Abstract
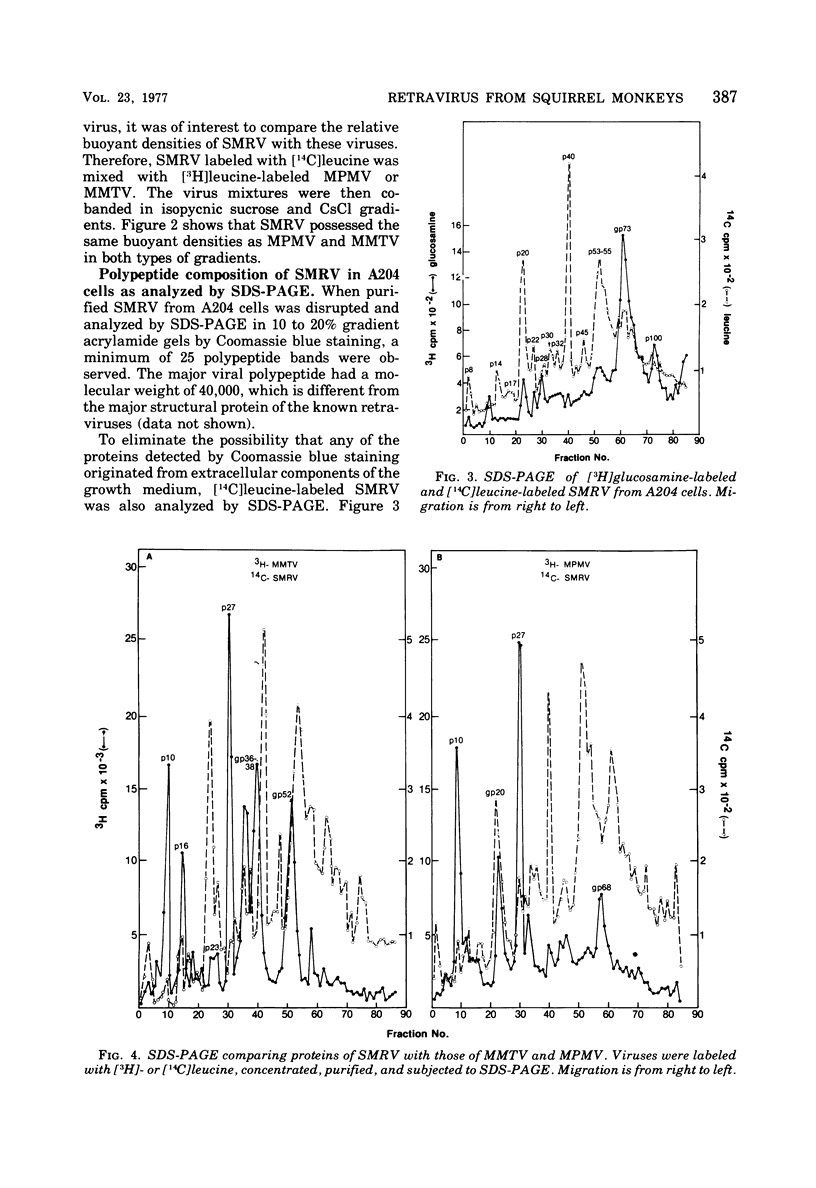
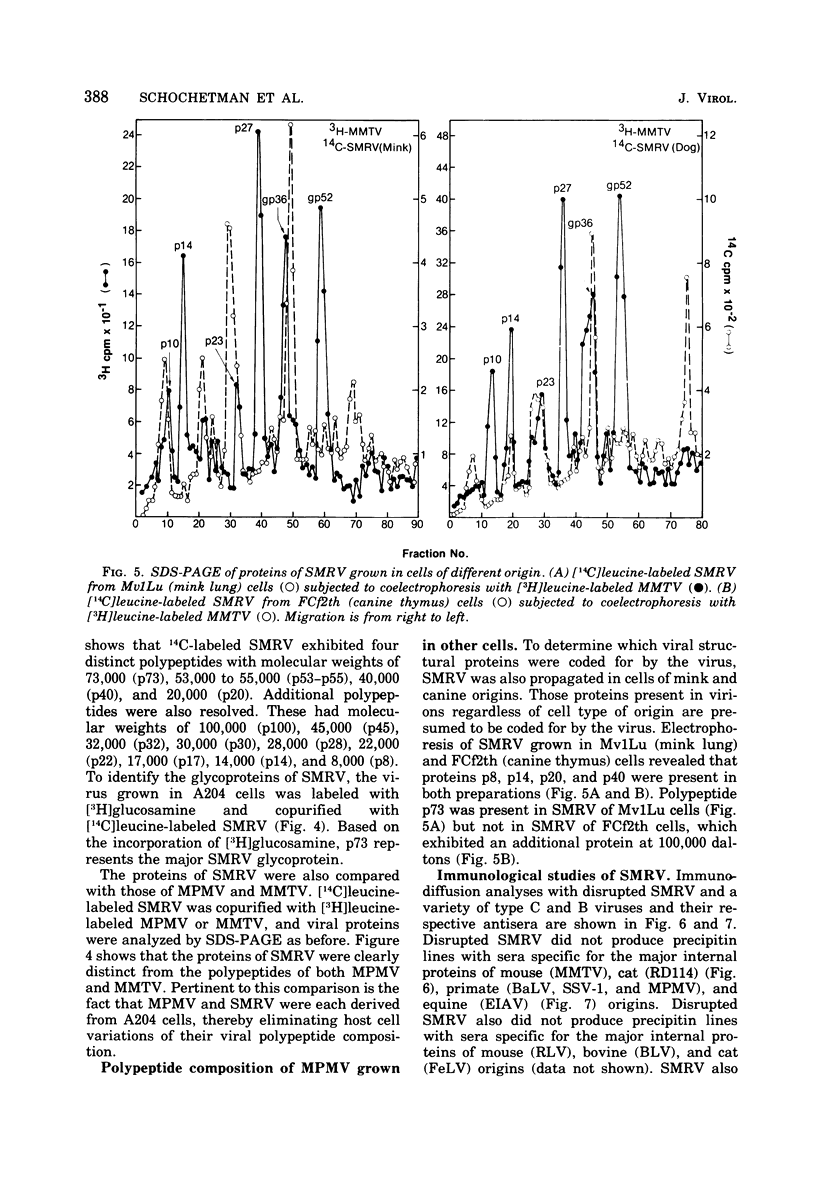
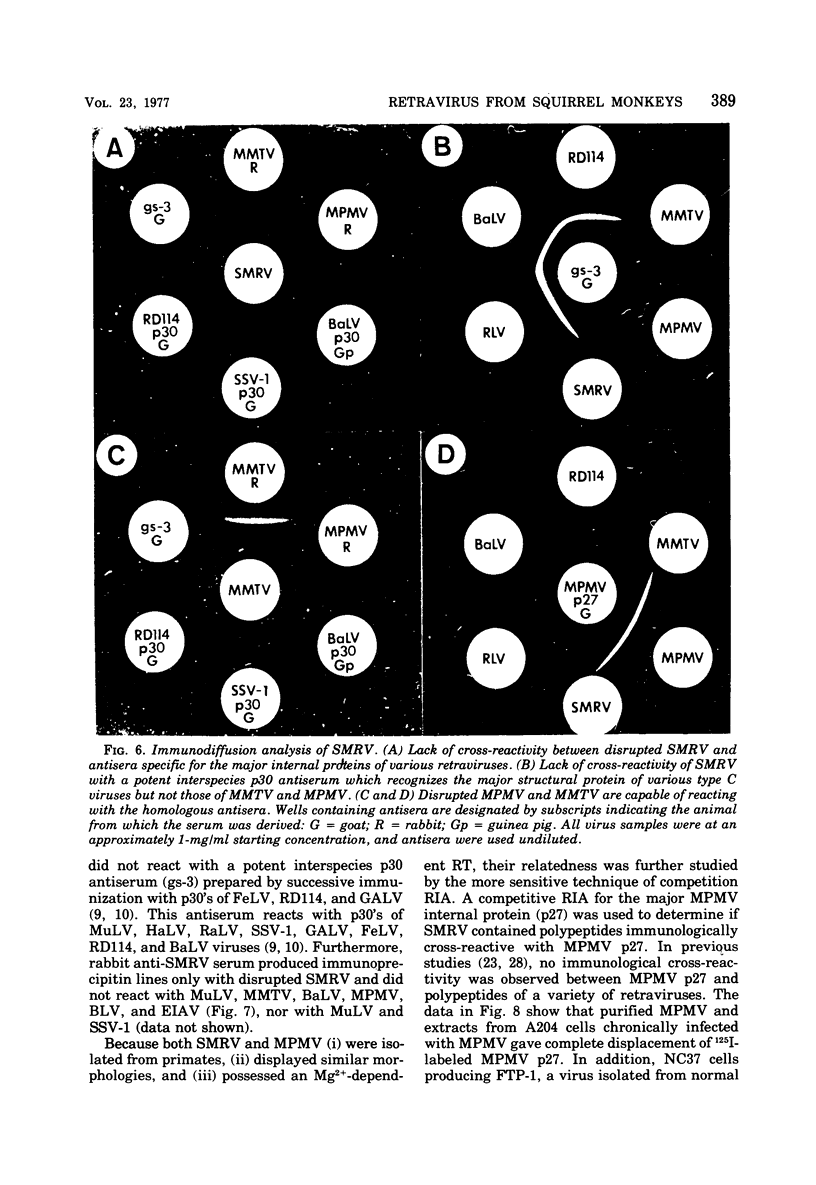
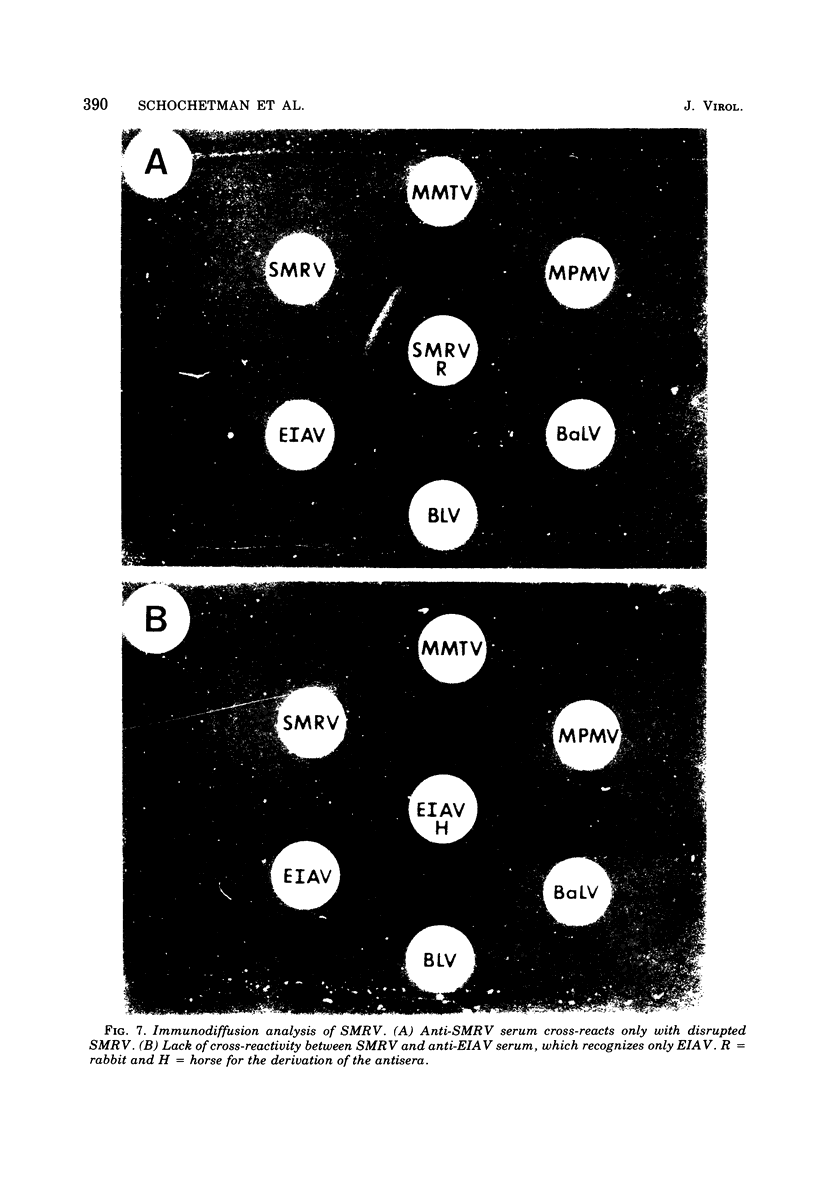
A new retravirus (SMRV) isolated from a squirrel monkey, Saimiri sciureus, has an Mg2+-dependen reverse transcriptase and a buoyant density of 1.17 g/cm3 in sucrose and 1.21 g/cm3 in cesium chloride, similar to the mouse mammary tumor virus and the Mason-Pfizer monkey virus. The polypeptide patter of SMRV as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis was distinct from the reported polypeptide patterns of known retraviruses. Four major polypeptides of molecular weights 40,000, 20,000, 14,000 and 8,000 were resolved in virus propagated in human, mink, and canine cells. In A204 human rhabdomyosarcoma cells, a protein of 73,000 daltons (gp73) represented the major viral glycoprotein as determined by [3H]glucosamine labeling. Additional proteins were also observed, but their presence depended on the cell type in which the virus was propagated. In both species-and interspecies-specific assays, no antigenic relatedness was observed between SMRV and Mason-Pfizer monkey virus, mouse mammary tumor virus, baboon endogenous virus (BaLV), woolly monkey virus (SSV-1), murine leukemia virus, endogenous feline type C virus (RD-114), bovine leukemia virus, and equine infectious anemia virus. These findings indicate that SMRV represents a new retravirus and the first isolate from a New World monkey.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Schidlovsky G., Korol W., Vidrine G., Cicmanec J. L. Occurrence of Mason-Pfizer monkey virus in healthy rhesus monkeys. Cancer Res. 1974 Dec;34(12):3504–3508. [PubMed] [Google Scholar]
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Bauer R. F., Arthur L. O., Fine D. L. Propagation of mouse mammary tumor cell lines and production of mouse mammary tumor virus in a serum-free medium. In Vitro. 1976 Aug;12(8):558–563. doi: 10.1007/BF02797439. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Livingston D. M., Sherr C. J., Todaro G. J., Kalter S. S. Infectious C-type virus isolated from a baboon placenta. Nature. 1974 Mar 1;248(5443):17–20. doi: 10.1038/248017a0. [DOI] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Dalton A. J., Melnick J. L., Bauer H., Beaudreau G., Bentvelzen P., Bolognesi D., Gallo R., Graffi A., Haguenau F., Heston W. The case for a family of reverse transcriptase viruses: Retraviridae. Intervirology. 1974;4(4):201–206. doi: 10.1159/000149963. [DOI] [PubMed] [Google Scholar]
- Fine D. L., Plowman J. K., Kelley S. P., Arthur L. O., Hillman E. A. Enhanced production of mouse mammary tumor virus in dexamethasone-treated, 5-iododeoxyuridine-stimulated mammary tumor cell cultures. J Natl Cancer Inst. 1974 Jun;52(6):1881–1886. doi: 10.1093/jnci/52.6.1881. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Gilden R. V. Interrelationships among RNA tumor viruses and host cells. Adv Cancer Res. 1975;22:157–202. doi: 10.1016/s0065-230x(08)60177-3. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Long C. W., Hanson M., Toni R., Charman H. P., Oroszlan S., Miller J. M., Van der Maaten M. J. Characteristics of the major internal protein and RNA-dependent DNA polymerase of bovine leukaemia virus. J Gen Virol. 1975 Dec;29(3):305–314. doi: 10.1099/0022-1317-29-3-305. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Arthur L. O., Zeve V. H., Fine D. L., Nagashima K. Surface localization of virus production on a glucocorticoid-stimulated oncornavirus-producing mouse mammary tumor cell line by scanning electron microscopy. Cancer Res. 1976 Mar;36(3):1084–1093. [PubMed] [Google Scholar]
- Heberling R. L., Barker S. T., Kalter S. S., Smith G. C., Helmke R. J. Oncornavirus: isolation from a squirrel monkey (Saimiri sciureus) lung culture. Science. 1977 Jan 21;195(4275):289–292. doi: 10.1126/science.63993. [DOI] [PubMed] [Google Scholar]
- Kalter S. S., Helmke R. J., Panigel M., Heberling R. L., Felsburg P. J., Axelrod L. R. Observations of apparent C-type particles in baboon (Papio cynocephalus) placentas. Science. 1973 Mar 30;179(4080):1332–1333. doi: 10.1126/science.179.4080.1332. [DOI] [PubMed] [Google Scholar]
- Kawakami T. G., Huff S. D., Buckley P. M., Dungworth D. L., Synder S. P., Gilden R. V. C-type virus associated with gibbon lymphosarcoma. Nat New Biol. 1972 Feb 9;235(58):170–171. doi: 10.1038/newbio235170a0. [DOI] [PubMed] [Google Scholar]
- Kubicek M. T., Fine D. L., Bennett D. G., Malan L. B., West D. M., Holloway A. M. Virus susceptibility of a new simian cell line of fetal origin. Appl Microbiol. 1973 Feb;25(2):275–278. doi: 10.1128/am.25.2.275-278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Gilden R. V., Bykovsky A. F., Miller G. G., Zhdanov V. M., Soloviev V. D., Scolnick E. M. Mason-Pfizer virus characterization: a similar virus in a human amniotic cell line. J Virol. 1973 Dec;12(6):1540–1547. doi: 10.1128/jvi.12.6.1540-1547.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D., Bridson W., Rayford P. L. Rapid calculation of radioimmunoassay results. J Lab Clin Med. 1969 Nov;74(5):770–781. [PubMed] [Google Scholar]
- Sarkar N. H., Moore D. H. Separation of B and C type virions by centrifugation in gentle density gradients. J Virol. 1974 May;13(5):1143–1147. doi: 10.1128/jvi.13.5.1143-1147.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Boehm-Truitt M., Schlom J. Antigenic analysis of the major structural protein of the Mason-Pfizer monkey virus. J Immunol. 1976 Jul;117(1):168–173. [PubMed] [Google Scholar]
- Schochetman G., Kortright K., Schlom J. Mason-Pfizer monkey virus: analysis and localization of virion proteins and glycoproteins. J Virol. 1975 Nov;16(5):1208–1219. doi: 10.1128/jvi.16.5.1208-1219.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Hino S., Garrett E. W., Aaronson S. A. Immunological cross reactivity of Mason-Pfizer monkey virus with type C RNA viruses endogenous to primates. Nature. 1976 Jun 17;261(5561):609–611. doi: 10.1038/261609a0. [DOI] [PubMed] [Google Scholar]
- Theilen G. H., Gould D., Fowler M., Dungworth D. L. C-type virus in tumor tissue of a woolly monkey (Lagothrix spp.) with fibrosarcoma. J Natl Cancer Inst. 1971 Oct;47(4):881–889. [PubMed] [Google Scholar]
- Todaro G. J., Lieber M. M., Benveniste R. E., Sherr C. J. Infectious primate type C viruses: Three isolates belonging to a new subgroup from the brains of normal gibbons. Virology. 1975 Oct;67(2):335–343. doi: 10.1016/0042-6822(75)90435-3. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E. Baboons and their close relatives are unusual among primates in their ability to release nondefective endogenous type C viruses. Virology. 1976 Jul 1;72(1):278–282. doi: 10.1016/0042-6822(76)90331-7. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Stephenson J. R., Aaronson S. A. Immunological properties of two polypeptides of Mason-Pfizer monkey virus. J Virol. 1974 Jul;14(1):125–132. doi: 10.1128/jvi.14.1.125-132.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J., Ahmed M., Lyles J., Larson D., Mayyasi S. A. Competition radioimmunoassay for mason-pfizer monkey virus: comparison with recent isolates. Int J Cancer. 1975 Apr 15;15(4):632–639. doi: 10.1002/ijc.2910150412. [DOI] [PubMed] [Google Scholar]





