Case Report
A 66 year old white female without a history of smoking presented with new onset chest discomfort and dyspnea. Chest imaging demonstrated a large left lower lobe mass, pleural deposits, and a left pleural effusion. Percutaneous biopsy (Figure 1A) and pleural fluid analyses demonstrated adenocarcinoma consistent with a lung primary (Figure 2A, C, E, G, I). The patient received 4 cycles of carboplatinpemetrexed with partial response. After two cycles of maintenance pemetrexed monotherapy, disease progression occurred. Molecular analysis of the original biopsy demonstrated an activating exon 19 EGFR mutation (Figure 3). Erlotinib was initiated, with excellent clinical and radiographic response (Figure 1B, C). Eight months later, the patient experienced disease progression. Repeat biopsy near the initial biopsy site (Figure 1D) demonstrated squamous cell carcinoma (Figure 2B, D, F, H, J) with persistent exon 19 EGFR mutation and no evidence of T790M mutation. The patient's functional status declined, and she developed paraneoplastic leukocytosis (WBC 73 × 103/mm) and hypercalcemia (calcium 12.5 mg/dL). She died shortly thereafter.
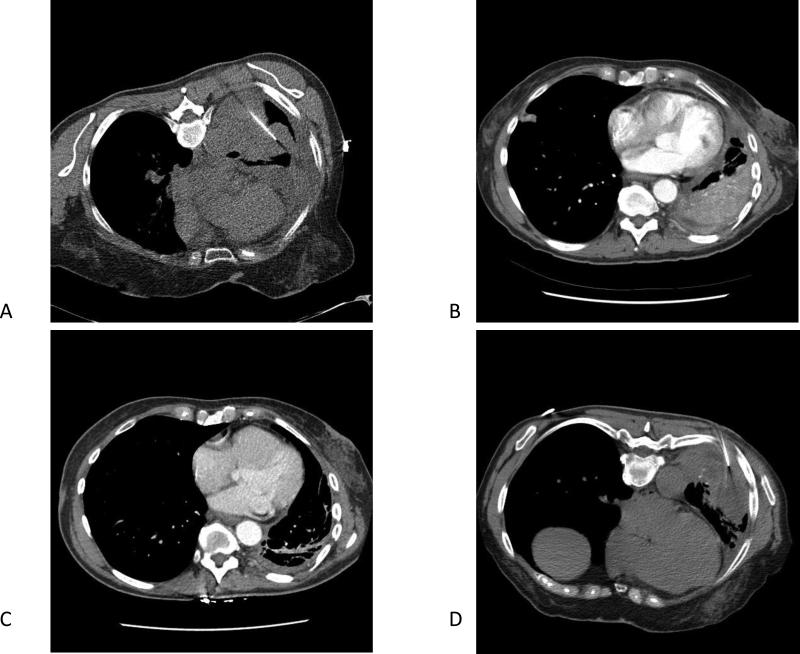
Figure 1.
(A) CT-guided biopsy of the lung lesion at the time of diagnosis. (B) CT chest before erlotinib administration. (C) CT chest three months after erlotinib initiated. (D) CT-guided biopsy of the lung cancer at the time of progression on erlotinib.
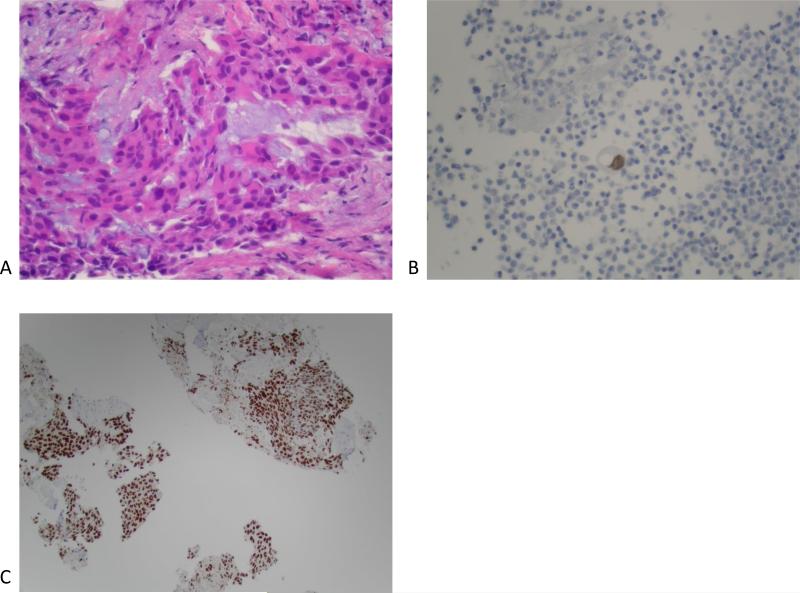
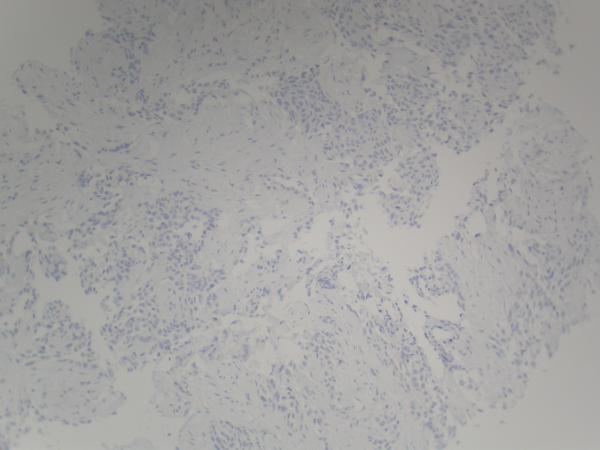
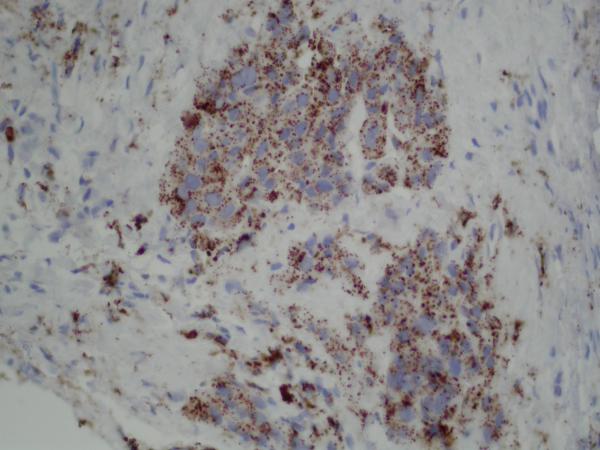
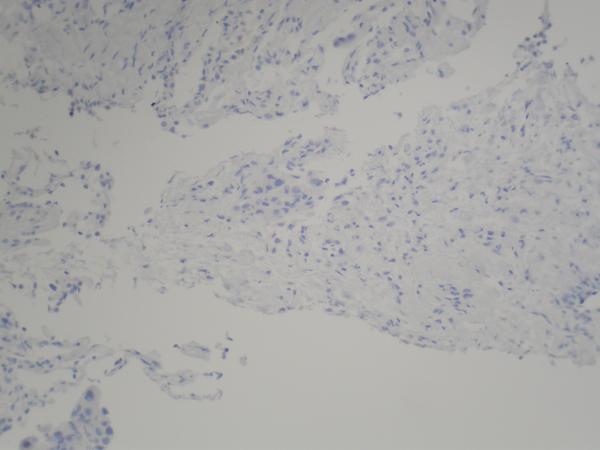
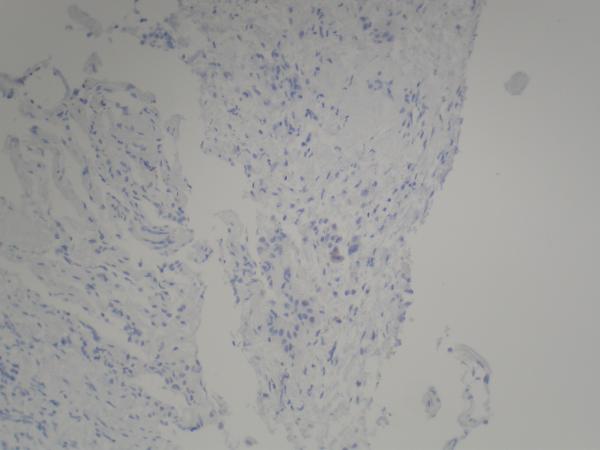
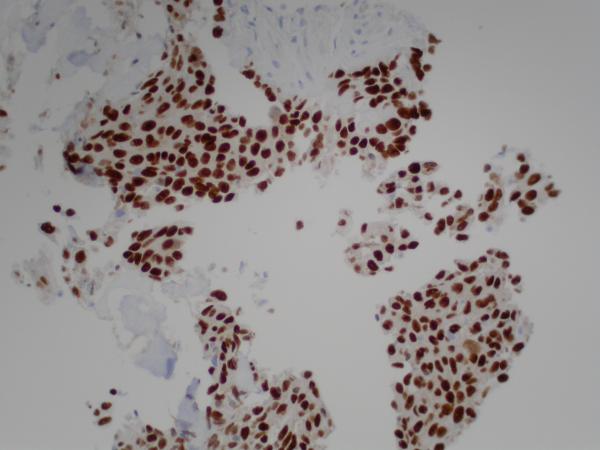
Figure 2.
(A) Pre-erlotinib treatment core needle biopsy of left lower lobe lesion demonstrating epithelial cells with pleomorphic nuclei and a mucoid background (H&E, 400X). (B) Post-erlotinib treatment core needle biopsy showing epithelioid cells with vacuolated cytoplasm, pleomorphic nuclei with occasional prominent nucleoli, and intercellular bridges (H&E, 600X). (C) Positive nuclear staining for TTF-1 in the pre-treatment (400X) and (D) negative in the post-treatment biopsy (200X). (E) Positive cytoplasmic staining for Napsin A in the pre-treatment (400X) and (F) negative in the post-treatment biopsy (200X). (G) Negative staining for CK5/6 in the pre-treatment (200X) and (H) positive stain in the post-treatment biopsy (200X). (I) Negative staining for p63 in the pre-treatment (200X) and (J) positive nuclear staining in the post-treatment core needle biopsy (200X).
Figure 3.
Colored peaks correspond to the exon 19 DNA sequence indicated as sample. The reference normal sequence is below. The 15 nucleotides deleted in the sample are underlined in the normal. The deletion appears homozygous.
Discussion
Despite initial efficacy, therapeutic resistance eventually emerges in essentially all cases of EGFR mutant NSCLC treated with EGFR inhibitors. Described mechanisms include secondary EGFR resistance mutations (ie, T790M) (49%), MET amplification or HGF over-expression (5%), up-regulation of the Axl kinase (20-25%), PI3K pathway hyperactivation via PIK3CA mutation or PTEN loss (5%), epithelial-tomesenchymal transition (40%), and histologic transformation to small cell lung cancer (5-14%).1 These rare cases of small cell transformation preserve the original sensitizing EGFR mutation, suggesting a monoclonal origin with the parent cells.
In this report, we describe the histologic transformation of EGFR mutant lung adenocarcinoma to squamous cell carcinoma as a mechanism of resistance to the EGFR inhibitor erlotinib. To our knowledge, only one similar case has been previously described.2 Potential explanations for this observation include (1) malignant cells acquire a different phenotype under the pressure of EGFR inhibition (metaplastic transformation); (2) both types of cells co-exist in the original tumor mass (ie, adenosquamous histology, representing 5-10% of NSCLC3), but only adenocarcinoma cells are sensitive to EGFR inhibition, giving selective advantage to the squamous cell component; or (3) development of a second primary cancer.
In this case, maintenance of the original EGFR mutation after histologic transformation makes the possibility of a second primary tumor unlikely. Instead, this phenomenon may indicate a population of pluripotent EGFR mutant cancer cells or cancer stem cells as the source of resistance.4 Although there are clear morphologic and immunohistochemical differences between the pre- and post-treatment specimens, the possibility of a mixed tumors cannot be ruled out due to the limited sampling provided by needle biopsies, even when performed in similar anatomic sites. We also acknowledge that the events in this case do not rule out the possibility that the pre-erlotinib chemotherapy may have played a role in selecting the squamous clone or driving alternative differentiation. Finally, our report is limited by the lack of additional tumor genomic analyses (eg, MET, PIK3CA), which have been described in a minority of cases featuring small cell transformation after treatment with EGFR inhibitors.1
Supplementary Material
Footnotes
Funding Support: None
Author Disclosures: None
References
- 1.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scher KS, Saldivar JS, Fishbein M, et al. EGFR-mutated lung cancer with T790M-acquired resistance in the brain and histologic transformation in the lung. J Natl Compr Canc Netw. 2013;11:1040–4. doi: 10.6004/jnccn.2013.0126. [DOI] [PubMed] [Google Scholar]
- 3.Bastide K, Ugolin N, Levalois C, et al. Are adenosquamous lung carcinomas a simple mix of adenocarcinomas and squamous cell carcinomas, or more complex at the molecular level? Lung Cancer. 2010;68:1–9. doi: 10.1016/j.lungcan.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.