Abstract
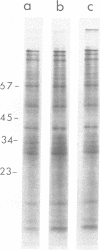
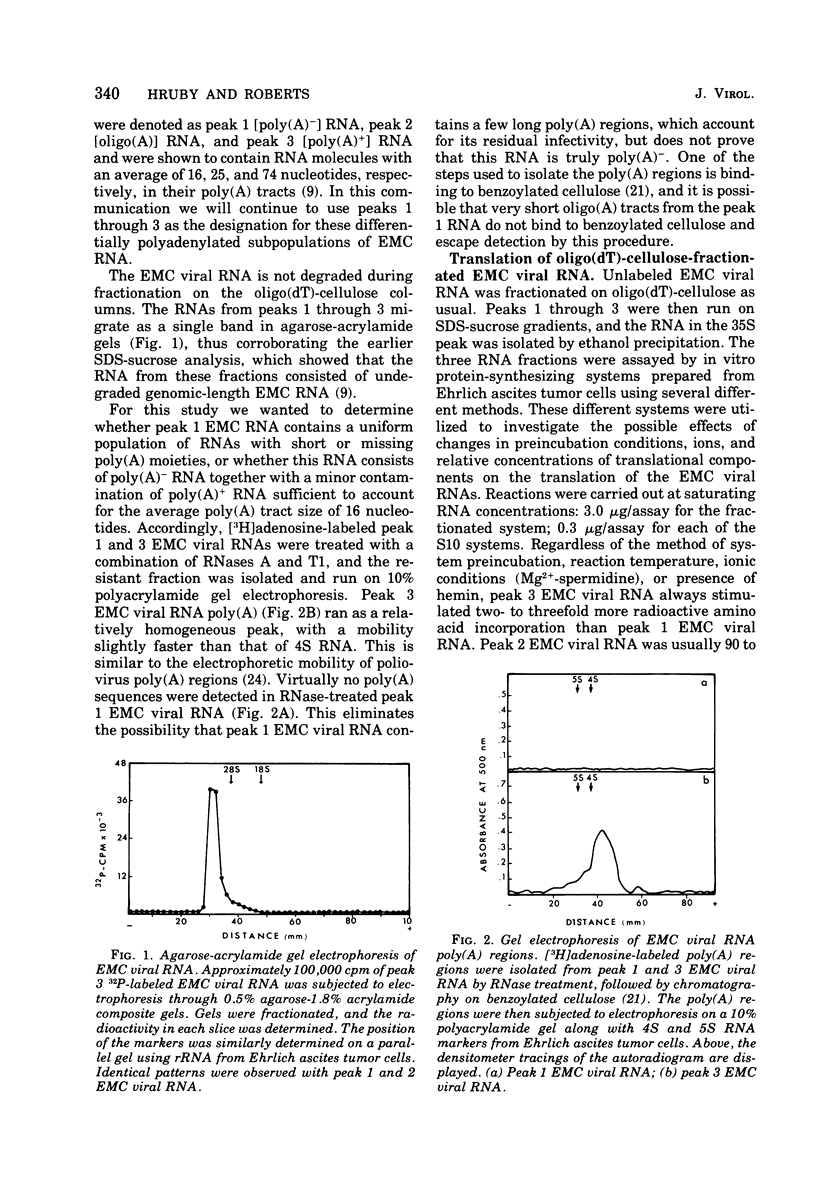
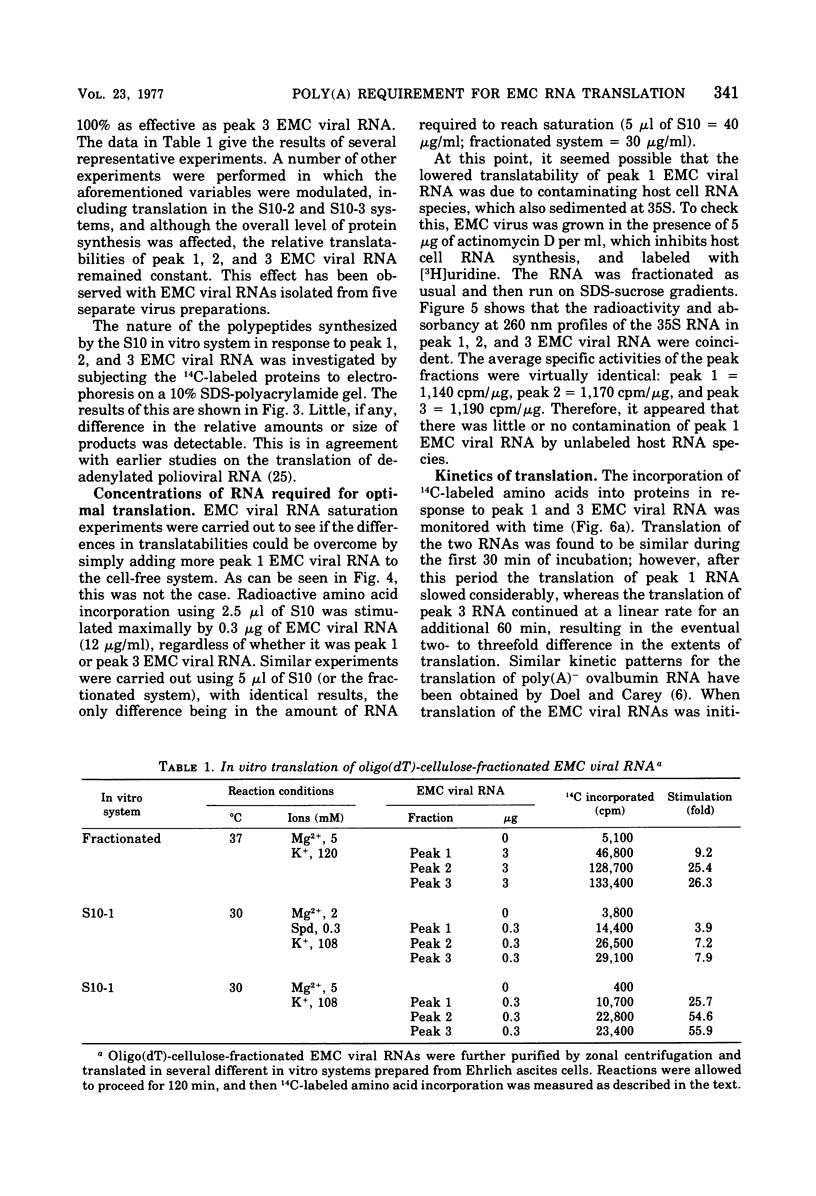
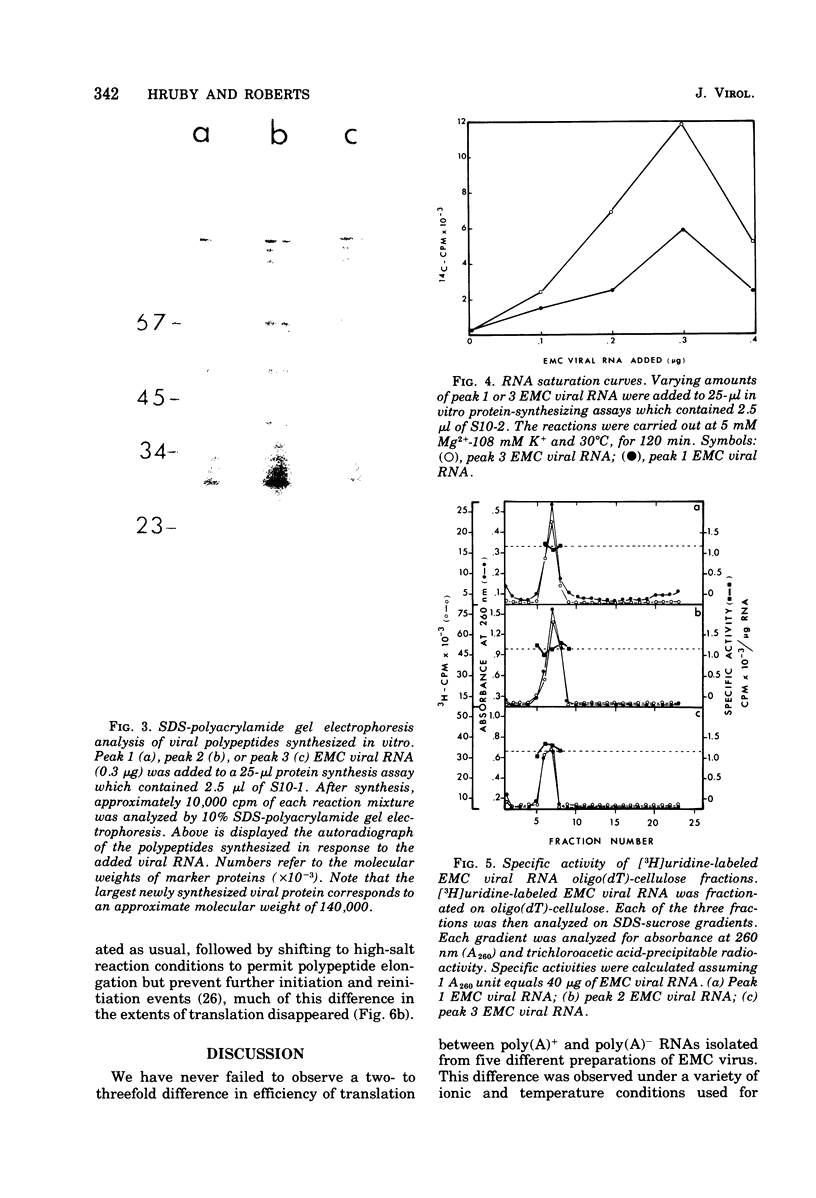
Differentially polyadenylated subpopulatons of encephalomyocarditis (EMC) viral RNA were isolated by affinity chromatography on oligodeoxythymidylic acid-cellulose. Translation of these RNA fractions in several in vitro protein-synthesizing systems, isolated from Ehrlich ascites tumor cells, demonstrated that poly(A)+EMC viral RNA was translated two to three times more efficiently than poly(A)-EMC viral RNA. Sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis of the polypetides synthesized by the in vitro system in response to the different RNAs showed no detectable differences in the size or relative amount- of the translational products. mRNA saturation curves indicated that the in vitro systems were stimulated maximally by equivalent amounts of RNA, wheter it be poly(A)-or poly(A)+ EMC viral RNA. Time course experiments showed that the differences in translatability were more pronounced late in the reaction when reinitiation was required, and that by eliminating reinitiation with high salt the apparent effect of poly(A) on translation was diminished. Together, these results suggest that poly(A) may be required for efficient initiation and reinitiation of protein synthesis in the cell-free systems. This interpretation is discussed relative to earlier data.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Characteristics and significance of the polyadenylate sequence in mammalian messenger RNA. Prog Nucleic Acid Res Mol Biol. 1976;17:117–148. doi: 10.1016/s0079-6603(08)60068-9. [DOI] [PubMed] [Google Scholar]
- Doel M. T., Carey N. H. The translational capacity of deadenylated ovalbumin messenger RNA. Cell. 1976 May;8(1):51–58. doi: 10.1016/0092-8674(76)90184-7. [DOI] [PubMed] [Google Scholar]
- Goldstein N. O., Pardoe I. U., Burness A. T. Requirement of an adenylic acid-rich segment for the infectivity of encephalomyocarditis virus RNA. J Gen Virol. 1976 May;31(2):271–276. doi: 10.1099/0022-1317-31-2-271. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., LeGendre S. M., Levy C. C. Stabilization of an RNA molecule by 3'-terminal poly (A)-induced inhibition of RNase activity. J Biol Chem. 1976 Jun 10;251(11):3287–3293. [PubMed] [Google Scholar]
- Hruby D. E., Roberts W. K. Encephalomyocarditis virus RNA: variations in polyadenylic acid content and biological activity. J Virol. 1976 Aug;19(2):325–330. doi: 10.1128/jvi.19.2.325-330.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Hubert E., Leclercq M., Nudel U., Soreq H., Salomon R., Lebleu B., Revel M., Littauer U. Z. Role of the polyadenylate segment in the translation of globin messenger RNA in Xenopus oocytes. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3143–3146. doi: 10.1073/pnas.71.8.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Seibert G., Steffen R., Zahn R. K. Endoribonuclease IV. 2. Further investigation on the specificity. Eur J Biochem. 1976 Nov 1;70(1):249–258. doi: 10.1111/j.1432-1033.1976.tb10976.x. [DOI] [PubMed] [Google Scholar]
- Nudel U., Soreq H., Littauer U. Z. Globin mRNA species containing poly(A) segments of different lengths. Their functional stability in Xenopus oocytes. Eur J Biochem. 1976 Apr 15;64(1):115–121. doi: 10.1111/j.1432-1033.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K. Studies on RNA synthesis in Ehrlich ascites cells extraction and properties of labeled RNA. Biochim Biophys Acta. 1965 Nov 8;108(3):474–488. doi: 10.1016/0005-2787(65)90039-0. [DOI] [PubMed] [Google Scholar]
- Roberts W. K. Use of benzoylated cellulose columns for the isolation of poly(adenylic acid) containing RNA and other polynucleotides with little secondary structure. Biochemistry. 1974 Aug 27;13(18):3677–3682. doi: 10.1021/bi00715a009. [DOI] [PubMed] [Google Scholar]
- Sharma O. K., Roberts W. K., Beezley D. N., Borek E. A transfer RNA-dependent protein synthesizing system from Ehrlich ascites extracts. Biochim Biophys Acta. 1975 May 16;390(3):327–331. doi: 10.1016/0005-2787(75)90353-6. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Polyadenylic acid on poliovirus RNA. II. poly(A) on intracellular RNAs. J Virol. 1975 Jun;15(6):1418–1431. doi: 10.1128/jvi.15.6.1418-1431.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Requirement of 3'-terminal poly(adenylic acid) for the infectivity of poliovirus RNA. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2983–2987. doi: 10.1073/pnas.71.8.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Villa-Komaroff L., Baltimore D. Studies on the function of polyadenylic acid on poliovirus RNA. Cell. 1975 Sep;6(1):41–44. doi: 10.1016/0092-8674(75)90071-9. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Guttman N., Baltimore D., Lodishi H. F. Complete translation of poliovirus RNA in a eukaryotic cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4157–4161. doi: 10.1073/pnas.72.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Crossley J., Humphries S. Translation of mouse globin messenger ribonucleic acid from which the poly(adenylic acid) sequence has been removed. Biochemistry. 1974 Feb 12;13(4):703–707. doi: 10.1021/bi00701a011. [DOI] [PubMed] [Google Scholar]