Abstract
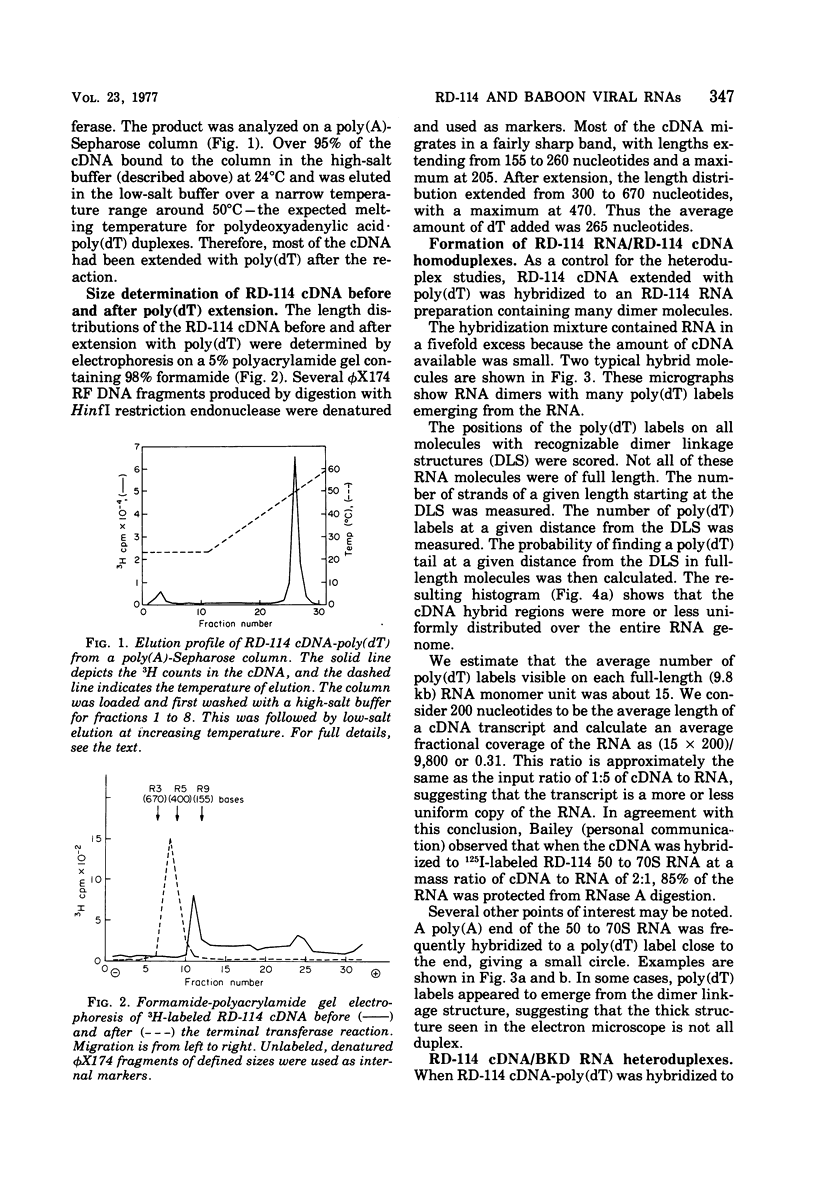
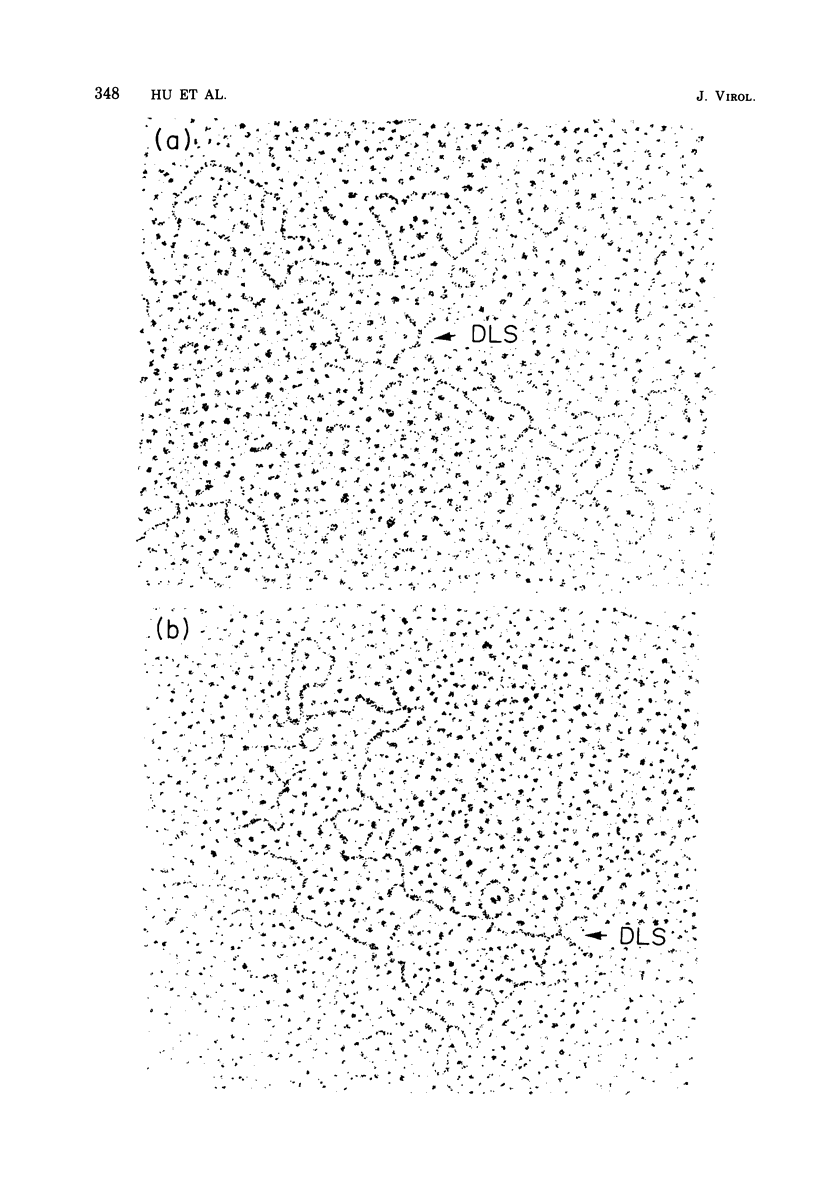
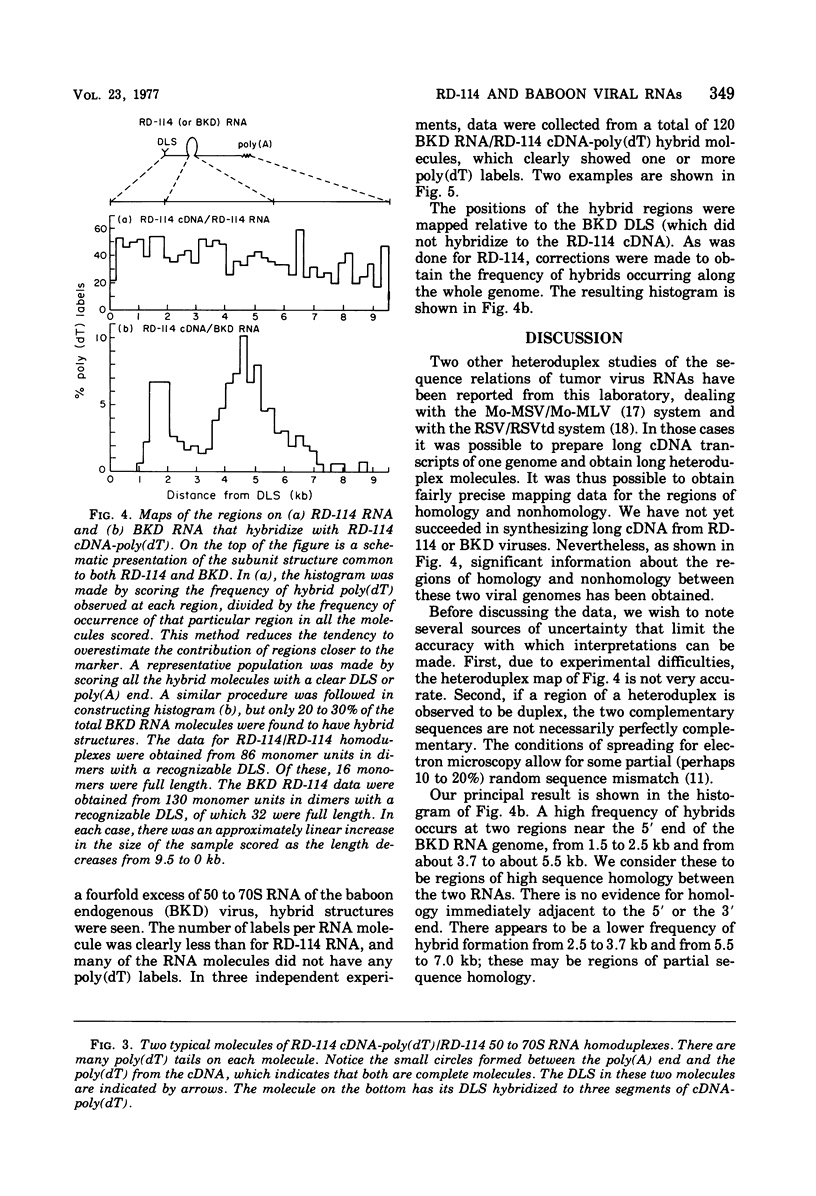
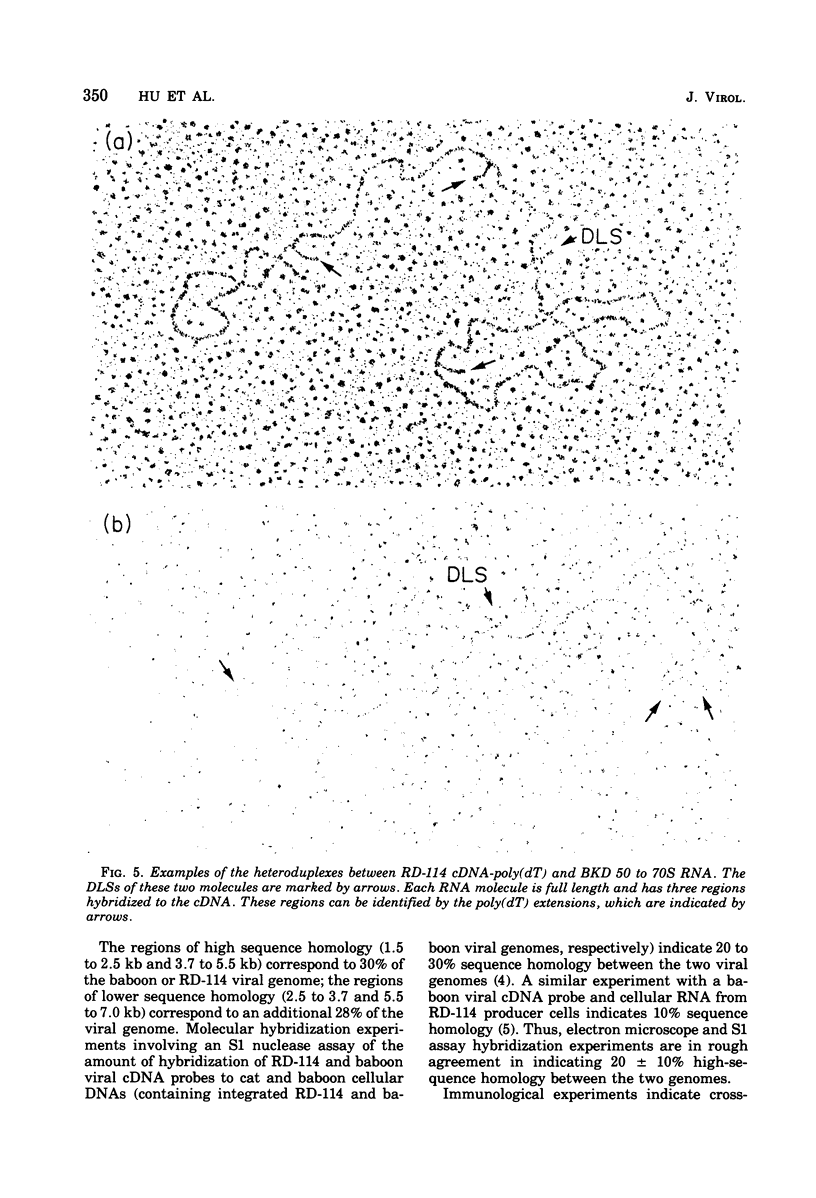
The regions of sequence homology and nonhomology between the RNA genomes of RD-114 and baboon endogenous type C viruses have been mapped by an electron microscope heteroduplex study. Short complementary DNA (cDNA) copies (approximately 150 to 200 nucleotides in length) of RD-114 RNA were prepared by an endogenous synthesis; labels of polydeoxythymidylic acid [poly(dT)] were attached to the 3' ends of the cDNA molecules by a reaction catalyzed by deoxynucleotidyl terminal transferase. The cDNA-poly(dT) was hybridized to RD-114 RNA and to baboon viral RNA dimer (50 to 70S) units, and the position- of the poly(dT) labels were observed by electron microscopy. With RD-114, labels were distributed uniformly along the genome. With baboon virus RNA (monomer length, 9.5 kilobases [kb]), the regions of high homology with RD-114 cDNA were observed to lie in the intervals from 1.5 to 2.5 kb and from 3.7 to 5.5 kb from the 5' end. The relations of these heteroduplex maps to the known antigenic similarities and differences among the several viral proteins and to the genetic maps of the viruses are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluda M. A., Roy-Burman P. Partial characterization of RD114 virus by DNA-RNA hybridization studies. Nat New Biol. 1973 Jul 11;244(132):59–62. doi: 10.1038/newbio244059a0. [DOI] [PubMed] [Google Scholar]
- Barbacid M., Stephenson J. R., Aaronson S. A. gag Gene of mammalian type-C RNA tumour viruses. Nature. 1976 Aug 12;262(5569):554–559. doi: 10.1038/262554a0. [DOI] [PubMed] [Google Scholar]
- Bender W., Davidson N. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell. 1976 Apr;7(4):595–607. doi: 10.1016/0092-8674(76)90210-5. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Livingston D. M., Sherr C. J., Todaro G. J., Kalter S. S. Infectious C-type virus isolated from a baboon placenta. Nature. 1974 Mar 1;248(5443):17–20. doi: 10.1038/248017a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of C-type viral genes: inheritance of exogenously acquired viral genes. Nature. 1974 Dec 6;252(5483):456–459. doi: 10.1038/252456a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: I. Nucleic acid from baboon type C virus as a measure of divergence among primate species. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4513–4518. doi: 10.1073/pnas.71.11.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Multiple divergent copies of endogenous C-type virogenes in mammalian cells. Nature. 1974 Nov 8;252(5479):170–173. doi: 10.1038/252170a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Segregation of RD-114 AND FeL-V-related sequences in crosses between domestic cat and leopard cat. Nature. 1975 Oct 9;257(5526):506–508. doi: 10.1038/257506a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. V., Aronson A. I. An assay for the endonucleolytic clevage of RNA to large oligonucleotides. Anal Biochem. 1973 Jun;53(2):603–612. doi: 10.1016/0003-2697(73)90112-7. [DOI] [PubMed] [Google Scholar]
- Dube S., Kung H. J., Bender W., Davidson N., Ostertag W. Size, subunit composition, and secondary structure of the Friend virus genome. J Virol. 1976 Oct;20(1):264–272. doi: 10.1128/jvi.20.1.264-272.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbert J. E., McAllister R. M., Nicolson M. O., Gilden R. V., Lennette E. H. RD-114 virus infectivity assay by measurements of DNA polymerase activity and virus group specific antigen. Proc Soc Exp Biol Med. 1974 Feb;145(2):366–370. doi: 10.3181/00379727-145-37812. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Peebles P. T., Nomura S., Haapala D. K. Isolation of RD-114-like oncornavirus from a cat cell line. J Virol. 1973 Jun;11(6):978–985. doi: 10.1128/jvi.11.6.978-985.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Gillespie S., Gallo R. C., East J. L., Dmochowski L. Genetic origin of RD114 and other RNA tumour viruses assayed by molecular hybridization. Nat New Biol. 1973 Jul 11;244(132):51–54. doi: 10.1038/newbio244051a0. [DOI] [PubMed] [Google Scholar]
- Hellman A., Peebles P. T., Strickland J. E., Fowler A. K., Kalter S. S., Oroszlan K. S., Gilden R. V. Baboon virus isolate M-7 with properties similar to feline virus RD-114. J Virol. 1974 Jul;14(1):133–138. doi: 10.1128/jvi.14.1.133-138.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Davidson N. A heteroduplex study of the sequence relationships between the RNAs of M-MSV and M-MLV. Cell. 1977 Mar;10(3):469–477. doi: 10.1016/0092-8674(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Junghans R. P., Hu S., Knight C. A., Davidson N. Heteroduplex analysis of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):477–481. doi: 10.1073/pnas.74.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalter S. S., Helmke R. J., Panigel M., Heberling R. L., Felsburg P. J., Axelrod L. R. Observations of apparent C-type particles in baboon (Papio cynocephalus) placentas. Science. 1973 Mar 30;179(4080):1332–1333. doi: 10.1126/science.179.4080.1332. [DOI] [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. Structure, subunit composition, and molecular weight of RD-114 RNA. J Virol. 1975 Aug;16(2):397–411. doi: 10.1128/jvi.16.2.397-411.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Hu S., Bender W., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. RD-114, baboon, and woolly monkey viral RNA's compared in size and structure. Cell. 1976 Apr;7(4):609–620. doi: 10.1016/0092-8674(76)90211-7. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Todaro G. J. Endogenous type C virus from a cat cell clone with properties distinct from previously described feline type C virus. Virology. 1973 May;53(1):142–151. doi: 10.1016/0042-6822(73)90473-x. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of RD114 virus nucleotide sequences in feline cellular DNA. Nat New Biol. 1973 Jul 11;244(132):62–64. doi: 10.1038/newbio244062a0. [DOI] [PubMed] [Google Scholar]
- Pal B. K., McAllister R. M., Gardner M. B., Roy-Burman P. Comparative studies on the structural phosphoproteins of mammalian type C viruses. J Virol. 1975 Jul;16(1):123–131. doi: 10.1128/jvi.16.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ruprecht R. M., Goodman N. C., Spiegelman S. Determination of natural host taxonomy of RNA tumor viruses by molecular hybridization: application to RD-114, a candidate human virus. Proc Natl Acad Sci U S A. 1973 May;70(5):1437–1441. doi: 10.1073/pnas.70.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Tseng J., Lee Y. K., Gilden R. V. Virus similar to RD114 virus in cat cells. Nat New Biol. 1973 Jul 11;244(132):56–59. doi: 10.1038/newbio244056a0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Lieber M. M., Benveniste R. E., Todaro G. J. Endogenous baboon type C virus (M7): biochemical and immunologic characterization. Virology. 1974 Apr;58(2):492–503. doi: 10.1016/0042-6822(74)90083-x. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z., Pinder J. C., Gratzer W. B. Molecular weight determination of nucleic acids by gel electrophoresis in non-aqueous solution. Nat New Biol. 1972 Jan 26;235(56):108–110. doi: 10.1038/newbio235108a0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Reynolds R. K., Aaronson S. A. Comparisons of the immunological properties of two structural polypeptides of type C RNA viruses endogenous to old world monkeys. J Virol. 1976 Feb;17(2):374–384. doi: 10.1128/jvi.17.2.374-384.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E., Lieber M. M., Melnick J. L. Type C viruses of baboons: isolation from normal cell cultures. Cell. 1974 May;2(1):55–61. doi: 10.1016/0092-8674(74)90008-7. [DOI] [PubMed] [Google Scholar]




