. 2014). Actually, co-release of neurotransmitters occurs for several neurotransmitters (Hnasko and Edwards 2012; Trudeau et al. 2014; Vaaga et al. 2014; Granger et al. 2016), but the specific role of co-release at spine synapses is not well studied.
Spines receiving dopaminergic terminals have been well studied especially in the striatum (rat, Freund et al. 1984; Groves et al. 1994) and cerebral cortex (rat, Verney et al. 1990; Carr and Sesack 1996; monkey, Macaca mulatta, Goldman-Rakic et al. 1989). In most cases, the labeled terminal forms a symmetric synapse on the side of the spine head or neck, and there is an unlabeled asymmetric synapse on the end of the spine head. Freund et al. (1984) found the twin input of a labeled symmetric synapse and an unlabeled asymmetric one on 39 % of the spines counted from one identified striatonigral neuron in the ventral striatum. Bergson et al. (1995) examined dopamine receptor distribution in a number of brain regions (monkey, Macaca mulatta), and found different distributions for D1 and D5 subtypes, with D1 more common in the head and neck of spines. Most spines in the dorsal striatum of the rat label with antibodies to either D1 or D2 receptors (Hersch et al. 1995). Yung et al. (1995) show examples in the rat striatum of pre-embedding immunogold labeling for D1 or D2 on the postsynaptic membrane of a symmetric synapse on the side of a head or neck of a spine that receives an asymmetric synapse on the head. Overall, the studies on distribution of dopamine receptors indicate that they are present on spines receiving only an asymmetric, glutamatergic synapse (“diad”) or both the latter and a symmetric, dopaminergic synapse (“triad”; reviewed in Yao et al. 2008). Those spines that do not have a direct, dopaminergic synapse would receive dopamine by asynaptic release from nearby dopaminergic axons; in fact, asynaptic release may be a common mechanism for many neurotransmitters (reviewed in Descarries et al. 2008). Ladepeche et al. (2013) provide evidence in studies of rat hippocampal cultures that dopamine and NMDA-type glutamate receptors form clusters together at the base of the spine, and release of dopamine then disrupts the interaction in the clusters, allowing NMDA receptors to migrate into the glutamatergic synapse on the spine head, and thus affecting its long-term plasticity. Zhang et al. (2015a) show that dopaminergic fibers from the rat ventral tegmental area to the nucleus accumbens may form both the dopaminergic terminals and one population of the glutamatergic terminals. The axons have two distinct subcellular compartments labeling for: (1) VGLUT2 (forming the asymmetric, glutamatergic synapse on the spine head) and (2) TH (tyrosine hydroxylase;); the latter forms the symmetric, dopaminergic synapse on the side of the spine compartment and this compartment also appears to label for a dopamine transporter and a vesicular dopamine transporter. Finally, in the lizard (Gekko gecko), dopamine-labeled terminals with mainly symmetric densities form on spine heads in the striatal complex and dorsal ventricular ridge (part of the cerebrum; Henselmans and Wouterlood 1994).
Cholinergic synapses, identified by immunocytochemistry against choline acetyltransferase (ChAT), can form on spines (as well as on dendrite shafts and sometimes on somata) throughout the cerebral cortex (Wainer et al. 1984; Houser et al. 1985; Clarke and Dunnett 1986; Umbriaco et al. 1994) as well as in other forebrain areas (Wainer et al. 1984) in rats. Most of these are symmetric. Both Wainer et al. (1984) and Houser et al. (1985) show examples with the labeled cholinergic terminal forming a symmetric synapse on the side of a spine head that also receives an unlabeled asymmetric synapse; Wainer et al. (1984) also show an example of a labeled, symmetric synapse that is the only terminal seen in that section of a spine. In the lizard (Gekko gecko) striatal complex and dorsal ventricular ridge, as for dopamine noted above, ChAT-labeled terminals with mainly symmetric densities form on spine heads (Henselmans and Wouterlood, 1994).
The cerebral cortex receives a noradrenergic input from the small locus coeruleus, but its prevalence at spine synapses (in the adult rat) is unclear. Thus, Séguéla et al. (1990) found that all labeled noradrenergic synapses are symmetric with most formed on dendrite shafts and only a few on spines. In contrast, Papadopoulos et al. (1989) found more labeled terminals on spines compared to dendrite shafts, and these included both symmetrical and asymmetrical synapses. The latter authors illustrate both an example of a labeled terminal on the side of a spine receiving an unlabeled asymmetric synapse, and another example in which the labeled terminal makes the only synaptic contact seen on a spine head, and it is asymmetric. Differences in the findings of these two studies may reflect in part, differences in interpretation because “different criteria were used for defining synaptic junctions.” (Séguéla et al. 1990).
Serotonin-labeled terminals (from the raphe nuclei) on spines have been described in the striatum of rats (Arluison and de la Manche 1980; Soghomonian et al. 1989) and monkeys (Macaca fascicularis; Pasik and Pasik 1982). Only about a tenth of labeled varicosities in the rat striatum make synapses; these are on either dendrite shafts or spines and are usually asymmetric. They usually are the only terminal seen to form a synapse on the spine, although Soghomonian et al. (1989) found one example of a spine head, followed in serial sections, forming synapses with two terminals: (1) an asymmetric synapse with a large, serotonin-labeled terminal that is partially invaginated by the spine; and (2) an unlabeled, symmetric synapse. In the monkey, the few synaptic contacts of labeled axon varicosities are all asymmetric on spines (Pasik and Pasik 1982). A similar labeling pattern for serotonin-labeled terminals with asymmetric densities is seen for spines in the cerebral cortex (Séguéla et al. 1989).
Finally, as noted in the previous section, Moshkov et al. (2013) describe some synapses on the ventral dendrite of the giant Mauthner neuron of the goldfish with invaginating spines that they call “crests.” These are symmetric, with apparently pleomorphic vesicles clustered on the sides and end, and they describe them as inhibitory; they also provide some evidence that these are glycinergic.
Why Do Synapses Form Spines?
Many synapses on dendrites and other neuronal processes function very well without developing spines. Even among excitatory, glutamatergic synapses in vertebrates, many kinds form directly on dendrite shafts without any trace of a spiny projection (Figs. 1, 6). So why are spine synapses favored in many kinds of synaptic connections? And of these, why do some kinds of spines invaginate into the presynaptic terminal and other kinds only contact the terminal surface? And why do some kinds of neurons, such as the CA3 pyramidal neurons in the hippocampus, have both kinds?
Why Do Some Kinds of Synapses Form Spines?
There are several benefits of spine synapses over flat contacts between pre- and postsynaptic processes (Coss and Perkel 985; Harris and Kater 1994; Shepherd 1996; Halpain 2000; Bloodgood et al. 2009). First, spines may allow more numerous and complex synaptic arrangements with a neuron, largely due to the overall increase in surface area of the postsynaptic neuron processes (Harris and Kater 1994; Chklovskii 2004). Thus, more synaptic contacts can be formed along a spiny dendrite and the dendrite shaft can be relatively thinner, allowing more complex circuitry per unit volume of neuropil (Harris and Kater 1994; Chklovskii 2004). Also, as we discussed in the last section, some excitatory spine synapses also have an inhibitory or regulatory synaptic terminal on the side of the head or neck, and this can add a level of control over individual synaptic inputs to the neuron (Harris and Kater 1994). Two other potential benefits in particular have been studied and discussed extensively: (1) the thin spine neck can increase electrical resistance or otherwise affect electrical compartmentalization of the spine, to filter overall neuronal synaptic signals differentially per spine input, and (2) spine structure is important for increased synaptic plasticity associated with learning and memory.
Computational studies predict that “excitable dendritic spines…provide an anatomical arrangement that economizes both excitable and synaptic channels.”; thus, relatively few channels on a spine head can produce a larger depolarization in the dendrite than for similar channels on the dendrite shaft, largely due to the greater input resistance of the spine head (i.e., dependent on the thin neck; Segev and Rall 1988; see also Rall and Rinzel 1971, 1973; Rall 1974). Indeed, these authors note that “…spine stem [neck] resistance…is perhaps the most important of several parameters that determine the electrical behavior of the spine…” A number of studies have examined the electrical properties of spines, especially those on the apical dendrites of CA1 pyramidal neurons of the hippocampus of rats and mice (Grunditz et al. 2008; Bloodgood et al. 2009; Harnett et al. 2012). Grunditz et al. (2008) found that the spine neck effects spine compartmentalization during postsynaptic depolarization, regulating calcium influx into the spine via NMDA receptor and R-type calcium channels. Bloodgood et al. (2009) found that opening of voltage-gated calcium channels on a spine depends on activation of that spine, since the neck acts as a barrier to current propagation, probably preventing their opening during subthreshold depolarization of the dendrite shaft or synaptic activity in neighboring spines. Such compartmentalization presumably would not be possible for synapses along a dendrite shaft. Spine neck resistance can be 500 MΩ, sufficient to allow passive amplification of spine head depolarization by almost 50 times (Harnett et al. 2012). Harnett et al. (2012) note that having synapses on spines allows control of both passive and active (via voltage-dependent conductance) amplification by regulation of spine neck resistance.
Thus, the spine synapse may be designed to regulate via structural and functional plasticity, the strength and contribution of individual synapses on neuron function. These ideas were first elucidated by Wilfrid Rall and colleagues in the 1970s and 1980s (Rall and Rinzel 1971; Rall 1974; reviewed in Segev and Rall 1988; Shepherd 1996). In the simplest model, a spine might be able to change amplification via a change in spine head width and neck length; Coss and Perkel (1985) describe how these basic dimensions can change with activity in two very different animals: (1) for escape behavior effects on jewel fish (Hemichromis bimaculatus) tectal interneuron spines; and (2) for flight behavior effects on honeybee Kenyon cell spines; they discuss how these changes might affect input resistance and transient membrane potential in the spine head. Interestingly, a study by Acker et al. (2011) on spines of pyramidal neurons from mouse cerebral cortical slices suggests that simple changes in spine head size and neck length may not affect transfer of voltage from the dendrite shaft to the spine head (Acker et al. 2011); but this does not consider the opposite transfer—from spine to shaft (Sala and Segal 2014). In contrast, Araya et al. (2006) also looked at pyramidal neuron spines in cortical slices, and found that voltage changes to or from spines depends on spine length. Gulledge et al. (2012) modelled a variety of known spiny neurons from different regions of the mammalian brain and suggest that a major function of spine synapses is to standardize the excitatory postsynaptic potentials (EPSPs) of synapses along extensive and structurally variable dendritic arborizations; in contrast, spineless synapses could produce a great variability in amplitude and kinetics of local EPSPs due to these complex dendritic geometries. Thus, putting the synapses at the ends of spines might reduce location-dependent variability in synapse properties, “…allowing more uniform activation of voltage-sensitive conductances at the site of synaptic input.” Further, the spine might maintain this uniformity even during LTP, when there is an increase in AMPA receptor conductance, by increasing spine neck width to decrease neck resistance.
In addition to current flow, the spine neck would be a barrier to movements of various molecules in the cytoplasm and on the cell surface; for example, diffusion in and out of the spine of AMPA receptors, the primary proteins at the postsynaptic membrane of the spine that mediate fast glutamatergic neurotransmission, is restricted by the neck (Ashby et al. 2006; Korkotian and Segal 2007; Sala and Segal 2014). A short spine neck also may allow faster diffusion of calcium from the spine head to the dendrite, and this may correlate with more effective increase in AMPA receptor delivery to spines during LTP.
In fact, there is a very large amount of evidence indicating that synaptic plasticity, such as occurs with long-term potentiation or depression (LTP and LTD) often involves large changes in spine size and structure, as well as number, especially for studies of the hippocampus and cerebral cortex. This may explain the variety of spine shapes, as we noted in an earlier section, including stubby, thin, mushroom, and branched. Also, among the larger spines, the PSD varies from simple to perforated to segmented, and also may invaginate spinules from the post-synaptic membrane into the presynaptic terminal. All of these variations may reflect modifications that affect the strength and specificity of function of an individual synapse. This large body of literature has been reviewed by others (e.g., Harris and Kater 1994; Sala and Segal 2014; Petralia et al. 2014, 2015), and we will not cover it in detail here. The morphological changes in spines during plasticity reflect extensive changes in synaptic molecules as well as internal reorganization of the spine cytoskeleton (Lai and Ip 2013; Sala and Segal 2014). In many cases, a major component of LTP is an increase in AMPA-type glutamate receptors in spines (i.e., the more AMPA receptors, the stronger the response to glutamate release from the presynaptic terminal; Korkotian and Segal 2007; Huganir and Nicoll 2013; Sala and Segal 2014); AMPA receptor density at synapses is correlated with size of the spine and the complexity of the PSD (Ganeshina et al. 2004a, b; Kopec et al. 2007; Korkotian and Segal 2007). The local changes in calcium levels in the restricted space of the spine is crucial for plasticity of individual synaptic inputs, and involves various calcium channels on the spine surface membrane and in organelles (Harris and Kater 1994; Sala and Segal 2014). For many spine synapses in the forebrain, at least, NMDA-type glutamate receptors are particularly important as a source for calcium entry into spines associated with plasticity (Paoletti et al. 2013; Horak et al. 2014; Lichnerova et al. 2015). There also are internal sources of calcium in the spine. As noted in an earlier section, large spines in the forebrain have a spine apparatus that can release calcium into the spine cytoplasm via ryanodine receptors, while cerebellar Purkinje cell spines have SER with IP3 receptors for release of calcium. Interestingly, a form of non-ionotropic NMDA receptor signaling, i.e., without calcium entry through the NMDA receptor, may mediate a reverse type of structural plasticity of spines, thus controlling spine shrinkage and elimination, necessary for refinement of complex neural circuitry (Stein et al. 2015).
In summary then, the synaptic spine has evolved for precise control of individual synaptic inputs to allow a neuron to better control multiple inputs and coordinate the fine responses needed for learning and other complex behaviors. But can all synaptic spines exhibit synaptic plasticity or are some designed to be static? Even if static, non-plastic spines exist, it seems unlikely that we can distinguish them by their structure. We have described numerous examples of plasticity in the spines of vertebrates, and a few in invertebrates, including in honeybees and a possible case in a mollusk. Changes in spine structure with plasticity seem to vary. In some cases, there can be profound modifications: compare thin and mushroom spines in the mammalian neocortex. In other cases, such as the honeybee, the changes may be subtler; so large shape changes may not be a requirement for spine plasticity. Note also that variation in spine shape and size is roughly similar across the animal kingdom, from worms to mammals (Fig. 4). So perhaps all spines are capable of realized or potential plasticity for learning and other adaptive behaviors. Shepherd (1996) suggests that spines have: “…a range of properties that must be critical for both rapid information processing and slower plastic changes underlying learning and memory.”
Why Do Some Kinds of Synaptic Spines Invaginate into the Terminal?
While there is abundant research on spine function and importance, there is little discussion in the literature on the significance of invaginating spine synapses. Actually, there are many kinds of invaginating processes associated with synapses and they can have several important functions. Invaginating spine synapses are just a small subset of these invaginating projections; the other kinds do not form a definitive chemical synapse (Petralia et al. 2015). These include trophic functions and removal of excess cell membranes, e.g., as may be a consequence of cell membrane proliferation during plasticity. But invaginating projections also function in many cases to direct a specific signal precisely between two particular cell processes, i.e., of two adjacent neuronal or glial cell processes. This would be especially pertinent for an invaginating spine synapse since by definition it is receiving a signal from another cell process (i.e., neurotransmitter reception). So one function of the invagination may be to ensure that the neurotrans-mitter signal activates the postsynaptic spine exclusively. But this is not the only strategy to insure exclusivity of neurotransmission. Thus, mammalian cerebellar Purkinje spine synapses are very closely enwrapped by processes of the Bergmann glia (Mugnaini 1972; Palay and Chan-Palay 1974), closely regulating and restricting diffusion of glutamate (Tzingounis and Wadiche 2007). But alternatively, the Purkinje spines can invaginate into the terminal, especially in early postnatal development. So perhaps the invagination is an early method of enwrapment in many of the Purkinje spines, and that typically it is replaced in the adult by the Bergmann glial wrapping.
Invaginating synaptic spines, and other invaginating projections not described in this review (Petralia et al. 2015), in some cases, may form or increase in number or complexity, in the changes associated with synaptic plasticity. We already described in an earlier section, the invaginating spine synapses associated with the gill-withdrawal reflex of Aplysia (Bailey and Thompson 1979; Bailey et al. 1979). As we noted, the invaginating spine synapses have about twice the number of presynaptic vesicles as regular synaptic contacts (with or without spines), and thus these authors suggest that the invaginating spine synapses are more effective and are formed in association with learning plasticity in the animal’s defensive gill-withdrawal reflex. We also have described examples of synaptic plasticity-related changes in the invaginating postsynaptic processes in the retina, such as during light–dark cycles.
The invaginating spine synapses that we have described here fall into two broad categories. The majority are simple spine structures with active zones and presynaptic vesicles mainly around the spine head region (Figs. 1, 2, 3, 9). But a second category includes more complex structures; the best example is the thorny excrescence of the CA3 mossy terminal (Figs. 6, 7a). In this case, the invaginating process is large and has several distinct, separate active zones and may be branched. Another very complex invaginating compound structure is seen at the retinal photoreceptor terminals, involving several postsynaptic processes that are arranged below the presynaptic ribbon/membrane, including invaginating processes and more basal ones. The invaginating crest synapse may be a third category, but it really is a subset of the typical crest synapses that have separate terminals on each side of the crest. However, in all cases, invaginating spines share this feature: a large extrasynaptic zone lacking glial or other processes, positioned between the invaginating spine and the presynaptic terminal membrane. This creates a potentially large glutamate spillover zone, allowing glutamate to diffuse over distances and activate glutamate receptors outside of the active zones. This functional arrangement has been described for the retinal ribbon synapses (of both pho-toreceptors and bipolar cells), where Müller glial cell processes, the primary mediators of glutamate uptake, are excluded from the complex of postsynaptic processes (Vardi et al. 1998; Haverkamp et al. 2000; DeVries et al. 2006; Veruki et al. 2006; Tzingounis and Wadiche 2007; Kramer and Davenport 2015). A similar phenomenon of spillover probably occurs in other complex synaptic structures that exclude glial processes, like the cerebellar granular layer glomeruli discussed in an earlier section (although the postsynaptic terminals here are not distinctly invaginating; Petralia et al. 2002; Petralia 2012; Tzingounis and Wadiche 2007). Thus, one possible function of an invaginating synapse is to foster glutamate spillover to reach receptors spread along the invaginating structures (but restricted to them, as discussed in the previous paragraph).
In addition, glutamate spillover may be just the first stage in a series of synaptic interactions that depend on the invagination of the components. This may be especially true in the highly organized vertebrate photoreceptor synapse where the two horizontal cell processes (spines) mediate lateral inhibition via negative feedback of the glutamatergic transmission. This feedback may involve GABA release, pH changes, and/or an electric field effect (ephaptic mechanism) within the restricted environment of the invaginating synapse (Gardner et al. 2015; Kramer and Davenport 2015). In fact, the latter mechanism involving electrical feedback (Byzov and Shura-Bura 1986; Gardner et al. 2015; Kramer and Davenport 2015) may explain best why there is such a consistent and regular arrangement of structures in the invagination of the vertebrate photore-ceptor (presynaptic ribbon and processes of horizontal and bipolar neurons); Kramer and Davenport (2015) note how it resembles an electronic transistor.
CA3 Mossy Terminals
The invaginating thorny excrescences (Figs. 6, 7a) of the CA3 mossy terminal synapses of the hippocampus form spine synapses on the proximal portion (stratum lucidum) of the apical dendrites of the CA3 pyramidal neurons (mossy fibers originate from dentate gyrus granule cells). It is interesting that more typical, non-invaginating spine synapses are formed on the remaining distal portions of these same dendrites (stratum radiatum). There must be a strong reason for a single dendrite to express two such different kinds of spine synapses. The answer may be this combination discussed above of spillover within the invaginated space and prevention of spillover outside of this. The arrangement of the mossy terminal and thorny excrescences is somewhat similar to that of a cerebellar mossy terminal glomerulus; one mossy terminal can make more than 37 synaptic contacts total with several different branched excrescences; and excrescences are fairly wide and so probably do not reduce charge transfer, unlike typical spines (Chicurel and Harris 1992). Henze et al. (2000) estimate that on one pyramidal neuron, there are about 700 synapses on excrescences from about 50 mossy fibers, all within the proximal 100 μm of the apical den-drite; this gives this input the potential for a strong excitatory drive to the soma. The unique structure of the mossy fiber-excrescence synapses, along with their specialized combination of receptors and associated proteins, gives the synapses unusual properties such as a large paired-pulse facilitation and frequency facilitation, where a modest increase in stimulation frequency can cause a large increase in synaptic strength (Nicoll and Schmitz 2005). Mossy fiber spontaneous miniature excitatory postsynaptic currents (EPSCs) vary in strength and a few can be very large; these may result from spillover of glutamate from the release site to reach adjacent active zone areas along the excrescence (Henze et al. 2000). Perhaps frequency facilitation also involves spillover. Since the contours of the invaginating excrescence match those of the surrounding mossy terminal membrane, glutamate is channeled naturally along these membranes, to reach the various post- and presynaptic receptors (Min et al. 1998; Vogt and Nicoll 1999; Darstein et al. 2003; Nicoll and Schmitz 2005). Rollenhagen et al. (2007) suggest that there is likely substantial “cross talk” among the active zones at a mossy fiber terminal due to both the close proximity of active zones and the lack of intervening glial processes; these factors may promote both the presynaptic diffusion of calcium and the postsynaptic spillover of the neurotransmitter. Overall, the unique features of these mossy terminal synapses may impart their unique function. A mossy fiber synapse may be “designed to have a higher net probability of release than most other cortical synapses…” (Henze et al. 2000), allowing a single granule cell to initiate action potentials in all of the CA3 pyramidal cells that it innervates with its mossy terminals. Or they may establish the subthreshold membrane potential of these neurons; the latter may be enough depolarization to allow NMDA receptor-mediated LTP to occur at regular spine synapses on the CA3 pyramidal cell apical dendrite (Henze et al. 2000). Interestingly, granule cell mossy fibers actually innervate more inhibitory interneurons in the CA3 region than pyramidal neurons (Acsády et al. 1998) and release probability is higher on interneurons, so that granule cells that fire at low frequency will have a net inhibitory output in the CA3 (Lawrence and McBain 2003; Bischofberger et al. 2006). But when the granule cell fires high-frequency bursts, the net effect on the CA3 will shift to excitation (Lawrence and McBain 2003; Bischofberger et al. 2006). A good example of this is when a rat moves into the place field of a particular granule cell, causing that cell to fire in short, high-frequency bursts (Henze et al. 2002; Lawrence and McBain 2003).
Plasticity is controlled very differently in these mossy terminal synapses compared to typical spine synapses in the hippocampus. For example, there is an NMDA receptor independent form of LTP here (Nicoll and Schmitz 2005). It is easy to conclude that this different mechanism may require some isolation from nearby standard synapses. Also, the mossy terminal synaptic vesicles release large amounts of zinc along with glutamate. This zinc affects the glutamate receptor plasticity at these synapses. It also is unique as a presynaptic ligand because it actually enters the postsynaptic process instead of just binding to a protein on the surface, as other ligands would do (Li et al. 2001). In addition, it can enter the postsynaptic neuron via a number of different kinds of channels. This makes it important to keep the space between the mossy terminal and excrescences closed off so that the zinc cannot readily reach adjacent neuronal and glial processes that possess channels capable of transporting it into the cell (Li et al. 2001). Nevertheless, the zinc does get out eventually; for example in late postnatal (P17–27) rat hippocampal slices, with 100 Hz electrical stimulation of mossy fibers for 2 s, zinc concentration in the stratum lucidum peaks in 5 s, and then spreads to the adjacent part of the stratum radiatum, up to 100 μm, but there is little increase more distally (Ueno et al. 2002).
Thus, the advantage of invagination of excrescences at mossy terminals in the hippocampus (1) may be to allow precise diffusion of neurotransmitter to effect the unique synaptic mechanism within a mossy terminal, and (2) may help isolate this unique mechanism from the immediate environment.
Why Not Have Spines?
Many kinds of neurons in the mammalian brain have few or no spines (Fig. 1). If spines provide so many advantages, why are they absent from many neurons? One reason is that presumably spines will not evolve to form in neural circuits if they are not necessary; spines may increase neuron surface area and volume and thus be energy inefficient for the neuron. In addition, circuits that need to be particularly fast may pass currents faster via flat, simple synapses. Examples include rapid relay synapses in sensory circuits and disynaptic inhibitory input to excitatory principal neurons. The medial nucleus of the trapezoid body is an auditory relay nucleus that receives giant glutamatergic calyces of Held on the somas of the glycinergic principal neurons. This synapse serves as a fast relay of the transduced sound signal from the ear, necessary to determine interaural intensity and time differences (i.e., between ears; Nakamura and Cramer 2011), and has a very fast form of AMPA-type glutamate receptor, high in the GluA4 flop subunit; they have a desensitization time of 1.7 ms (compared to 15.2 ms for CA3 pyramidal neurons; Geiger et al. 1995). Note however that at least during postnatal development (P9 rat), a small number (<6 %) of synaptic contacts in the calyx of Held are on invaginating spines (Sätzler et al. 2002), similar to what we discussed earlier for the endbulb of Held in the anteroventral cochlear nucleus.
In the forebrain, the lack or paucity of spines on most inhibitory interneurons may contribute to the generation of very fast excitatory postsynaptic potentials (EPSPs; Geiger et al. 1997; Hu et al. 2014). For example, in the hippocampus, the half duration of EPSPs at granule cell-basket cell (a type of interneuron) synapses is 3.7 ms, while that of synapses between glutamatergic principal neurons is about seven times longer (Geiger et al. 1997). Not only do spineless synapses avoid the reduction of charge transfer found in spines (as discussed in an earlier section), but the glutamate probably clears more quickly by diffusion from the synaptic cleft in spineless synapses (Geiger et al. 1997). This fast speed is necessary because the interneuron functions in a disynaptic circuit. For example, in the CA1 region of the hippocampus, Schaeffer collaterals of CA3 pyramidal neuron axons form synapses both on interneurons and pyramidal neurons, so that these GABAergic interneurons can modulate the response of the pyramidal neurons. The interneurons must respond quickly so that they can generate substantial inhibitory conductance in the pyramidal neurons before the latter can generate action potentials (Hu et al. 2014).
Pathology of Invaginating and Other Spine Synapses
Dysfunctions of spine synapses have been implicated in a wide range of neurological diseases including Alzheimer’s disease, schizophrenia, epilepsy, depression, and neurodevelopmental disorders (intellectual disability, autism spectrum disorders, fragile X-syndrome, Rett syndrome). For the standard spine synapses and their pathology, we refer the reader to recent, comprehensive reviews (Lai and Ip 2013; Sala and Segal 2014; Duman and Duman 2015). These disorders can lead to a reduction in spine number and/or a change in spine type or connectivity. As an example, a recent study on spine synapse dysfunction and Alzheimer’s disease showed that loss of the large, mushroom synaptic spines of the CA1 region of the hippocampus, possibly associated with memory storage, occurs in two (presenilin and amyloid precursor protein) knock-in mouse models of Alzheimer’s disease, due to downregulation of a specific, neuronal store-operated calcium entry pathway (Zhang et al. 2015b).
For the invaginating spine synapses, while it is not entirely clear why some areas of the nervous system have these unique structures, their disruption could be linked to several neurological disorders. For instance, in epilepsy, the invaginating thorny excrescence/mossy fiber terminal synapses of the hippocampus can show profound changes (Nitsch and Rinne 1981; Eid et al. 2002). In human temporal lobe epilepsy, one characteristic change is the extensive remodeling of mossy fibers and terminals in the hippocampus, notably with abnormal innervation of the inner molecular layer of the dentate gyrus (Sutula et al. 1989; Babb et al. 1991). The enlarged sprouting mossy fibers form synaptic contacts in this latter area with numerous enlarged, complex, and often invaginating spines that overall resemble the thorny excrescences of mossy terminal synapses of the CA3 and CA4 seen in normal animals (Zhang and Houser 1999). This suggests that for synapses in the hippocampus at least, spine invagination is induced by mossy terminal formation (Zhao et al. 2012; see also Nek et al. 1993). Related to this idea, Zhang and Houser (1999) discuss how the formation of the complex, excrescence-like spines (with perforated densities, spinules, invaginating spine processes, and more organelles) indicate synaptic plasticity leading to greater synaptic efficacy. The abnormal formation of these mossy terminal synapses in the molecular layer of the dentate gyrus may contribute to the hyperexcitability that is seen in granule cells of the dentate gyrus in epilepsy. There also are changes in the mossy terminal synapses in the CA3 and CA4 of epileptic humans, including the appearance of a variety of invaginating spines and excrescences where there is an increased expression of the GluR1-containing AMPA receptors, and this probably contributes to the increased excitation of these neuronal pathways (Eid et al. 2002). Other conditions, such as in animals under chronic stress, can induce a shrinkage or even retraction of the thorny excrescences in the CA3 hippocampus (Stewart et al. 2005).
Similarly, changes and dysfunctions in the invaginating spines and associated processes of the synaptic structure in the photoreceptors have been reported in a number of retinal diseases in humans, such as various forms of macular degeneration (Weber et al. 2002; Sullivan et al. 2007). In the retina of age-related macular degeneration, which may affect 20–50 % of people ≥75 years old, the displacement of rod photoreceptor synapses appears prominent (Sullivan et al. 2007). The multiple, invaginating postsynaptic processes in these displaced rod synapses appear to originate only from bipolar neurons, whereas normal rod postsynaptic processes originate from both bipolar and horizontal cells. Other aging-associated pathologies of the retina also include abnormalities in the photoreceptor synaptic regions (Nag and Wadhwa 2012). Among the retinal dystrophies, retinitis pigmentosa is probably the most common, and involves the loss of photoreceptors themselves, beginning with the rods of the peripheral retina (Berson 1993). But in cone-rod dystrophy, which initially affects the central retina, the cone photoreceptor pedicles with invaginating postsynaptic processes seem to be particularly affected and are visibly distorted (Gregory-Evans et al. 1998).
In the deaf white cat model of congenital deafness, the invaginating somatic spines and associated structures in the endbulbs of Held of the anteroventral cochlear nucleus are reduced in size and extent in early postnatal deaf cats (Baker et al. 2010). These changes appear prior to obvious abnormal changes in the cochlea itself, and could be due to a disruption of spontaneous discharges in the auditory nerve.
These examples of neurological disorders reveal an association of the pathology with invaginating spine synapses. Despite this correlation, however, it is not clearly known whether the pathology of the invaginating spines is a by-product of the neurological diseases or if the damaged invaginating spines cause these diseases. It also remains to be investigated whether the specific architecture of the invaginating spines makes them sensitive and vulnerable to certain disease-inflicted insults. We have suggested in this review that the degree of spine invagination may be related generally to overall synaptic activity and plasticity of the terminal. This would be consistent with the disruptions seen in many of the pathologies discussed above, all of which seem to involve large, complex presynaptic terminals with large populations of synaptic vesicles (i.e., mossy, photoreceptor, and endbulb terminals). However, we also have discussed more specific functions of the invaginating spine related to increased precision in signaling, such as the control of spillover of neurotransmitters and isolation from the influence of other chemical factors in the local environment. But we do not really know yet whether the invagination is critical to this and whether this is the factor most affected in pathologies. The knowledge of the invaginating spine synapses, from their evolutionary history to their ultrastructural characteristics, should raise interest and excitement in understanding both their roles in normal physiology and pathological conditions.
Conclusions
Based on the available, albeit limited, information on spine synapses in invertebrates and non-mammalian vertebrates, we suggest that there are two basic functional kinds of spine synapse: invaginating spine synapses appeared first in the evolution of the nervous system and then non-invaginating spine synapses appeared in the first brains. The earliest synaptic contacts probably had no spines, and the spineless chemical synapse still is common throughout all animal groups. But early in animal evolution, some chemical synapses became more complex by forming a spine on the postsynaptic process and invaginating this spine into the presynaptic process. Some sponges (Porifera), though they lack any definitive nervous system with chemical synapses, have cells that extend long processes and these can bear small invaginating, spine-like processes. Whether or not these structures are common, they open the possibility that invaginating spines were capable of developing readily along with the first true chemical synapses, which already are prevalent and diverse in the Ctenophora and Cnidaria. Ctenophores do not seem to possess any definitive spine synapses, but in any case, ctenophore evolution is problematic. The few spine synapses that have been described so far in Cnidaria, possess invaginating spines. They are found in cnidarians known either for their superior vision or unusual, active prey capture behavior. This suggests that invagination of spines may be an adaptation for some more advanced behaviors in the Cnidaria. The association of these invaginating synapses with photoreceptor neurotransmission is particularly interesting, for there is a tendency toward invaginating synapses in photoreceptor neurotransmission in higher invertebrates and most vertebrates. This also seems to be a trend in the evolution of advanced mechanoreception associated with hearing, balance and rapid motion sensing (e.g., gill-withdrawal reflex in the sea hare). In addition, vertebrates have invaginating synapses associated with a number of neural pathways.
The advantages of the invaginating spine have not been well studied. We have suggested that studies on some mammalian invaginating spine synapse systems, such as the hippocampal mossy fiber-thorny excrescence synapse and retinal synapses, indicate that the invagination helps regulate the degree of neurotransmitter spillover and activation of receptors at various distances from the release site. It also helps regulate the levels of various controlling ions such as calcium and zinc. The invagination promotes the passage of some neurotransmitters and ions within it, but it also protects the synapse from other, extrinsic sources of molecules and ions by sealing in the synaptic area entirely within the presynaptic terminal. Our discussion of pathologies of invaginating synapses further support the contribution of the invagination to the proper function of these neural circuits.
Finally, we suggest that the proliferation of non-invaginating spine synapse coincides with the evolution of the bilaterally symmetrical animal and the brain, with the simplest examples in the flatworms. Thus, as the nervous system and its component neurons evolved greater complexity to adapt to the consequences of bilateral symmetry, i.e., cephalization and more directed locomotion, it formed into a true brain and one or more associated nerve cords. The interconnection of neuron processes became more complicated in the brain, and spine synapses were one adaptation to facilitate its function. Otherwise, whether non-invaginating or invaginating, the synaptic spine morphology has changed little in evolution, evolving greater structural complexity only in a few animal groups, such as the addition of the spine apparatus in the larger spines of the forebrain of mammals.
Acknowledgments
This work was supported by the Intramural Research Programs of NIDCD/NIH and NIA/NIH. The code for the Advanced Imaging Core of NIDCD is ZIC DC000081-03. We thank Megan Wyeth for helpful discussion about hippocampal CA3 mossy terminal synapse function and inhibitory interneurons.
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that there is no conflict of interest.
References
- Achatz JG, Martinez P. The nervous system of Isodiametra pulchra (Acoela) with a discussion on the neu-roanatomy of the Xenacoelomorpha and its evolutionary implications. Frontiers in Zoology. 2012;9:27. doi: 10.1186/1742-9994-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acker CD, Yan P, Loew LM. Single-voxel Recording of voltage transients in dendritic spines. Biophysical Journal. 2011;101(2):L11–L13. doi: 10.1016/j.bpj.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acsády L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. Journal of Neuroscience. 1998;18(9):3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akert K, Pfenning K, Sandri C. Fine structure of synapses in subfornical organ of cat. Zeitschrift Fur Zell-forschung Und Mikroskopische Anatomie. 1967a;81(4):537–556. doi: 10.1007/BF00541013. [DOI] [PubMed] [Google Scholar]
- Akert K, Pfenninger K, Sandri C. Crest synapses with subjunctional bodies in the subfornical organ. Brain Research. 1967b;5(1):118–120. doi: 10.1016/0006-8993(67)90224-7. [DOI] [PubMed] [Google Scholar]
- Alberstein R, Grey R, Zimmet A, Simmons DK, Mayer ML. Glycine activated ion channel subunits encoded by ctenophore glutamate receptor genes. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(44):E6048–E6057. doi: 10.1073/pnas.1513771112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson DG, Thomson JN. Pharynx of caenorhabditis elegans. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1976;275(938):299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure, and functions. New York: CRC Press; 1997. [Google Scholar]
- Alvarez-Otero R, Regueira SD, Anadon R. New structural aspects of the synaptic contacts on Purkinje cells in an elasmobranch cerebellum. Journal of Anatomy. 1993;182(Pt 1):13–21. [PMC free article] [PubMed] [Google Scholar]
- Anderson PAV, Grunert U. 3-Dimensional structure of bidirectional, excitatory chemical Synapses in the jellyfish Cyanea-Capillata. Synapse. 1988;2(6):606–613. doi: 10.1002/syn.890020605. [DOI] [PubMed] [Google Scholar]
- Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters membrane potentials. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(47):17961–17966. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D, Tosches MA, Marlow H. From nerve net to nerve ring, nerve cord and brain - evolution of the nervous system. Nature Reviews Neuroscience. 2016;17:61–72. doi: 10.1038/nrn.2015.15. [DOI] [PubMed] [Google Scholar]
- Arluison M, de la Manche IS. High-resolution radioautographic study of the serotonin innervation of the rat corpus striatum after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1980;5(2):229–240. doi: 10.1016/0306-4522(80)90100-1. [DOI] [PubMed] [Google Scholar]
- Aronin N, DiFiglia M, Liotta AS, Martin JB. Ultrastructural localization and biochemical features of immunoreactive LEU-enkephalin in monkey dorsal horn. Journal of Neuroscience. 1981;1(6):561–577. doi: 10.1523/JNEUROSCI.01-06-00561.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, Maier SR, Nishimune A, Henley JM. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. Journal of Neuroscience. 2006;26(26):7046–7055. doi: 10.1523/JNEUROSCI.1235-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in Human epileptic Fascia-Dentata. Neuroscience. 1991;42(2):351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Harris KM. Structural components of synaptic plasticity and memory consolidation. Cold Spring Harbor Perspectives in Biology. 2015;7:a021758. doi: 10.1101/cshperspect.a021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Thompson EB. Indented synapses in Aplysia. Brain Research. 1979;173(1):13–20. doi: 10.1016/0006-8993(79)91091-6. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Thompson EB, Castellucci VF, Kandel ER. Ultrastructure of the synapses of sensory neurons that mediate the Gill-withdrawal reflex in Aplysia. Journal of Neurocytology. 1979;8(4):415–444. doi: 10.1007/BF01214801. [DOI] [PubMed] [Google Scholar]
- Baker CA, Montey KL, Pongstaporn T, Ryugo DK. Postnatal development of the endbulb of held in congenitally deaf cats. Front Neuroanatical. 2010;4:19. doi: 10.3389/fnana.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedini C, Lanfranchi A. The central and peripheral nervous-system of Acoela (Plathelminthes). An electron-microscopic study. Acta Zoologica. 1991;72(2):101–106. [Google Scholar]
- Bedini C, Lanfranchi A. Ultrastructural study of the brain of a typhloplanid flatworm. Acta Zoologica. 1998;79(3):243–249. [Google Scholar]
- Bell CC, Han V, Sawtell NB. Cerebellum-like structures and their implications for cerebellar function. Annual Review of Neuroscience. 2008;31:1–24. doi: 10.1146/annurev.neuro.30.051606.094225. [DOI] [PubMed] [Google Scholar]
- Bellot A, Guivernau B, Tajes M, Bosch-Morato M, Valls-Comamala V, Munoz FJ. The structure and function of actin cytoskeleton in mature glutamatergic dendritic spines. Brain Research. 2014;1573:1–16. doi: 10.1016/j.brainres.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Suprachiasmatic nucleus and melatonin reciprocal interactions and clinical correlations. Neurology. 2008;71(8):594–598. doi: 10.1212/01.wnl.0000324283.57261.37. [DOI] [PubMed] [Google Scholar]
- Benwitz G. Electron-microscopic investigations on development of colloblasts in Ctenophore Pleurobrachia-Pileus (Tenta-culifera, Cydippea) Zoomorphologie. 1978;89(3):257–278. [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. Journal of Neuroscience. 1995;15(12):7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson EL. Retinitis-Pigmentosa. The Friedenwald Lecture. Investigative Ophthalmology & Visual Science. 1993;34(5):1659–1676. [PubMed] [Google Scholar]
- Bery A, Cardona A, Martinez P, Hartenstein V. Structure of the central nervous system of a juvenile acoel. Symsagittifera roscoffensis. Development Genes and Evolution. 2010;220(3–4):61–76. doi: 10.1007/s00427-010-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Frotscher M, Jonas P. Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal network. Pflugers Archiv-European Journal of Physiology. 2006;453(3):361–372. doi: 10.1007/s00424-006-0093-2. [DOI] [PubMed] [Google Scholar]
- Blanks JC, Adinolfi AM, Lolley RN. Synaptogenesis in the photoreceptor terminal of the mouse retina. Journal of Comparative Neurology. 1974;156(1):81–93. doi: 10.1002/cne.901560107. [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Giessel AJ, Sabatini BL. Biphasic synaptic Ca influx arising from compartmentalized electrical signals in dendritic spines. PLoS Biology. 2009;7(9):e1000190. doi: 10.1371/journal.pbio.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A, Helmstaedter M. Common circuit design in fly and mammalian motion vision. Nature Neuroscience. 2015;18(8):1067–1076. doi: 10.1038/nn.4050. [DOI] [PubMed] [Google Scholar]
- Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron. 2014;82(2):444–459. doi: 10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschek CB. Fine structure of peripheral retina and Lamina Ganglionaris of fly, Musca-Domestica. zeitschrift Fur Zell-forschung Und Mikroskopische Anatomie. 1971;118(3):369–409. doi: 10.1007/BF00331193. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annual Review of Neuroscience. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott BB, Guillery RW, Gray EG. Synaptic structure and its alteration with environmental temperature: A study by light and electron microscopy of central nervous system of lizards. Proceedings of the Royal Society Series B-Biological Sciences. 1961;154(955):151. + [Google Scholar]
- Brandon JG, Coss RG. Rapid dendritic spine stem shortening during one-trial learning: the honeybee’s first orientation flight. Brain Research. 1982;252(1):51–61. doi: 10.1016/0006-8993(82)90977-5. [DOI] [PubMed] [Google Scholar]
- Brown S, Wolff G. Fine structural organization of the hemiellipsoid body of the land hermit crab, Coenobita clypeatus. Journal of Comparative Neurology. 2012;520(13):2847–2863. doi: 10.1002/cne.23058. [DOI] [PubMed] [Google Scholar]
- Budelmann BU, Sachse M, Staudigl M. The angular-acceleration receptor system of the statocyst of Octopus-Vulgaris: Morphometry, ultrastructure, and neuronal and synaptic organization. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1987;315(1174):305–343. [Google Scholar]
- Budelmann BU, Thies G. Secondary sensory cells in gravity receptor system of statocyst of Octopus-Vulgaris. Cell and Tissue Research. 1977;182(1):93–98. doi: 10.1007/BF00222057. [DOI] [PubMed] [Google Scholar]
- Burighel P, Lane NJ, Fabio G, Stefano T, Zaniolo G, Carnevali MD, Manni L. Novel, secondary sensory cell organ in ascidians: in search of the ancestor of the vertebrate lateral line. Journal of Comparative Neurology. 2003;461(2):236–249. doi: 10.1002/cne.10666. [DOI] [PubMed] [Google Scholar]
- Buttarelli FR, Pellicano C, Pontieri FE. Neurophar-macology and behavior in planarians: Translations to mammals. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2008;147(4):399–408. doi: 10.1016/j.cbpc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Byzov AL, Shura-Bura TM. Electrical feedback mechanism in the processing of signals in the outer plexiform layer of the retina. Vision Research. 1986;26(1):33–44. doi: 10.1016/0042-6989(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Campellone KG, Leong JM. Tails of two Tirs: actin pedestal formation by enteropathogenic E. coli and enterohem-orrhagic E. coli O157:H7. Current Opinion in Microbiology. 2003;6(1):82–90. doi: 10.1016/s1369-5274(03)00005-5. [DOI] [PubMed] [Google Scholar]
- Cannon JT, Vellutini BC, Smith J, 3rd, Ronquist F, Jondelius U, Hejnol A. Xenacoelomorpha is the sister group to Nephrozoa. Nature. 2016;530(7588):89–93. doi: 10.1038/nature16520. [DOI] [PubMed] [Google Scholar]
- Cano J, Pasik P, Pasik T. Early postnatal development of the monkey globus pallidus: A golgi and electron microscopic study. Journal of Comparative Neurology. 1989;279(3):353–367. doi: 10.1002/cne.902790303. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: Synaptic targets and relation to dopamine terminals. Journal of Comparative Neurology. 1996;369(1):1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Case NM, Young JZ, Gray EG. Ultrastructure and synaptic relations in optic lobe of brain of Eledone and Octopus. Journal of Ultrastructure Research. 1972;39(1–2):115–123. doi: 10.1016/s0022-5320(72)80012-1. [DOI] [PubMed] [Google Scholar]
- Castejón OJ, Apkarian RP. Conventional and high resolution field emission scanning electron microscopy of vertebrate cerebellar parallel fiber-Purkinje spine synapses. Cellular Molecular Biology (Noisy-le-grand) 1993;39(8):863–873. [PubMed] [Google Scholar]
- Castejón OJ, Villegas GM. Fine structure of synaptic contacts in stellate ganglion of squid. Journal of Ultrastructure Research. 1964;10(5–6):585–598. doi: 10.1016/s0022-5320(64)80032-0. [DOI] [PubMed] [Google Scholar]
- Cerminara NL, Lang EJ, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nature Reviews Neuroscience. 2015;16(2):79–93. doi: 10.1038/nrn3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazeau A, Giannone G. Organization and dynamics of the actin cytoskeleton during dendritic spine morphological remodeling. Cellular and Molecular Life Science. 2016 doi: 10.1007/s00018-00016-02214-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Levy JM, Hou A, Winters C, Azzam R, Sousa AA, et al. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proceedings of National Academy of Science USA. 2015;112(50):E6983–E6992. doi: 10.1073/pnas.1517045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WR, Xiong WH, Shepherd GM. Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron. 2000;25(3):625–633. doi: 10.1016/s0896-6273(00)81065-x. [DOI] [PubMed] [Google Scholar]
- Chevy Q, Heubl M, Goutierre M, Backer S, Moutkine I, Eugene E, et al. KCC2 Gates Activity-Driven AMPA Receptor Traffic through Cofilin Phosphorylation. Journal of Neuroscience. 2015;35(48):15772–15786. doi: 10.1523/JNEUROSCI.1735-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. Journal of Comparative Neurology. 1992;325(2):169–182. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GC, Higley MJ. Compartmentalization of GABAer-gic inhibition by dendritic spines. Science. 2013;340(6133):759–762. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB. Synaptic connectivity and neuronal morphology: Viewpoint two sides of the same coin. Neuron. 2004;43(5):609–617. doi: 10.1016/j.neuron.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Chu DTW, Klymkowsky MW. The appearance of acetylated alpha-tubulin during early development and cellular-differentiation in Xenopus. Developmental Biology. 1989;136(1):104–117. doi: 10.1016/0012-1606(89)90134-6. [DOI] [PubMed] [Google Scholar]
- Clarke DJ, Dunnett SB. Ultrastructural organization of choline-acetyltransferase-immunoreactive fibres innervating the neocortex from embryonic ventral forebrain grafts. Journal of Comparative Neurology. 1986;250(2):192–205. doi: 10.1002/cne.902500206. [DOI] [PubMed] [Google Scholar]
- Clément P. Ultrastructural research on rotifers. Archive Hydrobiol Beib Ergebn Limnol. 1977;8:270–297. [Google Scholar]
- Coates MM. Visual ecology and functional morphology of cubozoa (cnidaria) Integrative and Comparative Biology. 2003;43(4):542–548. doi: 10.1093/icb/43.4.542. [DOI] [PubMed] [Google Scholar]
- Cobb JLS, Stubbs TR. The giant-neuron system in Ophiuroids. 3. The detailed connections of the circumoral nerve ring. Cell and Tissue Research. 1982;226(3):675–687. doi: 10.1007/BF00214794. [DOI] [PubMed] [Google Scholar]
- Cohen AI. Ultrastructural analysis of photoreceptors of squid and their synaptic connections. 3. Photoreceptor terminations in optic lobes. Journal of Comparative Neurology. 1973;147(3):399–425. doi: 10.1002/cne.901470306. [DOI] [PubMed] [Google Scholar]
- Colmers WF. Neuronal and synaptic organization in gravity receptor system of statocyst of Octopus-Vulgaris. Cell and Tissue Research. 1977;185(4):491–503. doi: 10.1007/BF00220653. [DOI] [PubMed] [Google Scholar]
- Colonnier M, Guillery RW. Synaptic organization in the lateral geniculate nucleus of the monkey. Z Zellforsch Mikrosk Anat. 1964;62:333–355. doi: 10.1007/BF00339284. [DOI] [PubMed] [Google Scholar]
- Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. Journal of Neuroscience. 2002;22(6):2215–2224. doi: 10.1523/JNEUROSCI.22-06-02215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss RG, Brandon JG, Globus A. Changes in morphology of dendritic spines on honeybee calycal interneurons associated with cumulative nursing and foraging experiences. Brain Research. 1980;192(1):49–59. doi: 10.1016/0006-8993(80)91007-0. [DOI] [PubMed] [Google Scholar]
- Coss RG, Perkel DH. The function of dendritic spines: A review of theoretical issues. Behavioral and Neural Biology. 1985;44(2):151–185. doi: 10.1016/s0163-1047(85)90170-0. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. Horizontal cells in the retina of the rabbit. Journal of Neuroscience. 1982;2(10):1486–1493. doi: 10.1523/JNEUROSCI.02-10-01486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. Journal of Neuroscience. 2003;23(22):8013–8019. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EDP, Bennett HS. Some features of the submicroscopic morphology of synapses in frog and earthworm. Journal of Biophysical and Biochemical Cytology. 1955;1(1):47–58. doi: 10.1083/jcb.1.1.47. + 43 plates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano ME, Luzzatto AC, Mugnaini E. Neuronal ultrastructure and somatostatin immunolocalization in the ciliary ganglion of chicken and quail. Journal of Neurocytology. 1993;22(10):868–892. doi: 10.1007/BF01186358. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Gerrits NM, Voogd J, Leonard CS, Simpson JI. The rostral dorsal cap and ventrolateral outgrowth of the rabbit inferior olive receive a GABAergic input from dorsal group Y and the ventral dentate nucleus. Journal of Comparative Neurology. 1994;341(3):420–432. doi: 10.1002/cne.903410311. [DOI] [PubMed] [Google Scholar]
- Dehay C, Douglas RJ, Martin KA, Nelson C. Excitation by geniculocortical synapses is not ‘vetoed’ at the level of dendritic spines in cat visual cortex. Journal of Physiology. 1991;440:723–734. doi: 10.1113/jphysiol.1991.sp018732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Merten T, Roth SU, Mundel P, Frotscher M. Actin-associated protein synaptopodin in the rat hippocampal formation: Localization in the spine neck and close association with the spine apparatus of principal neurons. Journal of Comparative Neurology. 2000;418(2):164–181. doi: 10.1002/(sici)1096-9861(20000306)418:2<164::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Deller T, Orth CB, Del Turco D, Vlachos A, Burbach GJ, Drakew A, et al. A role for synaptopodin and the spine apparatus in hippocampal synaptic plasticity. Annals of Anatomy-Anatomischer Anzeiger. 2007;189(1):5–16. doi: 10.1016/j.aanat.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Dent EW, Merriam EB, Hu X. The dynamic cytoskeleton: backbone of dendritic spine plasticity. Current Opinion in Neurobiology. 2011;21(1):175–181. doi: 10.1016/j.conb.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Berube-Carriere N, Riad M, Bo GD, Mendez JA, Trudeau LE. Glutamate in dopamine neurons: Synaptic versus diffuse transmission. Brain Research Reviews. 2008;58(2):290–302. doi: 10.1016/j.brainresrev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: A light and electron microscope radioautographic study. Journal of Comparative Neurology. 1982;207(3):239–254. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50(5):735–748. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- DiGregorio DA, Nusser Z, Silver RA. Spillover of glutamate onto synaptic AMPA receptors enhances fast transmission at a cerebellar synapse. Neuron. 2002;35(3):521–533. doi: 10.1016/s0896-6273(02)00787-0. [DOI] [PubMed] [Google Scholar]
- Dilly PN. Synapses in Cerebral Ganglion of Adult Ciona Intestinalis. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie. 1969;93(1):142–150. doi: 10.1007/BF00325029. [DOI] [PubMed] [Google Scholar]
- Dilly PN. Structures of Tentacles of “Rhabdopleura-Compacta(Hemichordata) with special reference to neurociliary control. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie. 1972;129(1):20–39. doi: 10.1007/BF00307107. [DOI] [PubMed] [Google Scholar]
- Dilly PN, Gray EG, Young JZ. Electron microscopy of optic nerves and optic lobes of octopus and Eledone. Proceedings of the Royal Society Series B-Biological Sciences. 1963;158(973):446. doi: 10.1098/rspb.1963.0057. + [DOI] [PubMed] [Google Scholar]
- Dilly PN, Welsch U, Storch V. Structure of nerve fibre layer and neurocord in enteropneusts. Zeitschrift Fur Zell-forschung Und Mikroskopische Anatomie. 1970;103(1):129–148. doi: 10.1007/BF00335406. [DOI] [PubMed] [Google Scholar]
- Dryer L, Graziadei PPC. Synaptology of the olfactory bulb of an elasmobranch fish Sphyrna tiburo. Anatomy and Embryology. 1996;193(2):101–114. doi: 10.1007/BF00214701. [DOI] [PubMed] [Google Scholar]
- Dubin MW. The inner plexiform layer of the vertebrate retina: a quantitative and comparative electron microscopic analysis. Journal of Comparative Neurology. 1970;140(4):479–505. doi: 10.1002/cne.901400406. [DOI] [PubMed] [Google Scholar]
- Duman CH, Duman RS. Spine synapse remodeling in the pathophysiology and treatment of depression. Neuroscience Letters. 2015;601:20–29. doi: 10.1016/j.neulet.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T, Kovacs I, Spencer DD, de Lanerolle NC. Novel expression of AMPA-receptor subunit GluR1 on mossy cells and CA3 pyramidal neurons in the human epileptogenic hippocampus. European Journal of Neuroscience. 2002;15(3):517–527. doi: 10.1046/j.0953-816x.2001.01887.x. [DOI] [PubMed] [Google Scholar]
- Ellwanger K, Eich A, Nickel M. GABA and glutamate specifically induce contractions in the sponge Tethya wilhelma. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2007;193(1):1–11. doi: 10.1007/s00359-006-0165-y. [DOI] [PubMed] [Google Scholar]
- Ellwanger K, Nickel M. Neuroactive substances specifically modulate rhythmic body contractions in the nerveless metazoon Tethya wilhelma (Demospongiae, Porifera) Frontiers in Zoology. 2006;3:7. doi: 10.1186/1742-9994-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes RD, Grant SG. Evolution of synapse complexity and diversity. Annual Review of Neuroscience. 2012;35:111–131. doi: 10.1146/annurev-neuro-062111-150433. [DOI] [PubMed] [Google Scholar]
- Fahrenbach WH. Brain of the Horseshoe Crab (Limulus-Polyphemus).3. Cellular and synaptic organization of the Corpora Pedunculata. Tissue and Cell. 1979;11(1):163–200. doi: 10.1016/0040-8166(79)90016-8. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Peters A. Synaptic glomerulus and intrinsic neuron in dorsal lateral geniculate nucleus of cat. Journal of Comparative Neurology. 1972;144(3):285–334. doi: 10.1002/cne.901440304. [DOI] [PubMed] [Google Scholar]
- Farris SM, Robinson GE, Fahrbach SE. Experience-and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. Journal of Neuroscience. 2001;21(16):6395–6404. doi: 10.1523/JNEUROSCI.21-16-06395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero E. Fine-structural analysis of statocyst in Turbellaria Acoela. Zoologica Scripta. 1973;2(1):5–16. [Google Scholar]
- Fischbach KF, Dittrich APM. The optic lobe of Drosophila-Melanogaster. 1. A golgi analysis of wild-Type structure. Cell and Tissue Research. 1989;258(3):441–475. [Google Scholar]
- Fischer FP. Quantitative analysis of the innervation of the chicken basilar papilla. Hearing Research. 1992;61(1–2):167–178. doi: 10.1016/0378-5955(92)90048-r. [DOI] [PubMed] [Google Scholar]
- Fishelson L. Observations on the moving colonies of the genus Tethya (Demospongia, Porifera).1. Behavior and cytology. Zoomorphology. 1981;98(1):89–99. [Google Scholar]
- Fisher SK, Boycott BB. Synaptic connections made by horizontal cells within outer plexiform layer of retina of cat and rabbit. Proceedings of the Royal Society Series B-Biological Sciences. 1974;186(1085):317. doi: 10.1098/rspb.1974.0052. [DOI] [PubMed] [Google Scholar]
- Flood PR. A peculiar mode of muscular innervation in Amphioxus. Light and electron microscopic studies of the so-called ventral roots. Journal of Comparative Neurology. 1966;126(2):181–217. doi: 10.1002/cne.901260204. [DOI] [PubMed] [Google Scholar]
- Frambach I, Rossler W, Winkler M, Schurmann FW. F-actin at identified synapses in the mushroom body neuropil of the insect brain. Journal of Comparative Neurology. 2004;475(3):303–314. doi: 10.1002/cne.20165. [DOI] [PubMed] [Google Scholar]
- Franc JM. Organization and function of ctenophore colloblasts: Ultrastructural-study. Biological Bulletin. 1978;155(3):527–541. [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13(4):1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Seress L, Schwerdtfeger WK, Buhl E. The mossy cells of the fascia-dentata: A comparative-study of their fine-structure and synaptic connections in rodents and primates. Journal of Comparative Neurology. 1991;312(1):145–163. doi: 10.1002/cne.903120111. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Studer D, Graber W, Chai X, Nestel S, Zhao S. Fine structure of synapses on dendritic spines. Frontiers in Neuroanatomy. 2014;8:94. doi: 10.3389/fnana.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA. A ‘calcium capacitor’ shapes cholinergic inhibition of cochlear hair cells. Journal of Physiology. 2014;592(Pt 16):3393–3401. doi: 10.1113/jphysiol.2013.267914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience. 2004a;125(3):615–623. doi: 10.1016/j.neuroscience.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. Journal of Comparative Neurology. 2004b;468(1):86–95. doi: 10.1002/cne.10950. [DOI] [PubMed] [Google Scholar]
- Gardner CL, Jones JR, Baer SM, Crook SM. Drift-diffusion simulation of the ephaptic effect in the triad synapse of the retina. Journal of Computational Neuroscience. 2015;38(1):129–142. doi: 10.1007/s10827-014-0531-7. [DOI] [PubMed] [Google Scholar]
- Garm A, Nilsson DE. Visual navigation in starfish: First evidence for the use of vision and eyes in starfish. Proceedings of the Royal Society B-Biological Sciences. 2014;281(1777):20133011. doi: 10.1098/rspb.2013.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Lubke J, Roth A, Frotscher M, Jonas P. Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron. 1997;18(6):1009–1023. doi: 10.1016/s0896-6273(00)80339-6. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15(1):193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proceedings of National Academy of Science USA. 1989;86(22):9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosney DL, de Grado M, Finlay BB. Putting E. coli on a pedestal: a unique system to study signal transduction and the actin cytoskeleton. Trends in Cell Biology. 1999;9(1):11–14. doi: 10.1016/s0962-8924(98)01418-4. [DOI] [PubMed] [Google Scholar]
- Gordon DP. Microarchitecture and function of lophophore in bryozoan Cryptosula-Pallasiana. Marine Biology. 1974;27(2):147–163. [Google Scholar]
- Goto J, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor-mediated calcium release in Purkinje cells: from molecular mechanism to behavior. Cerebellum. 2011;10(4):820–833. doi: 10.1007/s12311-011-0270-5. [DOI] [PubMed] [Google Scholar]
- Granger AJ, Mulder N, Saunders A, Sabatini BL. Cotransmission of acetylcholine and GABA. Neuropharmacology. 2016;100:40–46. doi: 10.1016/j.neuropharm.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: An electron microscope study. Journal of Anatomy. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Gray EG. The granule cells, mossy synapses and Purkinje spine synapses of the cerebellum: Light and electron microscope observations. Journal of Anatomy. 1961;95:345–356. [PMC free article] [PubMed] [Google Scholar]
- Gray EG, Guillery RW. A note on the dendritic spine apparatus. Journal of Anatomy. 1963;97:389–392. [PMC free article] [PubMed] [Google Scholar]
- Gray GC, Martin VJ, Satterlie RA. Ultrastructure of the retinal synapses in cubozoans. Biological Bulletin. 2009;217(1):35–49. doi: 10.1086/BBLv217n1p35. [DOI] [PubMed] [Google Scholar]
- Gregory-Evans K, Fariss RN, Possin DE, Gregory-Evans CY, Milam AH. Abnormal cone synapses in human cone-rod dystrophy. Ophthalmology. 1998;105(12):2306–2312. doi: 10.1016/S0161-6420(98)91233-7. [DOI] [PubMed] [Google Scholar]
- Grigoryan G, Segal M. Ryanodine-mediated conversion of STP to LTP is lacking in synaptopodin-deficient mice. Brain Structure and Function. 2016;221(4):2393–2397. doi: 10.1007/s00429-015-1026-7. [DOI] [PubMed] [Google Scholar]
- Groves PM, Linder JC, Young SJ. 5-hydroxy-dopamine-labeled dopaminergic axons: Three-dimensional reconstructions of axons, synapses and postsynaptic targets in rat neostriatum. Neuroscience. 1994;58(3):593–604. doi: 10.1016/0306-4522(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Grunditz A, Holbro N, Tian L, Zuo Y, Oertner TG. Spine neck plasticity controls postsynaptic calcium signals through electrical compartmentalization. Journal of Neuroscience. 2008;28(50):13457–13466. doi: 10.1523/JNEUROSCI.2702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güldner FH. Synaptology of Rat Suprachiasmatic Nucleus. Cell and Tissue Research. 1976;165(4):509–544. doi: 10.1007/BF00224478. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Carnevale NT, Stuart GJ. Electrical advantages of dendritic spines. PLoS One. 2012;7(4):e36007. doi: 10.1371/journal.pone.0036007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Miettinen R, Jacobowitz DM, Freund TF. Calretinin is present in nonpyramidal cells of the rat hippocampus. 1. A new type of neuron specifically associated with the mossy fiber system. Neuroscience. 1992;48(1):1–27. doi: 10.1016/0306-4522(92)90334-x. [DOI] [PubMed] [Google Scholar]
- Günther J, Schürmann FW. Zur Feinstruktur des dorsalen riesenfasersystems im bauchmark des regenwurms. II. Synaptische beziehungen der proximalen riesenfaserkollateralen. Z Zellforsch. 1973;139:369–396. [PubMed] [Google Scholar]
- Haas MA, Bell D, Slender A, Lana-Elola E, Watson-Scales S, Fisher EMC, et al. Alterations to dendritic spine morphology, but not dendrite patterning, of cortical projection neurons in Tc1 and Ts1Rhr mouse models of down syndrome. PLoS One. 2013;8(10):e78561. doi: 10.1371/journal.pone.0078561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner GS. The ultrastructure of retinula cell endings in the compound eye of the crayfish. Journal of Neurocytology. 1974;3(3):295–311. doi: 10.1007/BF01097915. [DOI] [PubMed] [Google Scholar]
- Hall DH, Russell RL. The posterior nervous-system of the nematode Caenorhabditis-Elegans: Serial reconstruction of identified neurons and complete pattern of synaptic-interactions. Journal of Neuroscience. 1991;11(1):1–22. doi: 10.1523/JNEUROSCI.11-01-00001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S. Actin and the agile spine: how and why do dendritic spines dance? Trends in Neurosciences. 2000;23(4):141–146. doi: 10.1016/s0166-2236(00)01576-9. [DOI] [PubMed] [Google Scholar]
- Halton DW, Gustafsson MKS. Functional morphology of the platyhelminth nervous system. Parasitology. 1996;113:S47–S72. [Google Scholar]
- Hama K. Some observations on fine structure of giant fibers of crayfishes (Cambarus Virilus and Cambarus Clarkii) with special reference to submicroscopic organization of synapses. Anatomical Record. 1961;141(4):275–293. doi: 10.1002/ar.1091410403. [DOI] [PubMed] [Google Scholar]
- Hama K. Some Observations on the fine structure of the giant synapse in the stellate ganglion of the squid, Doryteuphis-Bleekeri. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie. 1962;56(4):437–444. doi: 10.1007/BF00335624. [DOI] [PubMed] [Google Scholar]
- Hamill GS, Lenn NJ. Synaptic plasticity within the interpeduncular nucleus after unilateral lesions of the habenula in neonatal rats. Journal of Neuroscience. 1983;3(11):2128–2145. doi: 10.1523/JNEUROSCI.03-11-02128.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DW. The calyceal synapse of type I vestibular hair cells. Journal of Ultrastructure Research. 1968;23(1):98–114. doi: 10.1016/s0022-5320(68)80034-6. [DOI] [PubMed] [Google Scholar]
- Hamlyn LH. Fine Structure of Mossy Fibre Endings in Hippocampus of Rabbit. Journal of Anatomy. 1962;96(Jan):112–120. + 116 plates. [PMC free article] [PubMed] [Google Scholar]
- Hamori J, Horridge GA. Lobster optic lamina. 2. Types of synapse. Journal of Cell Science. 1966;1(2):257–270. doi: 10.1242/jcs.1.3.275. [DOI] [PubMed] [Google Scholar]
- Hamori J, Pasik T, Pasik P, Szentagothai J. Triadic synaptic arrangements and their possible significance in the lateral geniculate nucleus of the monkey. Brain Research. 1974;80(3):379–393. doi: 10.1016/0006-8993(74)91024-5. [DOI] [PubMed] [Google Scholar]
- Harnett MT, Makara JK, Spruston N, Kath WL, Magee JC. Synaptic amplification by dendritic spines enhances input cooperativity. Nature. 2012;491(7425):599. doi: 10.1038/nature11554. + [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. Journal of Neuroscience. 1992;12(7):2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annual Review of Neuroscience. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Harris KM, Weinberg RJ. Ultrastructure of synapses in the mammalian brain. Cold Spring Harbor Perspectives in Biology. 2012;4(5):a005587. doi: 10.1101/cshperspect.a005587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Current Biology. 2007;17(1):R29–R35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Hausen K, Wolburgbuchholz K, Ribi WA. The synaptic organization of visual interneurons in the lobula complex of flies: A light and electron microscopical study using silver-intensified Cobalt-Impregnations. Cell and Tissue Research. 1980;208(3):371–387. doi: 10.1007/BF00233871. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Grunert U, Wassle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27(1):85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Heimberg AM, Cowper-Sallari R, Semon M, Donoghue PCJ, Peterson KJ. microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(45):19379–19383. doi: 10.1073/pnas.1010350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henselmans JML, Wouterlood FG. Light and electron-microscopic characterization of cholinergic and dopaminergic structures in the striatal complex and the dorsal ventricular ridge of the lizard Gekko-Gecko. Journal of Comparative Neurology. 1994;345(1):69–83. doi: 10.1002/cne.903450105. [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: A review. Neuroscience. 2000;98(3):407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsaki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nature Neuroscience. 2002;5(8):790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dendritic spines: Structure, dynamics and regulation. Nature Reviews Neuroscience. 2001;2(12):880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Hernandez-Nicaise ML. The nervous system of ctenophores. III. Ultrastructure of synapses. Journal of Neurocytology. 1973;2(3):249–263. doi: 10.1007/BF01104029. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, et al. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. Journal of Neuroscience. 1995;15(7 Pt 2):5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. The ultrastructure of the basilar papilla of the chick. Journal of Comparative Neurology. 1978;181(2):361–374. doi: 10.1002/cne.901810208. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. Neurotransmitter corelease: Mechanism and physiological role. Annual Review of Physiology. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg K. Hagfish retina: Fine structure of retinal cells in Myxine-Glutinosa, L, with special reference to receptor and epithelial cells. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie. 1970;111(4):519–538. doi: 10.1007/BF00330929. [DOI] [PubMed] [Google Scholar]
- Holmberg K. Hagfish retina: Electron microscopic study comparing receptor and epithelial cells in Pacific Hagfish, Polistotrema-Stouti, with those in Atlantic Hagfish, Myxine-Glutinosa. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie. 1971;121(2):249–269. doi: 10.1007/BF00340676. [DOI] [PubMed] [Google Scholar]
- Holmberg K, Ohman P. Fine-structure of retinal synaptic organelles in lamprey and hagfish photoreceptors. Vision Research. 1976;16(3):237–239. doi: 10.1016/0042-6989(76)90105-x. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Thurm U. Mono- and oligo-vesicular synapses and their connectivity in a Cnidarian sensory epithelium (Coryne tubulosa) Journal of Comparative Neurology. 2001;432(4):537–549. doi: 10.1002/cne.1118. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Akhmanova A. Dendritic spine plasticity: New regulatory roles of dynamic microtubules. Neuroscientist. 2010;16(6):650–661. doi: 10.1177/1073858410386357. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Bradke F. Control of neuronal polarity and plasticity–a renaissance for microtubules? Trends in Cell Biology. 2009;19(12):669–676. doi: 10.1016/j.tcb.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Horak M, Petralia RS, Kaniakova M, Sans N. ER to synapse trafficking of NMDA receptors. Frontiers in Cell Neuroscience. 2014;8:394. doi: 10.3389/fncel.2014.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn G, Bradley P, Mccabe BJ. Changes in the structure of synapses associated with learning. Journal of Neuroscience. 1985;5(12):3161–3168. doi: 10.1523/JNEUROSCI.05-12-03161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. Immunocytochemical localization of choline acetyltrans-ferase in rat cerebral cortex: A study of cholinergic neurons and synapses. Journal of Comparative Neurology. 1985;234(1):17–34. doi: 10.1002/cne.902340103. [DOI] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345(6196):1255263. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: The last 25 years. Neuron. 2013;80(3):704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa M. Fine-structure of olfactory-bulb in goldfish Carassius-Auratus. Brain Research. 1976;115(1):53–56. doi: 10.1016/0006-8993(76)90821-0. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Nicoll RA. An Intracellular analysis of dendrodendritic inhibition in the turtle invitro Olfactory-Bulb. Journal of Physiology-London. 1982;326(May):213–234. doi: 10.1113/jphysiol.1982.sp014187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka P, Vlachos A, Schwarzacher SW, Deller T. A role for the spine apparatus in LTP and spatial learning. Behavioural Brain Research. 2008;192(1):12–19. doi: 10.1016/j.bbr.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Johnston D, Amaral DG. Hippocampus. In: Shepherd GM, editor. The synaptic organization of the brain. 5. New York: Oxford University Press; 2004. pp. 455–498. [Google Scholar]
- Jones EG, Powell TP. Morphological variations in the dendritic spines of the neocortex. Journal of Cell Science. 1969;5(2):509–529. doi: 10.1242/jcs.5.2.509. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TP. Electron microscopy of the somatic sensory cortex of the cat. I. Cell types and synaptic organization. Philosophical Transactions of the Royal Society of London Series B, Biological sciences. 1970;257(812):1–11. doi: 10.1098/rstb.1970.0003. [DOI] [PubMed] [Google Scholar]
- Jorgensen EM. Animal evolution: looking for the first nervous system. Current Biology. 2014;24(14):R655–R658. doi: 10.1016/j.cub.2014.06.036. [DOI] [PubMed] [Google Scholar]
- Kaifu K, Akamatsu T, Segawa S. Underwater sound detection by cephalopod statocyst. Fisheries Science. 2008;74(4):781–786. [Google Scholar]
- Kapadia SE, de Lanerolle NC, LaMotte CC. Immunocytochemical and electron microscopic study of serotonin neuronal organization in the dorsal raphe nucleus of the monkey. Neuroscience. 1985;15(3):729–746. doi: 10.1016/0306-4522(85)90075-2. [DOI] [PubMed] [Google Scholar]
- Kasa P, Dobo E, Wolff JR. Cholinergic innervation of the mouse superior cervical ganglion: light- and electron-microscopic immunocytochemistry for choline acetyltransferase. Cell and Tissue Research. 1991;265(1):151–158. doi: 10.1007/BF00318149. [DOI] [PubMed] [Google Scholar]
- Keenan CL, Coss R, Koopowitz H. Cytoarchitecture of primitive brains: Golgi studies in flatworms. Journal of Comparative Neurology. 1981;195(4):697–716. doi: 10.1002/cne.901950412. [DOI] [PubMed] [Google Scholar]
- Kelava I, Rentzsch F, Technau U. Evolution of eumetazoan nervous systems: Insights from cnidarians. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2015;370(1684):20150065. doi: 10.1098/rstb.2015.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DE, Smith SW. Fine structure of the pineal organs of the adult frog, Rana Pipiens. Journal of Cell Biology. 1964;22:653–674. doi: 10.1083/jcb.22.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34(2):265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Koizumi O, Sato N, Goto C. Chemical anatomy of hydra nervous system using antibodies against hydra neuropeptides: A review. Hydrobiologia. 2004;530:41–47. [Google Scholar]
- Kolb H. The organization of the outer plexiform layer in the retina of the cat: Electron microscopic observations. Journal of Neurocytology. 1977;6(2):131–153. doi: 10.1007/BF01261502. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. Journal of Neuroscience. 2007;27(50):13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkotian E, Frotscher M, Segal M. Synaptopodin regulates spine plasticity: Mediation by calcium stores. Journal of Neuroscience. 2014;34(35):11641–11651. doi: 10.1523/JNEUROSCI.0381-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkotian E, Segal M. Morphological constraints on calcium dependent glutamate receptor trafficking into individual dendritic spine. Cell Calcium. 2007;42(1):41–57. doi: 10.1016/j.ceca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Kotsyuba EP, Kotsyuba AE. Ultrastructural characteristics of interneuronal connections of the central nervous system of bivalve molluscs. Journal of Evolutionary Biochemistry and Physiology. 2002;38(3):330–335. [Google Scholar]
- Kramer RH, Davenport CM. Lateral inhibition in the vertebrate retina: The case of the missing neurotransmitter. PLoS Biology. 2015;13(12):e1002322. doi: 10.1371/journal.pbio.1002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Hatada S, Kondo S, Karube F, Kawaguchi Y. Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. Journal of Neuroscience. 2007;27(5):1139–1150. doi: 10.1523/JNEUROSCI.3846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacalli TC. The dorsal compartment locomotory control system in amphioxus larvae. Journal of Morphology. 2002;252(3):227–237. doi: 10.1002/jmor.1101. [DOI] [PubMed] [Google Scholar]
- Lacalli TC, Kelly SJ. Sensory pathways in amphioxus larvae II. Dorsal tracts and translumenal cells. Acta Zoologica. 2003;84(1):1–13. [Google Scholar]
- Ladepeche L, Dupuis JP, Bouchet D, Doudnikoff E, Yang L, Campagne Y, et al. Single-molecule imaging of the functional crosstalk between surface NMDA and dopamine D1 receptors. Proc Natl Acad Sci U S A. 2013;110(44):18005–18010. doi: 10.1073/pnas.1310145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KO, Ip NY. Structural plasticity of dendritic spines: The underlying mechanisms and its dysregulation in brain disorders. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2013;1832(12):2257–2263. doi: 10.1016/j.bbadis.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Collin SP, Pugh EN. Evolution of the vertebrate eye: Opsins, photoreceptors, retina and eye cup. Nature Reviews Neuroscience. 2007;8(12):960–975. doi: 10.1038/nrn2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN, Jr, Collin SP. The origin of the vertebrate eye. Evolution Eduation and Outreach. 2008;1:415–426. [Google Scholar]
- Lannoo MJ, Hawkes R. A search for primitive Purkinje cells: Zebrin II expression in sea lampreys (Petromyzon marinus) Neuroscience Letters. 1997;237(1):53–55. doi: 10.1016/s0304-3940(97)00802-1. [DOI] [PubMed] [Google Scholar]
- Laughlin SB. Neural integration in first optic neuropil of dragonflies. 1. signal amplification in Dark-Adapted second-order Neurons. Journal of Comparative Physiology. 1973;84(4):335–355. [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: Containing the detonation–feedforward inhibition in the CA3 hippocampus. Trends in Neurosciences. 2003;26(11):631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Hess M, Wanner G, Melzer RR. Dissecting a neuron network: FIB-SEM-based 3D-reconstruction of the visual neuropils in the sea spider Achelia langi (Dohrn, 1881) (Pycnogonida) BMC Biology. 2014;12:59. doi: 10.1186/s12915-014-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiss F, Groh C, Butcher NJ, Meinertzhagen IA, Tavosanis G. Synaptic organization in the adult Drosophila mushroom body calyx. Journal of Comparative Neurology. 2009a;517(6):808–824. doi: 10.1002/cne.22184. [DOI] [PubMed] [Google Scholar]
- Leiss F, Koper E, Hein I, Fouquet W, Lindner J, Sigrist S, Tavosanis G. Characterization of dendritic spines in the Drosophila central nervous system. Dev Neurobiol. 2009b;69(4):221–234. doi: 10.1002/dneu.20699. [DOI] [PubMed] [Google Scholar]
- Lenn NJ, Leranth C, Zaborszky L. Choline acetyltransferase immunoreactivity is localized to four types of synapses in the rat interpeduncular nucleus. Journal of Neurocytology. 1985;14(6):909–919. doi: 10.1007/BF01224804. [DOI] [PubMed] [Google Scholar]
- Leys SP. Elements of a ‘nervous system’ in sponges. Journal of Experimental Biology. 2015;218(Pt 4):581–591. doi: 10.1242/jeb.110817. [DOI] [PubMed] [Google Scholar]
- Li Y, Hough CJ, Frederickson CJ, Sarvey JM. Induction of mossy fiber →Ca3 long-term potentiation requires translocation of synaptically released Zn2+ Journal of Neuroscience. 2001;21(20):8015–8025. doi: 10.1523/JNEUROSCI.21-20-08015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichnerova K, Kaniakova M, Park SP, Skrenkova K, Wang YX, Petralia RS, et al. Two N-glycosylation sites in the GluN1 subunit are essential for releasing N-methyl-d-aspartate (NMDA) receptors from the Endoplasmic reticulum. Journal of Biological Chemistry. 2015;290(30):18379–18390. doi: 10.1074/jbc.M115.656546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman AR. Microtubule-associated smooth endoplasmic reticulum in the frog’s brain. Z Zellforsch Mikrosk Anat. 1971;116(4):564–577. doi: 10.1007/BF00335058. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Walton KD, Lang EJ. Cerebellum. In: Shepherd GM, editor. The synaptic organization of the brain. 5. New York: Oxford University Press; 2004. pp. 271–309. [Google Scholar]
- Lopez-Garcia C, Martinez-Guijarro FJ. Neurons in the medial cortex give rise to Timm-positive boutons in the cerebral cortex of lizards. Brain Research. 1988;463(2):205–217. doi: 10.1016/0006-8993(88)90393-9. [DOI] [PubMed] [Google Scholar]
- Mackie GO, Lawn ID, Dececcatty MP. Studies on Hexactinellid Sponges. 2. Excitability, conduction and coordination of responses in Rhabdocalyptus-Dawsoni (Lambe, 1873) Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1983;301(1107):401–418. [Google Scholar]
- Maggio N, Vlachos A. Synaptic plasticity at the interface of health and disease: New insights on the role of endoplasmic reticulum intracellular calcium stores. Neuroscience. 2014;281C:135–146. doi: 10.1016/j.neuroscience.2014.09.041. [DOI] [PubMed] [Google Scholar]
- Mäntylä K, Reuter M, Halton DW, Maule AG, Brennan GP, Shaw C, Gustafsson MKS. The nervous system of Procerodes littoralis (Maricola, Tricladida). An ultrastructural and immunoelectron microscopical study. Acta Zoologica. 1998;79(1):1–8. [Google Scholar]
- Marlow HQ, Srivastava M, Matus DQ, Rokhsar D, Martindale MQ. Anatomy and development of the nervous system of Nematostella vectensis, an Anthozoan Cnidarian. Developmental Neurobiology. 2009;69(4):235–254. doi: 10.1002/dneu.20698. [DOI] [PubMed] [Google Scholar]
- Martin VJ. Photoreceptors of cnidarians. Canadian Journal of Zoology. 2002;80(10):1703–1722. [Google Scholar]
- Martin VJ. Photoreceptors of cubozoan jellyfish. Hydrobiologia. 2004;530:135–144. [Google Scholar]
- Martinez Guijarro FJ, Berbel PJ, Molowny A, Lopez Garcia C. Apical dendritic spines and axonic terminals in the bipyramidal neurons of the dorsomedial cortex of lizards (Lacerta) Anatomy and Embryology (Berl) 1984;170(3):321–326. doi: 10.1007/BF00318737. [DOI] [PubMed] [Google Scholar]
- Mashanov VS, Zueva OR, Heinzeller T. Regeneration of the radial nerve cord in a holothurian: A promising new model system for studying post-traumatic recovery in the adult nervous system. Tissue and Cell. 2008;40(5):351–372. doi: 10.1016/j.tice.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Mashanov VS, Zueva OR, Heinzeller T, Dolmatov IY. Ultrastructure of the circumoral nerve ring and the radial nerve cords in holothurians (Echinodermata) Zoomorphology. 2006;125(1):27–38. [Google Scholar]
- Meek J, Nieuwenhuys R. Palisade pattern of mormyrid Purkinje cells: a correlated light and electron microscopic study. Journal of Comparative Neurology. 1991;306(1):156–192. doi: 10.1002/cne.903060111. [DOI] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102(3):527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, O’Neil SD. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. Journal of Comparative Neurology. 1991;305(2):232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- Miglietta MP, Della Tommasa L, Denitto F, Gravili C, Pagliara P, Bouillon J, Boero F. Approaches to the ethology of hydroids and medusae (Cnidaria, Hydrozoa) Scientia Marina. 2000;64:63–71. [Google Scholar]
- Mikami Y, Yoshida T, Matsuda N, Mishina M. Expression of zebrafish glutamate receptor delta2 in neurons with cerebellum-like wiring. Biochem Biophys Res Commun. 2004;322(1):168–176. doi: 10.1016/j.bbrc.2004.07.095. [DOI] [PubMed] [Google Scholar]
- Milhaud M, Pappas GD. Postsynaptic bodies in Habenula and interpeduncular Nuclei of cat. Journal of Cell Biology. 1966;30(2):437–441. doi: 10.1083/jcb.30.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min MY, Rusakov DA, Kullmann DM. Activation of AMPA, kainate, and metabotropic receptors at hippocampal mossy fiber synapses: Role of glutamate diffusion. Neuron. 1998;21(3):561–570. doi: 10.1016/s0896-6273(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Nakamura Y. An electron microscope study of the locus coeruleus in the rabbit, with special reference to direct hypothalamic and mesencephalic projections. Archives of Histology Japan. 1972;34(5):433–448. doi: 10.1679/aohc1950.34.433. [DOI] [PubMed] [Google Scholar]
- Moraczewski J, Czubaj A, Bakowska J. Organization and ultrastructure of nervous-system in Catenulida (Turbellaria) Zoomorphologie. 1977;87(1):87–95. [Google Scholar]
- Morita M, Best JB. Electron microscopic studies of planaria. 3. Some observations on fine structure of planarian nervous tissue. Journal of Experimental Zoology. 1966;161(3):391–412. doi: 10.1002/jez.1401610307. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Kocot KM, Citarella MR, Dosung S, Norekian TP, Povolotskaya IS, et al. The ctenophore genome and the evolutionary origins of neural systems. Nature. 2014;510(7503):109–114. doi: 10.1038/nature13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz LL, Kohn AB. Independent origins of neurons and synapses: Insights from ctenophores. Philosophical Transactions of the Royal Society of London Series B, Biological sciences. 2016;371(1685):20150041. doi: 10.1098/rstb.2015.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshkov DA, Shtanchaev RS, Mikheeva IB, Bezgina EN, Kokanova NA, Mikhailova GZ, et al. Visual input controls the functional activity of goldfish Mauthner neuron through the reciprocal synaptic mechanism. Journal of Integrative Neuroscience. 2013;12(1):17–34. doi: 10.1142/S0219635213500039. [DOI] [PubMed] [Google Scholar]
- Mugnaini E. The histology and cytology of the cerebellar cortex. In: Larsell O, Jansen J, editors. The comparative anatomy and histology of the cerebellum. The human cerebellum, cerebellar connections, and cerebellar cortex. Minneapolis: The University of Minnesota Press; 1972. pp. 201–264. + 267 plates. [Google Scholar]
- Muller JF, Mascagni F, Zaric V, Mott DD, McDonald AJ. Localization of the M2 muscarinic cholinergic receptor in dendrites, cholinergic terminals, and noncholinergic terminals in the rat basolateral amygdala: An ultrastructural analysis. Journal of Comparative Neurology. 2016 doi: 10.1002/cne.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller KJ, McMahan UJ. The shapes of sensory and motor neurones and the distribution of their synapses in ganglia of the leech: a study using intracellular injection of horseradish peroxidase. Proceedings of the Royal Society of London Series B: Biological Sciences. 1976;194(1117):481–499. doi: 10.1098/rspb.1976.0090. [DOI] [PubMed] [Google Scholar]
- Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W. Synaptopodin: An actin-associated protein in telencephalic dendrites and renal podocytes. Journal of Cell Biology. 1997;139(1):193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M, Zimmer J, Raisman G. Quantitative electron-microscopic evidence for Re-innervation in the adult-Rat interpeduncular nucleus after lesions of the fasciculus retroflexus. Journal Of Comparative Neurology. 1979;187(2):447–468. doi: 10.1002/cne.901870211. [DOI] [PubMed] [Google Scholar]
- Nag TC, Wadhwa S. Ultrastructure of the human retina in aging and various pathological states. Micron. 2012;43(7):759–781. doi: 10.1016/j.micron.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Nakamura PA, Cramer KS. Formation and maturation of the calyx of Held. Hearing Research. 2011;276(1–2):70–78. doi: 10.1016/j.heares.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nässel DR. Types and arrangements of neurons in crayfish optic lamina. Cell and Tissue Research. 1977;179(1):45–75. doi: 10.1007/BF00278462. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Strausfeld NJ. A pair of descending neurons with dendrites in the optic lobes projecting directly to thoracic ganglia of dipterous insects. Cell and Tissue Research. 1982;226(2):355–362. doi: 10.1007/BF00218365. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Waterman TH. Golgi em evidence for visual formation channeling in crayfish lamina ganglionaris. Brain Research. 1977;130(3):556–563. doi: 10.1016/0006-8993(77)90118-4. [DOI] [PubMed] [Google Scholar]
- Nek N, Schwegler H, Crusio WE, Frotscher M. Are the fine-structural characteristics of mouse hippocampal mossy fiber synapses determined by the density of mossy fiber axons. Neuroscience Letters. 1993;158(1):75–78. doi: 10.1016/0304-3940(93)90616-s. [DOI] [PubMed] [Google Scholar]
- Nelson R, Lutzow AV, Kolb H, Gouras P. Horizontal cells in cat retina with independent dendritic systems. Science. 1975;189(4197):137–139. doi: 10.1126/science.1138370. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Spine microdomains for postsynaptic signaling and plasticity. Trends in Cell Biology. 2009;19(5):218–227. doi: 10.1016/j.tcb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Nickel M. Kinetics and rhythm of body contractions in the sponge Tethya wilhelma (Porifera: Demospongiae) Journal of Experimental Biology. 2004;207(26):4515–4524. doi: 10.1242/jeb.01289. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nature Reviews Neuroscience. 2005;6(11):863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Nilsson DE, Gislen L, Coates MM, Skogh C, Garm A. Advanced optics in a jellyfish eye. Nature. 2005;435(7039):201–205. doi: 10.1038/nature03484. [DOI] [PubMed] [Google Scholar]
- Nitsch C, Rinne U. Large Dense-Core vesicle exocytosis and membrane recycling in the mossy fiber synapses of the rabbit hippocampus during epileptiform seizures. Journal of Neurocytology. 1981;10(2):201–219. doi: 10.1007/BF01257967. [DOI] [PubMed] [Google Scholar]
- Nomokonova LM, Ozirskaya EV. Morphological study of middle and posterior hypothalamic projections to forebrain in the pond turtle. Neuroscience and Behavioral Physiology. 1984;14(4):282–290. doi: 10.1007/BF01149612. [DOI] [PubMed] [Google Scholar]
- Oksche A, von Harnack M. Elektronenmikroskopische Untersuchungen Am Stirnorgan Von Anuren - (Zur Frage Der Lichtrezeptoren) Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie. 1963;59(2):239–288. [PubMed] [Google Scholar]
- Okubo Y, Suzuki J, Kanemaru K, Nakamura N, Shibata T, Iino M. Visualization of Ca2+ filling mechanisms upon synaptic inputs in the endoplasmic reticulum of cerebellar Purkinje cells. Journal of Neuroscience. 2015;35(48):15837–15846. doi: 10.1523/JNEUROSCI.3487-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Brinkmann M, Sieger T, Thurm U. Hydrozoan nematocytes send and receive synaptic signals induced by mechanochemical stimuli. Journal of Experimental Biology. 2008;211(17):2876–2888. doi: 10.1242/jeb.018515. [DOI] [PubMed] [Google Scholar]
- Orth CB, Schultz C, Muller CM, Frotscher M, Deller T. Loss of the cisternal organelle in the axon initial segment of cortical neurons in synaptopodin-deficient mice. Journal of Comparative Neurology. 2007;504(5):441–449. doi: 10.1002/cne.21445. [DOI] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar cortex. cytology and organization. New York: Springer; 1974. [Google Scholar]
- Palay SL, Sotelo C, Peters A, Orkand PM. Axon hillock and initial segment. Journal of Cell Biology. 1968;38(1):193–201. doi: 10.1083/jcb.38.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nature Reviews Neuroscience. 2013;14(6):383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Papadopoulos GC, Parnavelas JG, Buijs RM. Light and electron microscopic immunocytochemical analysis of the noradrenaline innervation of the rat visual cortex. Journal of Neurocytology. 1989;18(1):1–10. doi: 10.1007/BF01188418. [DOI] [PubMed] [Google Scholar]
- Pardos F, Roldan C, Benito J, Emig CC. Fine-Structure of the tentacles of Phoronis-Australis Haswell (Phoronida, Lophophorata) Acta Zoologica. 1991;72(2):81–90. [Google Scholar]
- Parkefelt L, Skogh C, Nilsson DE, Ekstrom P. Bilateral symmetric organization of neural elements in the visual system of a coelenterate, Tripedalia cystophora (Cubozoa) Journal of Comparative Neurology. 2005;492(3):251–262. doi: 10.1002/cne.20658. [DOI] [PubMed] [Google Scholar]
- Pasch E, Muenz TS, Rossler W. CaMKII is differentially localized in synaptic regions of Kenyon cells within the mushroom bodies of the honeybee brain. Journal of Comparative Neurology. 2011;519(18):3700–3712. doi: 10.1002/cne.22683. [DOI] [PubMed] [Google Scholar]
- Pasik T, Pasik P. Serotoninergic afferents in the monkey neostriatum. Acta biologica Academiae Scientiarum Hungaricae. 1982;33(2–3):277–288. [PubMed] [Google Scholar]
- Pavans de Ceccatty M. Ultrastructures et rapports des cellules mesenchymateuses de type nerveux de l’eponge Tethya lyncurium Link. Annals Science Nature Zoology. 1966;8:577–614. [Google Scholar]
- Peña-Contreras Z, Mendoza-Briceno RV, Miranda-Contreras L, Palacios-Pru EL. Synaptic dimorphism in Ony-chophoran cephalic ganglia. Revista de Biologia Tropical. 2007;55(1):261–267. doi: 10.15517/rbt.v55i1.6078. [DOI] [PubMed] [Google Scholar]
- Peters BH, Campbell AC. Morphology of the nervous and muscular systems in the heads of pedicellariae from the Sea-Urchin Echinus-Esculentus L. Journal of Morphology. 1987;193(1):35–51. doi: 10.1002/jmor.1051930105. [DOI] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. American Journal of Anatomy. 1970;127(4):321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster Hd. The fine structure of the nervous system. Neurons and their supporting cells. 3. New York: Oxford; 1991. [Google Scholar]
- Petralia RS. Distribution of extrasynaptic NMDA receptors on neurons. Scientific World Journal. 2012 doi: 10.1100/2012/267120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Mattson MP, Yao PJ. Communication breakdown: The impact of ageing on synapse structure. Ageing Research and Reviews. 2014;14:31–42. doi: 10.1016/j.arr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Hua F, Yi Z, Zhou A, Ge L, et al. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167(1):68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Mattson MP, Yao PJ. Sonic hedgehog distribution within mature hippocampal neurons. Communicative and Integrative Biology. 2011;4(6):775–777. doi: 10.4161/cib.17832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Mattson MP, Yao PJ. Structure, distribution, and function of neuronal/synaptic spinules and related invaginating projections. Neuromolecular Medicine. 2015;17(3):211–240. doi: 10.1007/s12017-015-8358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Sans N, Worley PF, Hammer JA, 3rd, Wenthold RJ. Glutamate receptor targeting in the postsynaptic spine involves mechanisms that are independent of myosin Va. European Journal of Neuroscience. 2001;13(9):1722–1732. doi: 10.1046/j.0953-816x.2001.01553.x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Wenthold RJ. NMDA receptors and PSD-95 are found in attachment plaques in cerebellar granular layer glomeruli. European Journal of Neuroscience. 2002;15(3):583–587. doi: 10.1046/j.1460-9568.2002.01896.x. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Zhao HM, Wenthold RJ. Ionotropic and metabotropic glutamate receptors show unique postsynaptic, presynaptic, and glial localizations in the dorsal cochlear nucleus. Journal of Comparative Neurology. 1996;372(3):356–383. doi: 10.1002/(SICI)1096-9861(19960826)372:3<356::AID-CNE3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of ampa-selective glutamate receptors in the rat-brain. Journal of Comparative Neurology. 1992;318(3):329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Glutamate receptor antibodies. Production and Immunocytochemistry. In: Ariano MA, editor. Receptor localization: Laboratory methods and procedures. New York: Wiley; 1998. pp. 46–74. [Google Scholar]
- Petralia RS, Wenthold RJ. Immunocytochemistry of NMDA receptors. Methods in Molecular Biology. 1999;128:73–92. doi: 10.1385/1-59259-683-5:73. [DOI] [PubMed] [Google Scholar]
- Pisani D, Pett W, Dohrmann M, Feuda R, Rota-Stabelli O, Philippe H, et al. Genomic data do not support comb jellies as the sister group to all other animals. Proceedings of National Academy of Science USA. 2015;112(50):15402–15407. doi: 10.1073/pnas.1518127112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Powell TPS. An electron-microscopic study of termination of afferent fibres to olfactory bulb from cerebral hemisphere. Journal of Cell Science. 1970a;7(1):157–187. doi: 10.1242/jcs.7.1.157. [DOI] [PubMed] [Google Scholar]
- Price JL, Powell TPS. Synaptology of granule cells of olfactory bulb. Journal of Cell Science. 1970b;7(1):125–155. doi: 10.1242/jcs.7.1.125. [DOI] [PubMed] [Google Scholar]
- Prince FP, Jones-Witters PH. The ultrstructure of the medical preoptic area of the rat. Cell and Tissue Research. 1974;153(4):517–530. doi: 10.1007/BF00231544. [DOI] [PubMed] [Google Scholar]
- Purves D, McMahan UJ. The distribution of synapses on a physiologically identified motor neuron in the central nervous system of the leech. An electron microscope study after the injection of the fluorescent dye procion yellow. Journal of Cell Biology. 1972;55(1):205–220. doi: 10.1083/jcb.55.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ. Microdomains in forebrain spines: An ultrastructural perspective. Molecular Neurobiology. 2013;47(1):77–89. doi: 10.1007/s12035-012-8345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raikova OI, Reuter M, Jondelius U, Gustafsson MKS. An immunocytochemical and ultrastructural study of the nervous and muscular systems of Xenoturbella westbladi (Bilateria inc. sed.) Zoomorphology. 2000;120(2):107–118. [Google Scholar]
- Rall W. Dendritic spines, synaptic potency and neuronal plasticity. In: Woody CD, Brown KA, Crow TJ, Knispel JD, editors. Cellular mechanisms subserving changes in neuronal activity. Los Angeles, CA: Brain Information Service; 1974. pp. 13–21. [Google Scholar]
- Rall W, Rinzel J. Dendritic spine function and synaptic attenuation calculations. Society for Neuroscience Abstracts. 1971;1:64. [Google Scholar]
- Rall W, Rinzel J. Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. Biophysical Journal. 1973;13(7):648–688. doi: 10.1016/S0006-3495(73)86014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W, Shepherd GM. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. Journal of Neurophysiology. 1968;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- Rall W, Shepherd GM, Reese TS, Brightma MW. Dendrodendritic synaptic pathway for inhibition in olfactory bulb. Experimental Neurology. 1966;14(1):44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Rehkämper G, Welsch U. On the fine-structure of the cerebral ganglion of sagitta (Chaetognatha) Zoomorphology. 1985;105(2):83–89. [Google Scholar]
- Reuter M. The nervous-system of microstomum-lineare (Turbellaria, Macrostomida).2. The ultrastructure of synapses and neurosecretory release sites. Cell and Tissue Research. 1981;218(2):375–387. doi: 10.1007/BF00210351. [DOI] [PubMed] [Google Scholar]
- Reuter M, Palmberg I. Synaptic and nonsynaptic release in Stenostomum-Leucops. A study of the nervous-system and sensory receptors. Early Brain. 1990;5:121–136. [Google Scholar]
- Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3(4):e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollenhagen A, Satzler K, Rodriguez EP, Jonas P, Frotscher M, Lubke JHR. Structural determinants of transmission at large hippocampal mossy fiber Synapses. Journal of Neuroscience. 2007;27(39):10434–10444. doi: 10.1523/JNEUROSCI.1946-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Zhang S, Wang HL, Hoffman AF, Lupica CR, Morales M. Single rodent mesohabenular axons release glutamate and GABA. Nature Neuroscience. 2014;17(11):1543–1551. doi: 10.1038/nn.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. Subsurface cisterns and their relationship to the neuronal plasma membrane. Journal of Cell Biology. 1962;13:405–421. doi: 10.1083/jcb.13.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. Ultrastructure of somatic muscle cells in Ascaris lumbricoides. II. Intermuscular junctions, neuromuscular junctions, and glycogen stores. Journal of Cell Biology. 1965;26(2):579–591. doi: 10.1083/jcb.26.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JF, Chiodin M. Where is my mind? How sponges and placozoans may have lost neural cell types. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2015;370(1684):20150059. doi: 10.1098/rstb.2015.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Montey KL, Wright AL, Bennett ML, Pongstaporn T. Postnatal development of a large auditory nerve terminal: the endbulb of held in cats. Hearing Research. 2006;216–217:100–115. doi: 10.1016/j.heares.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sala C, Segal M. Dendritic spines: The locus of structural and functional plasticity. Physiological Reviews. 2014;94(1):141–188. doi: 10.1152/physrev.00012.2013. [DOI] [PubMed] [Google Scholar]
- Sätzler K, Söhl LF, Bollmann JH, Borst JGG, Frotscher M, Sakmann B, Lübke JHR. Three-dimensional reconstruction of a calyx of held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. Journal of Neuroscience. 2002;22(24):10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer SF, Raviola E. Ultrastructural analysis of functional changes in the synaptic endings of turtle cone cells. Cold Spring Harbor Symposia on Quantitative Biology. 1976;40:521–528. doi: 10.1101/sqb.1976.040.01.048. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Marrone DF, Markus EJ. Disambiguating the similar: the dentate gyrus and pattern separation. Behavioural Brain Research. 2012;226(1):56–65. doi: 10.1016/j.bbr.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Schürmann FW. Note on structure of synapses in ventral nerve cord of onychophoran Peripatoides-Leuckarti. Cell and Tissue Research. 1978;186(3):527–534. doi: 10.1007/BF00224940. [DOI] [PubMed] [Google Scholar]
- Scott EK, Reuter JE, Luo L. Small GTPase Cdc42 is required for multiple aspects of dendritic morphogenesis. Journal of Neuroscience. 2003;23(8):3118–3123. doi: 10.1523/JNEUROSCI.23-08-03118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Korkotian E. Endoplasmic reticulum calcium stores in dendritic spines. Front Neuroanat. 2014;8:64. doi: 10.3389/fnana.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Vlachos A, Korkotian E. The spine apparatus, synaptopodin, and dendritic spine plasticity. Neuroscientist. 2010;16(2):125–131. doi: 10.1177/1073858409355829. [DOI] [PubMed] [Google Scholar]
- Segev I, Rall W. Computational study of an excitable dendritic spine. Journal of Neurophysiology. 1988;60(2):499–523. doi: 10.1152/jn.1988.60.2.499. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Watkins KC, Descarries L. Ultrastructural relationships of serotonin axon terminals in the cerebral-cortex of the adult-rat. Journal of Comparative Neurology. 1989;289(1):129–142. doi: 10.1002/cne.902890111. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Watkins KC, Geffard M, Descarries L. Noradrenaline axon terminals in adult-rat neocortex: An immunocytochemical analysis in serial thin-sections. Neuroscience. 1990;35(2):249–264. doi: 10.1016/0306-4522(90)90079-j. [DOI] [PubMed] [Google Scholar]
- Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. Journal of Neuroscience. 1993;13(7):3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SR. Extracellular-space and blood-eye barrier in an insect retina: Ultrastructural-study. Cell and Tissue Research. 1978;188(1):35–61. doi: 10.1007/BF00220513. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. The dendritic spine: A multifunctional integrative unit. Journal of Neurophysiology. 1996;75(6):2197–2210. doi: 10.1152/jn.1996.75.6.2197. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. The synaptic organization of the brain. 5. New York: Oxford University Press; 2004. [Google Scholar]
- Shepherd GM, Chen WR, Willhite D, Migliore M, Greer CA. The olfactory granule cell: From classical enigma to central role in olfactory processing. Brain Research Reviews. 2007;55(2):373–382. doi: 10.1016/j.brainresrev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Singla CL. Fine structure of the neuromuscular system of Polyorchis penicillatus (Hydromedusae, Cnidaria) Cell and Tissue Research. 1978;193(1):163–174. doi: 10.1007/BF00221609. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bennett BD, Bolam JP, Parent A, Sadikot AF. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. Journal of Comparative Neurology. 1994;344(1):1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- Smith CL, Pivovarova N, Reese TS. Coordinated feeding behavior in trichoplax, an animal without synapses. PLoS One. 2015;10(9):e0136098. doi: 10.1371/journal.pone.0136098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Varoqueaux F, Kittelmann M, Azzam RN, Cooper B, Winters CA, et al. Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens. Current Biology. 2014;24(14):1565–1572. doi: 10.1016/j.cub.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, Slapnick SM, August BK. Reciprocal synapses between inner hair cell spines and afferent dendrites in the organ of corti of the mouse. Synapse. 2003;50(1):53–66. doi: 10.1002/syn.10241. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Descarries L, Watkins KC. Serotonin innervation in adult-rat neostriatum. 2. Ultrastructural features: A autoradiographic and immunocytochemical study. Brain Research. 1989;481(1):67–86. doi: 10.1016/0006-8993(89)90486-1. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10(5):501–511. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Spacek J. ‘Free’ postsynaptic-like densities in normal adult brain: their occurrence, distribution, structure and association with subsurface cisterns. Journal of Neurocytology. 1982;11(5):693–706. doi: 10.1007/BF01153514. [DOI] [PubMed] [Google Scholar]
- Spacek J. Three-dimensional analysis of dendritic spines. II. Spine apparatus and other cytoplasmic components. Anatomy and Embryology (Berl) 1985;171(2):235–243. doi: 10.1007/BF00341418. [DOI] [PubMed] [Google Scholar]
- Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. Journal of Neuroscience. 1997;17(1):190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J, Lieberman AR. Ultrastructure and three-dimensional organization of synaptic glomeruli in rat somatosensory thalamus. Journal of Anatomy. 1974;117(Pt 3):487–516. [PMC free article] [PubMed] [Google Scholar]
- Stefanelli A, Caravita S. Ultrastructural features of the synaptic complex of the vestibular nuclei of Lampetra planeri (Bloch) Z Zellforsch Mikrosk Anat. 1970;108(2):282–296. doi: 10.1007/BF00335299. [DOI] [PubMed] [Google Scholar]
- Stein IS, Gray JA, Zito K. Non-ionotropic NMDA receptor signaling drives activity-induced dendritic spine shrinkage. Journal of Neuroscience. 2015;35(35):12303–12308. doi: 10.1523/JNEUROSCI.4289-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Demb JB. Retina. In: Shepherd GM, editor. The synaptic organization of the brain. 5. New York: Oxford University Press; 2004. pp. 217–269. [Google Scholar]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends in Neurosciences. 2005;28(1):20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Steward O, Reeves TM. Protein-synthetic machinery beneath postsynaptic sites on cns neurons: Association between polyribosomes and other organelles at the synaptic site. Journal of Neuroscience. 1988;8(1):176–184. doi: 10.1523/JNEUROSCI.08-01-00176.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, et al. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: A three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131(1):43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Stieb SM, Muenz TS, Wehner R, Rossler W. Visual experience and age affect synaptic organization in the mushroom bodies of the desert ant Cataglyphis fortis. Developmental Neurobiology. 2010;70(6):408–423. doi: 10.1002/dneu.20785. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Barth FG. 2 Visual systems in one brain: Neuropils serving the secondary eyes of the spider Cupiennius-Salei. Journal of Comparative Neurology. 1993;328(1):43–62. doi: 10.1002/cne.903280104. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Strausfeld CM, Stowe S, Rowell D, Loesel R. The organization and evolutionary implications of neuropils and their neurons in the brain of the onychophoran Euperipatoides rowelli. Arthropod Structure & Development. 2006;35(3):169–196. doi: 10.1016/j.asd.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Weltzien P, Barth FG. 2 visual systems in one brain: Neuropils serving the principal eyes of the spider Cupiennius-Salei. Journal of Comparative Neurology. 1993;328(1):63–75. doi: 10.1002/cne.903280105. [DOI] [PubMed] [Google Scholar]
- Sullivan RKP, WoldeMussie E, Pow DV. Dendritic and synaptic plasticity of neurons in the human age-related macular degeneration retina. Investigative Ophthalmology & Visual Science. 2007;48(6):2782–2791. doi: 10.1167/iovs.06-1283. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human Temporal-Lobe. Annals of Neurology. 1989;26(3):321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Special somatic spine synapses in ciliary ganglion of Chick. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie. 1967;83(1):70–75. doi: 10.1007/BF00334741. [DOI] [PubMed] [Google Scholar]
- Takasaka T, Smith CA. The structure and innervation of the pigeon’s basilar papilla. Journal of Ultrastructure Research. 1971;35(1):20–65. doi: 10.1016/s0022-5320(71)80141-7. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Smith CA. Structure of the chicken’s inner ear: SEM and TEM study. American Journal of Anatomy. 1978;153(2):251–271. doi: 10.1002/aja.1001530206. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Gallant PE, Brightman MW, Dosemeci A, Reese TS. Structural changes at synapses after delayed perfusion fixation in different regions of the mouse brain. Journal of Comparative Neurology. 2007;501(5):731–740. doi: 10.1002/cne.21276. [DOI] [PubMed] [Google Scholar]
- Tardent P, Schmid V. Ultrastructure of mechanoreceptors of Polyp Coryne-Pintneri (Hydrozoa, Athecata) Experimental Cell Research. 1972;72(1):265–275. doi: 10.1016/0014-4827(72)90589-7. [DOI] [PubMed] [Google Scholar]
- Tatsuoka H, Reese TS. New structural features of synapses in the anteroventral cochlear nucleus prepared by direct freezing and freeze-substitution. Journal of Comparative Neurology. 1989;290(3):343–357. doi: 10.1002/cne.902900304. [DOI] [PubMed] [Google Scholar]
- Toth ML, Melentijevic I, Shah L, Bhatia A, Lu K, Talwar A, Driscoll M. Neurite sprouting and synapse deterioration in the aging caenorhabditis Elegans nervous system. Journal of Neuroscience. 2012;32(26):8778–8790. doi: 10.1523/JNEUROSCI.1494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Tashiro A, Witter MP, Moser EI. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154(4):1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Hnasko TS, Wallen-Mackenzie A, Morales M, Rayport S, Sulzer D. The multilingual nature of dopamine neurons. Progress in Brain Research. 2014;211:141–164. doi: 10.1016/B978-0-444-63425-2.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo-Cenoz O. Some aspects of structural organization of arthropod eye. Cold Spring Harbor Symposia on Quantitative Biology. 1965;30:371–382. doi: 10.1101/sqb.1965.030.01.037. [DOI] [PubMed] [Google Scholar]
- Trujillo-Cenoz O, Melamed J. Fine structure of visual system of Lycosa (Araneae - Lycosidae). 2. Primary visual centers. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie. 1967;76(3):377–388. doi: 10.1007/BF00339295. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: Confining runaway excitation by shaping synaptic transmission. Nature Reviews Neuroscience. 2007;8(12):935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Ueno S, Tsukamoto M, Hirano T, Kikuchi K, Yamada MK, Nishiyama N, et al. Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-D-aspartate receptor activity in hippocampal CA3 circuits. Journal of Cell Biology. 2002;158(2):215–220. doi: 10.1083/jcb.200204066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbriaco D, Watkins KC, Descarries L, Cozzari C, Hartman BK. Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. Journal of Comparative Neurology. 1994;348(3):351–373. doi: 10.1002/cne.903480304. [DOI] [PubMed] [Google Scholar]
- Vaaga CE, Borisovska M, Westbrook GL. Dual-transmitter neurons: functional implications of co-release and co-transmission. Current Opinion in Neurobiology. 2014;29:25–32. doi: 10.1016/j.conb.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Morigiwa K, Wang TL, Shi YJ, Sterling P. Neurochemistry of the mammalian cone ‘synaptic complex’. Vision Research. 1998;38(10):1359–1369. doi: 10.1016/s0042-6989(98)00007-8. [DOI] [PubMed] [Google Scholar]
- Verney C, Alvarez C, Geffard M, Berger B. Ultrastructural double-labelling study of dopamine terminals and GABA-containing neurons in Rat anteromedial cerebral cortex. European Journal of Neuroscience. 1990;2(11):960–972. doi: 10.1111/j.1460-9568.1990.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Morkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nature Neuroscience. 2006;9(11):1388–1396. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- Vigh B, Manzano MJ, Zadori A, Frank CL, Lukats A, Rohlich P, David C. Nonvisual photoreceptors of the deep brain, pineal organs and retina. Histology and Histopathology. 2002;17(2):555–590. doi: 10.14670/HH-17.555. [DOI] [PubMed] [Google Scholar]
- Vlachos A, Korkotian E, Schonfeld E, Copanaki E, Deller T, Segal M. Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons. Journal of Neuroscience. 2009;29(4):1017–1033. doi: 10.1523/JNEUROSCI.5528-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt KE, Nicoll RA. Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proceedings of National Academy of Science USA. 1999;96(3):1118–1122. doi: 10.1073/pnas.96.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainer BH, Bolam JP, Freund TF, Henderson Z, Totterdell S, Smith AD. Cholinergic synapses in the rat brain: A correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Research. 1984;308(1):69–76. doi: 10.1016/0006-8993(84)90918-1. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Chun MH, Boycott BB. The rod pathway of the macaque monkey retina: Identification of AII-amacrine cells with antibodies against calretinin. Journal of Comparative Neurology. 1995;361(3):537–551. doi: 10.1002/cne.903610315. [DOI] [PubMed] [Google Scholar]
- Weber W, Grosmann M. Ultrastructure of basiepithelial nerve plexus of sea-urchin. Centrostephanus-Longispinus. Cell and Tissue Research. 1977;175(4):551–562. doi: 10.1007/BF00222418. [DOI] [PubMed] [Google Scholar]
- Weber BHF, Schrewe H, Molday LL, Gehrig A, White KL, Seeliger MW, et al. Inactivation of the murine X-linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):6222–6227. doi: 10.1073/pnas.092528599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellis DP, Kauer JS. Gabaergic and glutamatergic synaptic input to identified granule cells in salamander olfactory-bulb. Journal of Physiology-London. 1994;475(3):419–430. doi: 10.1113/jphysiol.1994.sp020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J, Parsons RL, Besso JA, Boldosse WG. Fine-structure of nerve cord of Myxicola-Infundibulum (Annelida, Polychaeta) Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie. 1972;131(2):141–148. doi: 10.1007/BF00306923. [DOI] [PubMed] [Google Scholar]
- Westfall JA. Ultrastructure of synapses in the first-evolved nervous systems. Journal of Neurocytology. 1996;25(12):735–746. doi: 10.1007/BF02284838. [DOI] [PubMed] [Google Scholar]
- Westfall JA. Neural pathways and innervation of cnidocytes in tentacles of sea anemones. Hydrobiologia. 2004;530:117–121. [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. Structure of ventral nerve cord of Caenorhabditis-Elegans. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1976;275(938):327–348. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous-system of the nematode Caenorhabditis-Elegans. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1986;314(1165):1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Morest DK. The growth of cochlear fibers and the formation of their synaptic endings in the avian inner ear: A study with the electron microscope. Neuroscience. 1985;14(1):277–300. doi: 10.1016/0306-4522(85)90178-2. [DOI] [PubMed] [Google Scholar]
- Wiera G, Mozrzymas JW. Extracellular proteolysis in structural and functional plasticity of mossy fiber synapses in hippocampus. Frontiers in Cellular Science. 2015 doi: 10.3389/fncel.2015.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Murakami F, Katsumaru H, Tsukahara N. Dendritic and somatic appendages of identified rubrospinal neurons of the cat. Neuroscience. 1987;22(1):113–130. doi: 10.1016/0306-4522(87)90202-8. [DOI] [PubMed] [Google Scholar]
- Wolff G, Harzsch S, Hansson BS, Brown S, Strausfeld N. Neuronal organization of the hemiellipsoid body of the land hermit crab, Coenobita clypeatus: Correspondence with the mushroom body ground pattern. Journal of Comparative Neurology. 2012;520(13):2824–2846. doi: 10.1002/cne.23059. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Maeda T, Tokunaga Y, Mano T. Fine structure of crest synapses in the locus coeruleus of the Japanese macaque (Macaca fuscata), with special reference to noradrenergic neurons. Kaibogaku Zasshi. 1997;72(3):199–208. [PubMed] [Google Scholar]
- Yamasu T, Yoshida M. Fine-structure of complex ocelli of a cubomedusan. Tamoya-Bursaria Haeckel. Cell and Tissue Research. 1976;170(3):325–339. doi: 10.1007/BF00219415. [DOI] [PubMed] [Google Scholar]
- Yao WD, Spealman RD, Zhang J. Dopaminergic signaling in dendritic spines. Biochemical Pharmacology. 2008;75(11):2055–2069. doi: 10.1016/j.bcp.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA, Schurmann FW. Synaptic connections of cholinergic antennal lobe relay neurons innervating the lateral horn neuropile in the brain of Drosophila melanogaster. Journal of Comparative Neurology. 2003;466(3):299–315. doi: 10.1002/cne.10867. [DOI] [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: Light and electron microscopy. Neuroscience. 1995;65(3):709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- Zhang NH, Houser CR. Ultrastructural localization of dynorphin in the dentate gyrus in human temporal lobe epilepsy: A study of reorganized mossy fiber synapses. Journal of Comparative Neurology. 1999;405(4):472–490. [PubMed] [Google Scholar]
- Zhang J, Petralia RS, Wang YX, Diamond JS. High-resolution quantitative immunogold analysis of membrane receptors at retinal ribbon synapses. JoVE. 2016;108(e53547):1–7. doi: 10.3791/53547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Morales M. Dopaminergic and glutamatergic micro-domains in a subset of rodent mesoaccumbens axons. Nature Neuroscience. 2015a;18(3):386–392. doi: 10.1038/nn.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu L, Pchitskaya E, Zakharova O, Saito T, Saido T, Bezprozvanny I. Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of Alzheimer’s disease. Journal of Neuroscience. 2015b;35(39):13275–13286. doi: 10.1523/JNEUROSCI.1034-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Studer D, Chai X, Graber W, Brose N, Nestel S, et al. Structural plasticity of hippocampal mossy fiber synapses as revealed by high-pressure freezing. Journal of Comparative Neurology. 2012;520(11):2340–2351. doi: 10.1002/cne.23040. [DOI] [PubMed] [Google Scholar]
- Zhao HM, Wenthold RJ, Petralia RS. Glutamate receptor targeting to synaptic populations on Purkinje cells is developmentally regulated. Journal of Neuroscience. 1998;18(14):5517–5528. doi: 10.1523/JNEUROSCI.18-14-05517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng CY, Seabold GK, Horak M, Petralia RS. MAGUKs, synaptic development, and synaptic plasticity. Neuroscientist. 2011;17(5):493–512. doi: 10.1177/1073858410386384. [DOI] [PMC free article] [PubMed] [Google Scholar]