Abstract
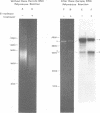
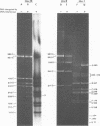
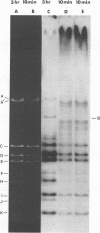
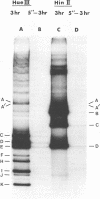
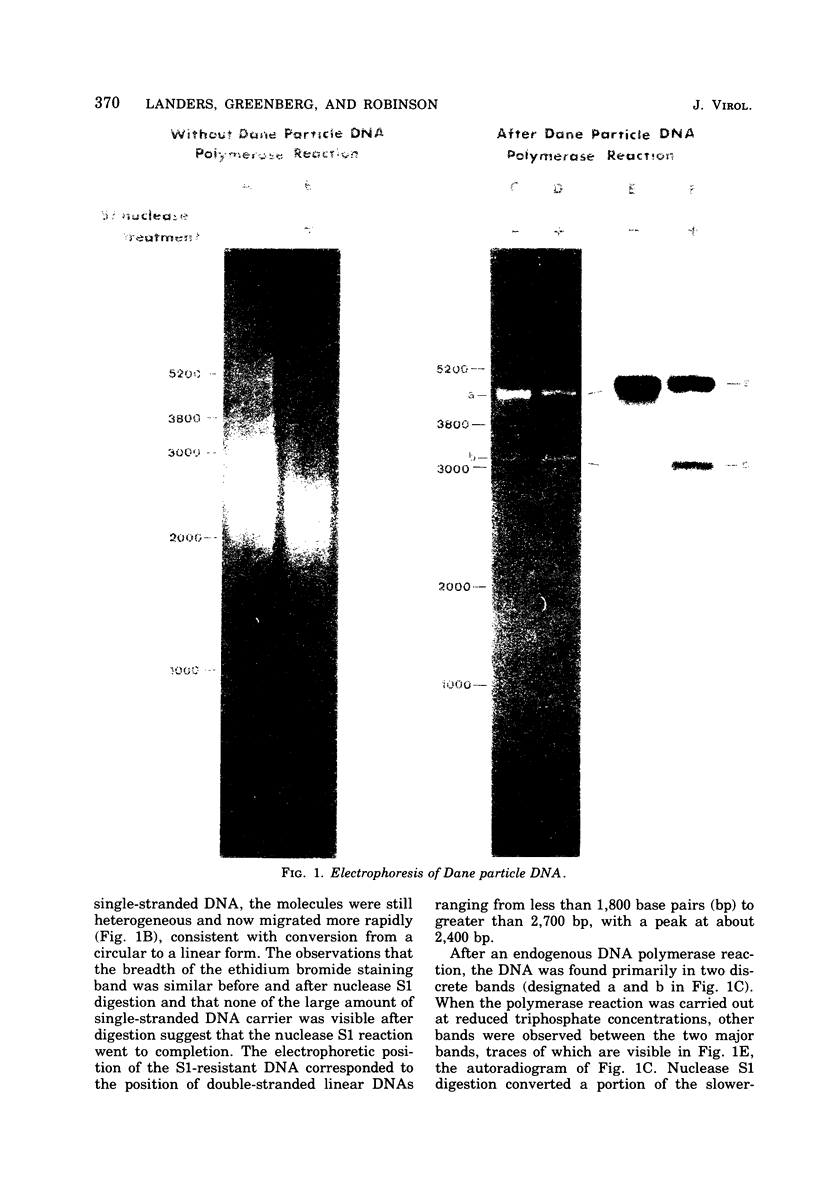
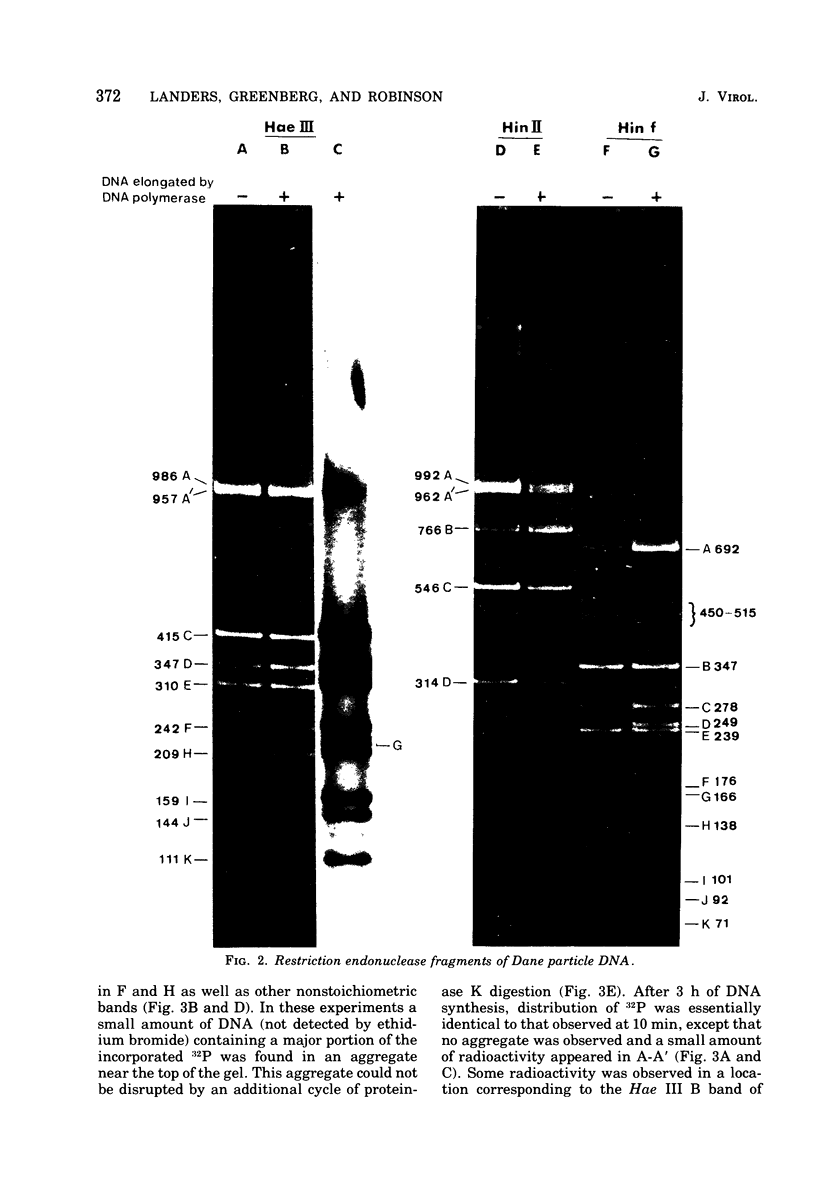
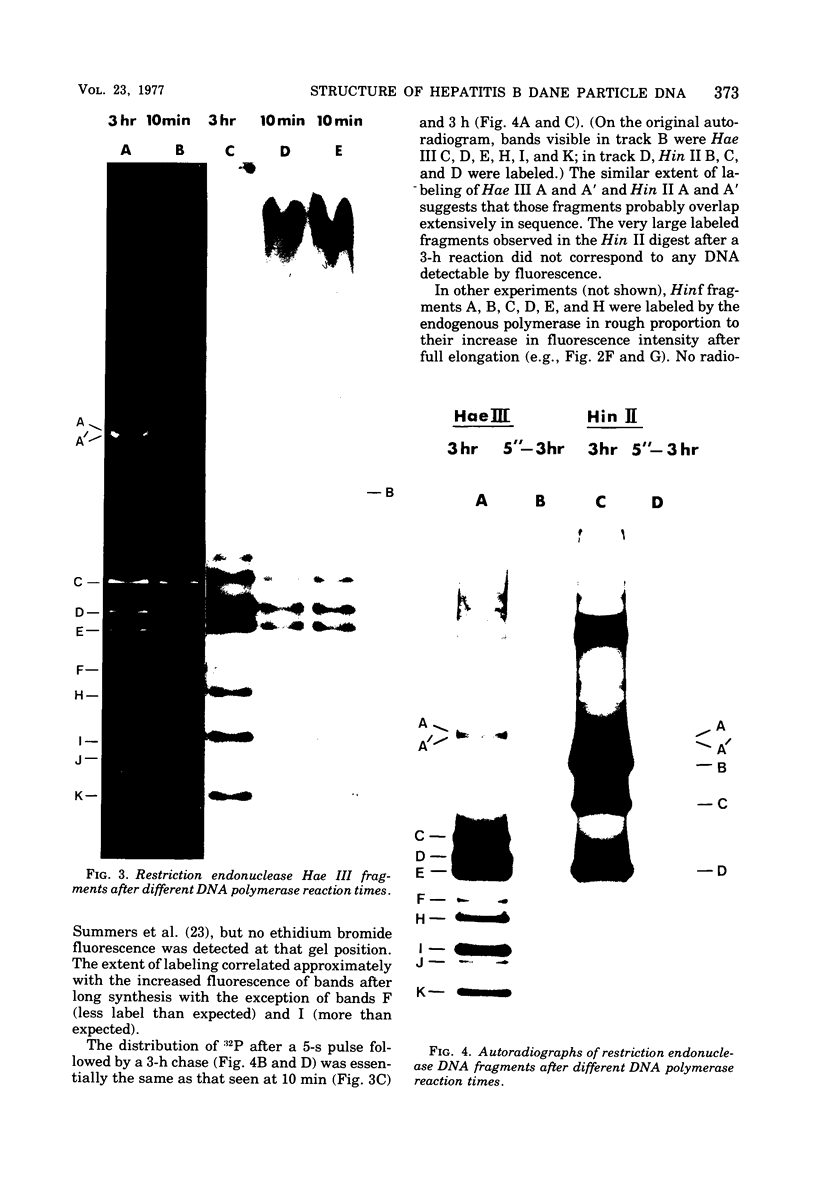
The circular DNA of hepatitis B Dane particles, which serves as the primer/template for an endogenous DNA polymerase, was analyzed by electrophoresis before and after a polymerase reaction and after digestion by restriction endonuclease or single-strand-specific endonuclease S1. The unreacted molecules extracted from the particles were electrophoretically heterogeneous, and treatment with S1 nuclease produced double-stranded linear DNA ranging in length from 1,700 to 2,800 base pairs (bp). After an endogenous DNA polymerase reaction, two discrete species of DNA molecules were found: a circular form and a linear form 3,200 bp long. The reaction resulted in a population of molecules with an elongated and more homogeneous double-stranded region. These results suggest that the circular molecules in Dane particles have single-stranded regions of varying lengths that are made double stranded during the DNA polymerase reaction. The endogenous DNA polymerase was found to initiate apparently at random in a region spanning more than a third of the molecule. Analysis of restriction endonuclease cleavage fragments of the fully elongated DNA revealed that although the molecules were of a uniform length, they were somewhat heterogeneous in sequence. The sum of the sizes of the 10 major endonuclease Hae III-generated fragments, detected by ethidium bromide, was 3,880 bp. Two additional fragments (B and G) detected by autoradiography after an endogenous DNA polymerase reaction with 32P-labeled deoxynucleoside triphosphates made the total 4,910 bp.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D. Individual morphological variations seen in Australia antigen positive sera. Am J Dis Child. 1972 Apr;123(4):303–309. doi: 10.1001/archpedi.1972.02110100035013. [DOI] [PubMed] [Google Scholar]
- Almeida J. D., Rubenstein D., Stott E. J. New antigen-antibody system in Australia-antigen-positive hepatitis. Lancet. 1971 Dec 4;2(7736):1225–1227. doi: 10.1016/s0140-6736(71)90543-5. [DOI] [PubMed] [Google Scholar]
- Alter H. J., Seeff L. B., Kaplan P. M., McAuliffe V. J., Wright E. C., Gerin J. L., Purcell R. H., Holland P. V., Zimmerman H. J. Type B hepatitis: the infectivity of blood positive for e antigen and DNA polymerase after accidental needlestick exposure. N Engl J Med. 1976 Oct 21;295(17):909–913. doi: 10.1056/NEJM197610212951701. [DOI] [PubMed] [Google Scholar]
- Barker L. F., Murray R. Relationship of virus dose to incubation time of clinical hepatitis and time of appearance of hepatitis--associated antigen. Am J Med Sci. 1972 Jan;263(1):27–33. doi: 10.1097/00000441-197201000-00005. [DOI] [PubMed] [Google Scholar]
- Dane D. S., Cameron C. H., Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970 Apr 4;1(7649):695–698. doi: 10.1016/s0140-6736(70)90926-8. [DOI] [PubMed] [Google Scholar]
- Hruska J. F., Clayton D. A., Rubenstein J. L., Robinson W. S. Structure of hepatitis B Dane particle DNA before and after the Dane particle DNA polymerase reaction. J Virol. 1977 Feb;21(2):666–672. doi: 10.1128/jvi.21.2.666-672.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. M., Greenman R. L., Gerin J. L., Purcell R. H., Robinson W. S. DNA polymerase associated with human hepatitis B antigen. J Virol. 1973 Nov;12(5):995–1005. doi: 10.1128/jvi.12.5.995-1005.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugman S., Hoofnagle J. H., Gerety R. J., Kaplan P. M., Gerin J. L. Viral hepatitis, type B, DNA polymerase activity and antibody to hepatitis B core antigen. N Engl J Med. 1974 Jun 13;290(24):1331–1335. doi: 10.1056/NEJM197406132902401. [DOI] [PubMed] [Google Scholar]
- Krugman S. Viral hepatitis type B: prospects for active immunization. Am J Med Sci. 1975 Sep-Oct;270(2):391–393. doi: 10.1097/00000441-197509000-00023. [DOI] [PubMed] [Google Scholar]
- MCCOLLUM R. W. The size of serum hepatitis virus. Proc Soc Exp Biol Med. 1952 Oct;81(1):157–160. doi: 10.3181/00379727-81-19809. [DOI] [PubMed] [Google Scholar]
- Middleton J. H., Edgell M. H., Hutchison C. A., 3rd Specific fragments of phi X174 deoxyribonucleic acid produced by a restriction enzyme from Haemophilus aegyptius, endonuclease Z. J Virol. 1972 Jul;10(1):42–50. doi: 10.1128/jvi.10.1.42-50.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Kamiyama I., Inomata M., Imai M., Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med. 1976 Apr 1;294(14):746–749. doi: 10.1056/NEJM197604012941402. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Clayton D. A., Greenman R. L. DNA of a human hepatitis B virus candidate. J Virol. 1974 Aug;14(2):384–391. doi: 10.1128/jvi.14.2.384-391.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S. DNA and DNA polymerase in the core of the Dane particle of hepatitis B. Am J Med Sci. 1975 Jul-Aug;270(1):151–159. doi: 10.1097/00000441-197507000-00021. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Greenman R. L. DNA polymerase in the core of the human hepatitis B virus candidate. J Virol. 1974 Jun;13(6):1231–1236. doi: 10.1128/jvi.13.6.1231-1236.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Lutwick L. I. The virus of hepatitis, type B (first of two parts). N Engl J Med. 1976 Nov 18;295(21):1168–1175. doi: 10.1056/NEJM197611182952105. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Summers J., O'Connell A., Millman I. Genome of hepatitis B virus: restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4597–4601. doi: 10.1073/pnas.72.11.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]