Abstract
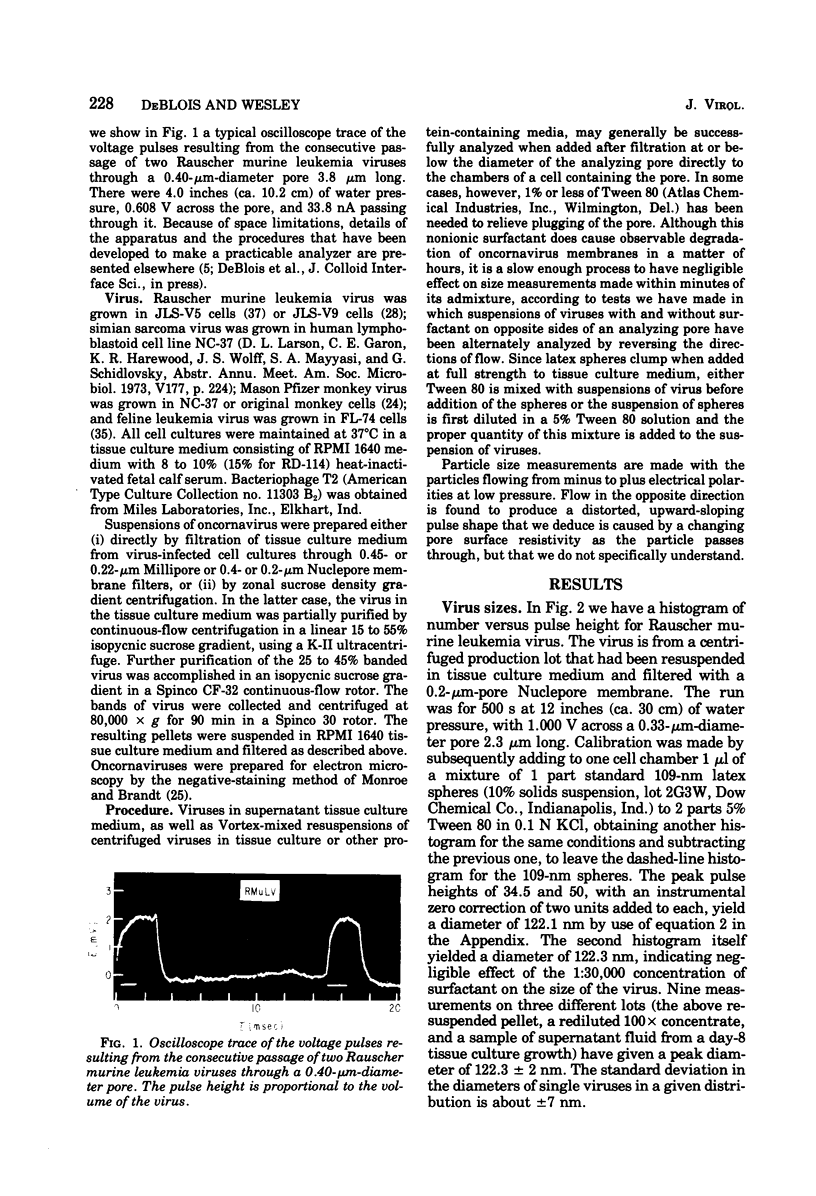
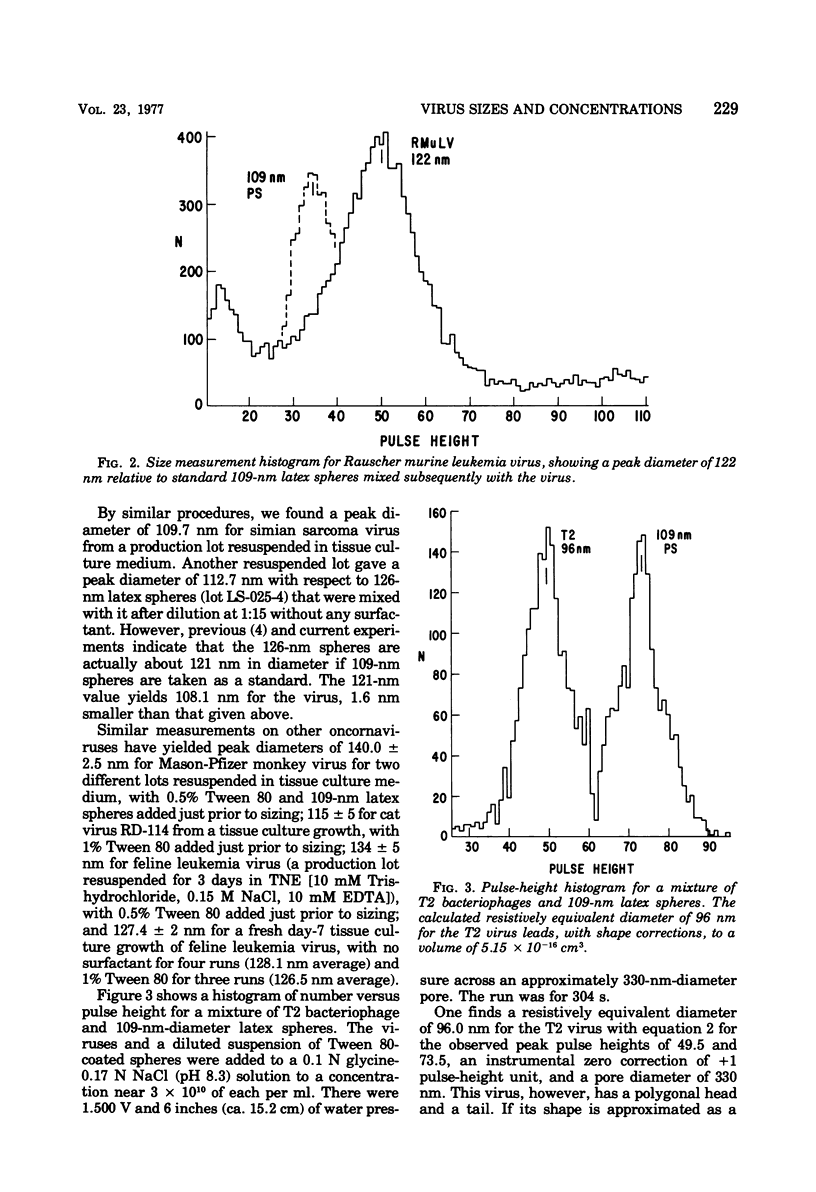
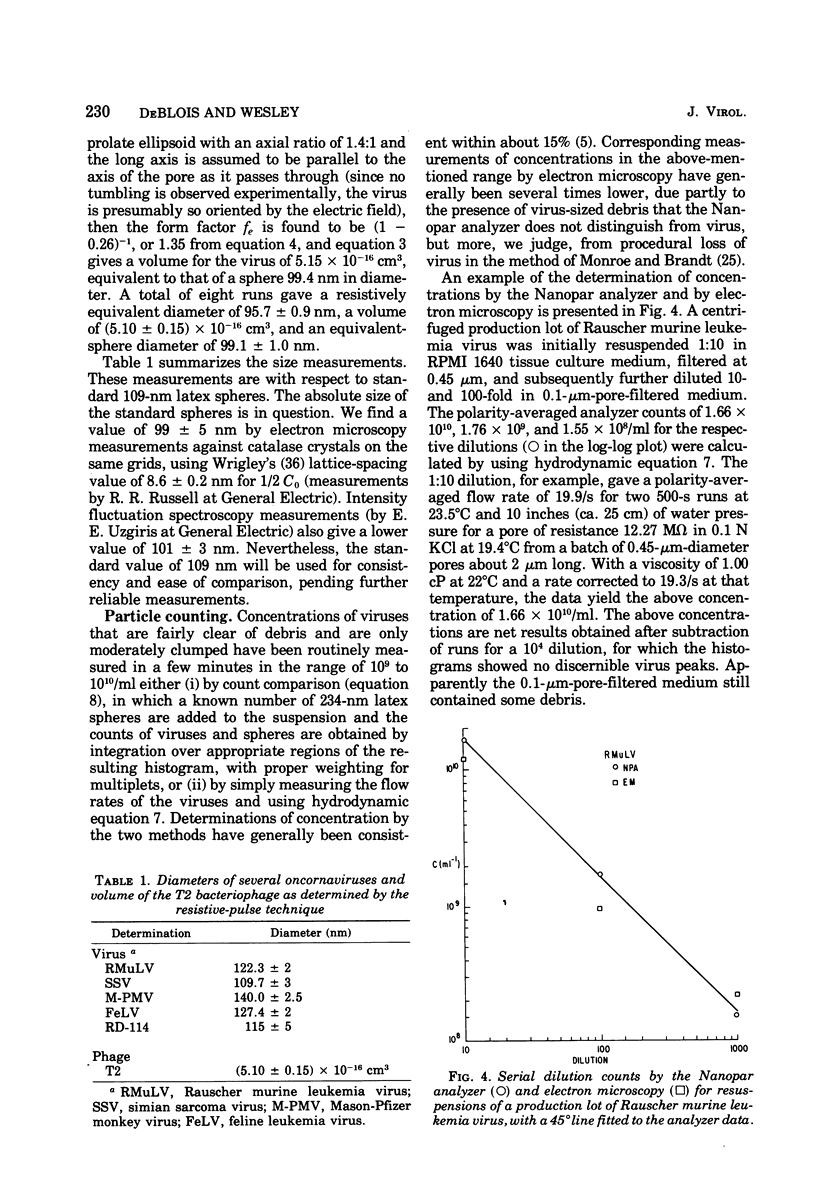
Viruses above about 60 nm in diameter may be rapidly sized to a few nanometers in their natural hydrated state as they pass one by one through a single pore in a newly developed nanometer-particle analyzer based on the resistive-pulse technique of the Coulter Counter and the use of submicron diameter pores made by the Nuclepore process. Size measurements for several type C oncornaviruses are: Rauscher murine leukemia, 122.3 +/- 2 nm; simian sarcoma, 109.7 +/- 3 nm; Mason-Pfizer monkey, 140.0 +/- 2.5 nm; RD-114, 115 +/- 5 nm; and feline leukemia, 127.4 +/- 2 nm, relative to standard 109-nm latex spheres. The T2 bacteriophage has a volume of (5.10 +/- 0.15) X 10(-16) cm3. Concentrations of viruses near 10(9) to 10(11)/ml that are fairly clear of debris are routinely measurable in a few minutes to an accuracy near 15%. A lower practical count limit is near 5 X 10(7) viruses per ml.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CUMMINGS D. J., KOZLOFF L. M. Biophysical properties of bacteriophage T2. Biochim Biophys Acta. 1960 Nov 18;44:445–458. doi: 10.1016/0006-3002(60)91599-7. [DOI] [PubMed] [Google Scholar]
- Carlson F. D. The application of intensity fluctuation spectroscopy to molecular biology. Annu Rev Biophys Bioeng. 1975;4(00):243–264. doi: 10.1146/annurev.bb.04.060175.001331. [DOI] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- De Harven E., Beju D., Evenson D. P., Basu S., Schidlovsky G. Structure of critical point dried oncornaviruses. Virology. 1973 Oct;55(2):535–540. doi: 10.1016/0042-6822(73)90198-0. [DOI] [PubMed] [Google Scholar]
- De-Thé G., O'Connor T. E. Structure of a murine leukemia virus after disruption with tween-ether and comparison with two myxoviruses. Virology. 1966 Apr;28(4):713–728. doi: 10.1016/0042-6822(66)90256-x. [DOI] [PubMed] [Google Scholar]
- Dougherty E., 3rd, Rickard C. G. Ultrastructural studies of feline leukemia virus. J Ultrastruct Res. 1970 Sep;32(5):472–476. doi: 10.1016/s0022-5320(70)80023-5. [DOI] [PubMed] [Google Scholar]
- Feller U., Dougherty R. M., Di Stefano H. S. Comparative morphology of avian and murine leukemia viruses. J Natl Cancer Inst. 1971 Dec;47(6):1289–1298. [PubMed] [Google Scholar]
- Fleischer R. L., Price P. B., Symes E. M. Novel Filter for Biological Materials. Science. 1964 Jan 17;143(3603):249–250. doi: 10.1126/science.143.3603.249. [DOI] [PubMed] [Google Scholar]
- Gordon C. N. Dimensions of the heads of the fast and slow sedimenting forms of bacteriophage T2L. J Mol Biol. 1972 Apr 14;65(3):435–445. doi: 10.1016/0022-2836(72)90200-8. [DOI] [PubMed] [Google Scholar]
- Harewood K. R., Vidrine J. G., Larson D. L., Wolff J. S., 3rd, Schidlovsky G., Mayyasi S. A. Biochemical and morphological studies of Simian Sarcoma Virus, Type 1. Biochim Biophys Acta. 1973 May 10;308(2):252–259. doi: 10.1016/0005-2787(73)90155-x. [DOI] [PubMed] [Google Scholar]
- Harvey J. D. Diffusion coefficients and hydrodynamic radii of three spherical RNA viruses by laser light scattering. Virology. 1973 Nov;56(1):365–368. doi: 10.1016/0042-6822(73)90313-9. [DOI] [PubMed] [Google Scholar]
- Harvey J. D., Farrell J. A., Bellamy A. R. Biophysical studies of reovirus type 3. II. Properties of the hydrated particle. Virology. 1974 Nov;62(1):154–160. doi: 10.1016/0042-6822(74)90311-0. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Laird H. M., Hay D., Crighton G. W. Replication of cat leukemia virus in cell cultures. Nature. 1968 Aug 3;219(5153):521–522. doi: 10.1038/219521a0. [DOI] [PubMed] [Google Scholar]
- Jensen E. M., Zelljadt I., Chopra H. C., Mason M. M. Isolation and propagation of a virus from a spontaneous mammary carcinoma of a rhesus monkey. Cancer Res. 1970 Sep;30(9):2388–2393. [PubMed] [Google Scholar]
- Kawakami T. G., Theilen G. H., Dungworth D. L., Munn R. J., Beall S. G. "C"-type viral particles in plasma of cats with feline leukemia. Science. 1967 Nov;158(3804):1049–1050. doi: 10.1126/science.158.3804.1049. [DOI] [PubMed] [Google Scholar]
- LEVY J. P., BOIRON M., SILVESTRE D., BERNARD J. THE ULTRASTRUCTURE OF RAUSCHER VIRUS. Virology. 1965 May;26:146–150. doi: 10.1016/0042-6822(65)90036-x. [DOI] [PubMed] [Google Scholar]
- Laird H. M., Jarrett O., Crighton G. W., Jarrett W. F. An electron microscopic study of virus particles in spontaneous leukemia in the cat. J Natl Cancer Inst. 1968 Oct;41(4):867–878. [PubMed] [Google Scholar]
- Luftig R. B., Kilham S. S. An electron microscope study of Rauscher leukemia virus. Virology. 1971 Nov;46(2):277–297. doi: 10.1016/0042-6822(71)90030-4. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., McMillan P. N., Culbreth K., Bolognesi D. P. A determination of the outer dimensions of oncornaviruses by several electron microscopic procedures. Cancer Res. 1974 Jul;34(7):1694–1706. [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M., Gardner M. B., Rongey R. W., Rasheed S., Sarma P. S., Huebner R. J., Hatanaka M., Oroszlan S., Gilden R. V. C-type virus released from cultured human rhabdomyosarcoma cells. Nat New Biol. 1972 Jan 5;235(53):3–6. doi: 10.1038/newbio235003a0. [DOI] [PubMed] [Google Scholar]
- Monroe J. H., Brandt P. M. Rapid semiquantitative method for screening large numbers of virus samples by negative staining electron microscopy. Appl Microbiol. 1970 Aug;20(2):259–262. doi: 10.1128/am.20.2.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Salmeen I., Gill D., Rimai L., McCormick J. J., Maher V. M., Arnold W. J., Hight M. E. Application of laser beat frequency spectroscopy to the detection and characterization of an oncogenic RNA virus. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1172–1178. doi: 10.1016/0006-291x(72)90958-8. [DOI] [PubMed] [Google Scholar]
- Salmeen I., Rimai L., Liebes L., Rich M. A., McCormick J. J. Hydrodynamic diameters of RNA tumor viruses. Studies by laser beat frequency light scattering spectroscopy of avian myeloblastosis and Rauscher murine leukemia viruses. Biochemistry. 1975 Jan 14;14(1):134–141. doi: 10.1021/bi00672a023. [DOI] [PubMed] [Google Scholar]
- Salmeen I., Rimai L., Luftig R. B., Libes L., Retzel E., Rich M., McCormick J. J. Hydrodynamic diameters of murine mammary, Rous sarcoma, and feline leukemia RNA tumor viruses: studies by laser beat frequency light-scattering spectroscopy and electron microscopy. J Virol. 1976 Feb;17(2):584–596. doi: 10.1128/jvi.17.2.584-596.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. H., Manthey W. J., Sheffield J. B. The morphology of murine oncornaviruses following different methods of preparation for electron microscopy. Cancer Res. 1975 Mar;35(3):740–749. [PubMed] [Google Scholar]
- Theilen G. H., Dungworth D. L., Kawakami T. G., Munn R. J., Ward J. M., Harrold J. B. Experimental induction of lymphosarcoma in the cat with "C"-type virus. Cancer Res. 1970 Feb;30(2):401–408. [PubMed] [Google Scholar]
- Theilen G. H., Gould D., Fowler M., Dungworth D. L. C-type virus in tumor tissue of a woolly monkey (Lagothrix spp.) with fibrosarcoma. J Natl Cancer Inst. 1971 Oct;47(4):881–889. [PubMed] [Google Scholar]
- Theilen G. H., Kawakami T. G., Rush J. D., Munn R. J. Replication of cat leukemia virus in cell suspension cultures. Nature. 1969 May 10;222(5193):589–590. doi: 10.1038/222589b0. [DOI] [PubMed] [Google Scholar]
- Wright B. S., Lasfargues J. C. Long-term propagation of the Rauscher murine leukemia virus in tissue culture. J Natl Cancer Inst. 1965 Aug;35(2):319–327. [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]
- ZEIGEL R. F., RAUSCHER F. J. ELECTRON MICROSCOPIC AND BIOASSAY STUDIES ON A MURINE LEUKEMIA VIRUS (RAUSCHER). I. EFFECTS OF PHYSICOCHEMICAL TREATMENTS ON THE MORPHOLOGY AND BIOLOGICAL ACTIVITY OF THE VIRUS. J Natl Cancer Inst. 1964 Jun;32:1277–1307. doi: 10.1093/jnci/32.6.1277. [DOI] [PubMed] [Google Scholar]



