Abstract
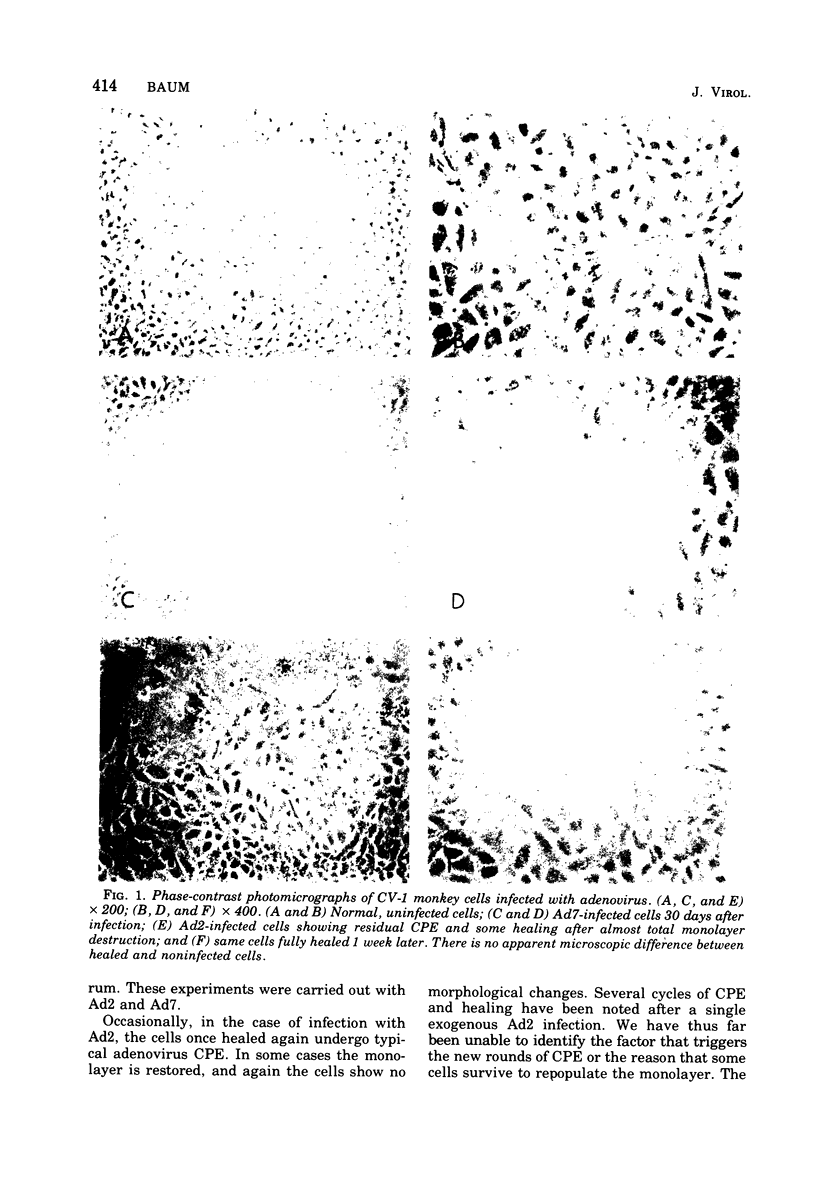
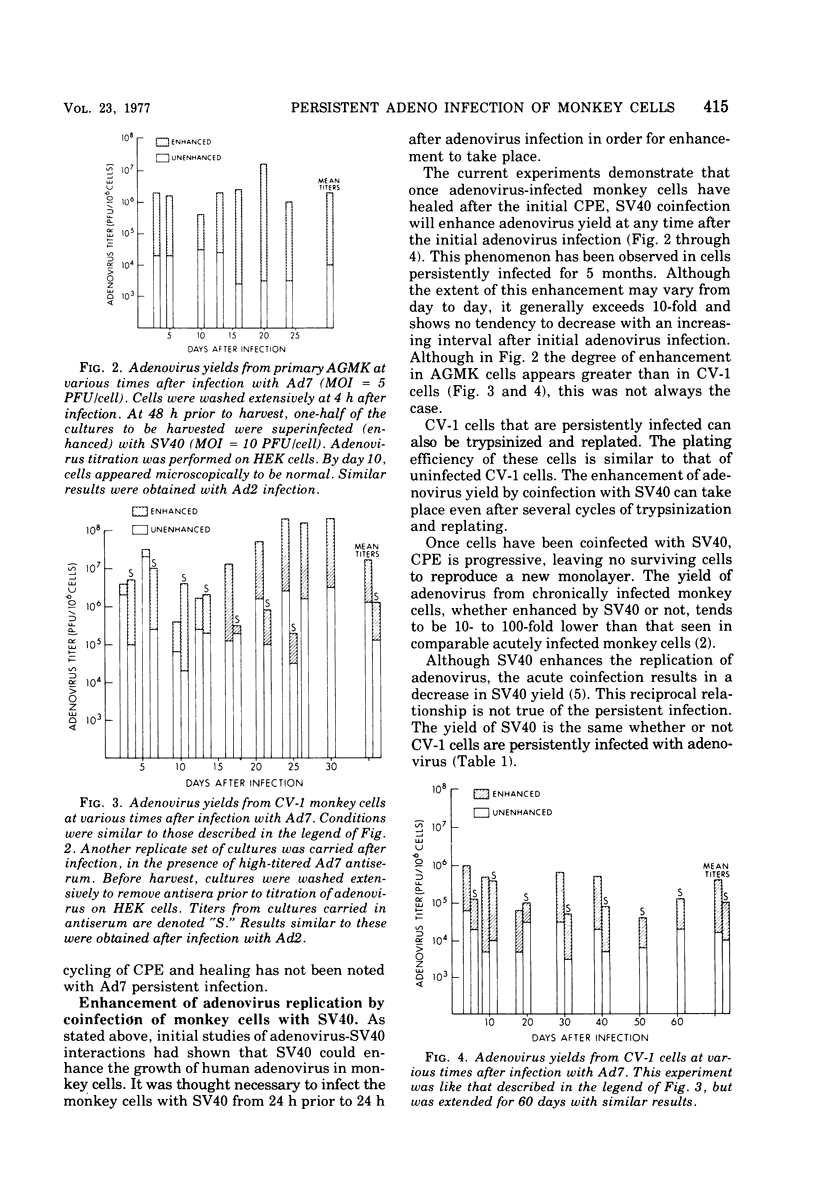
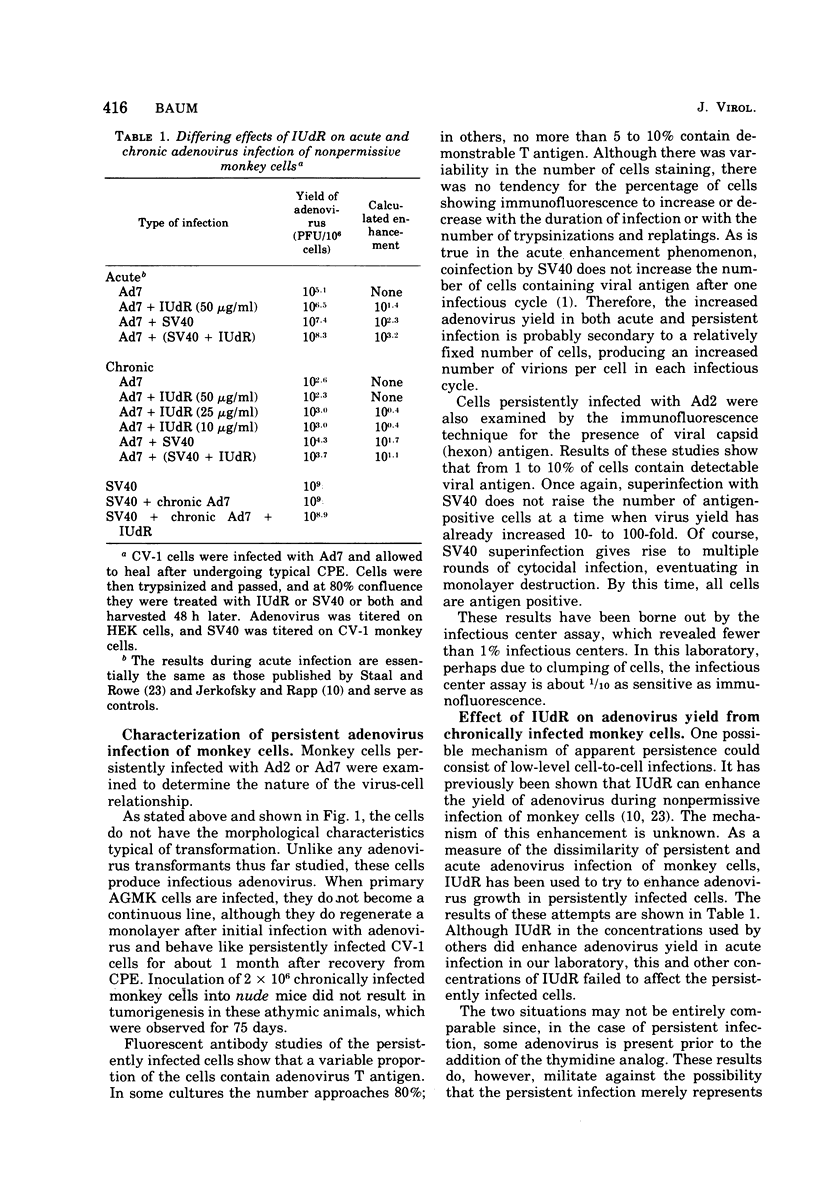
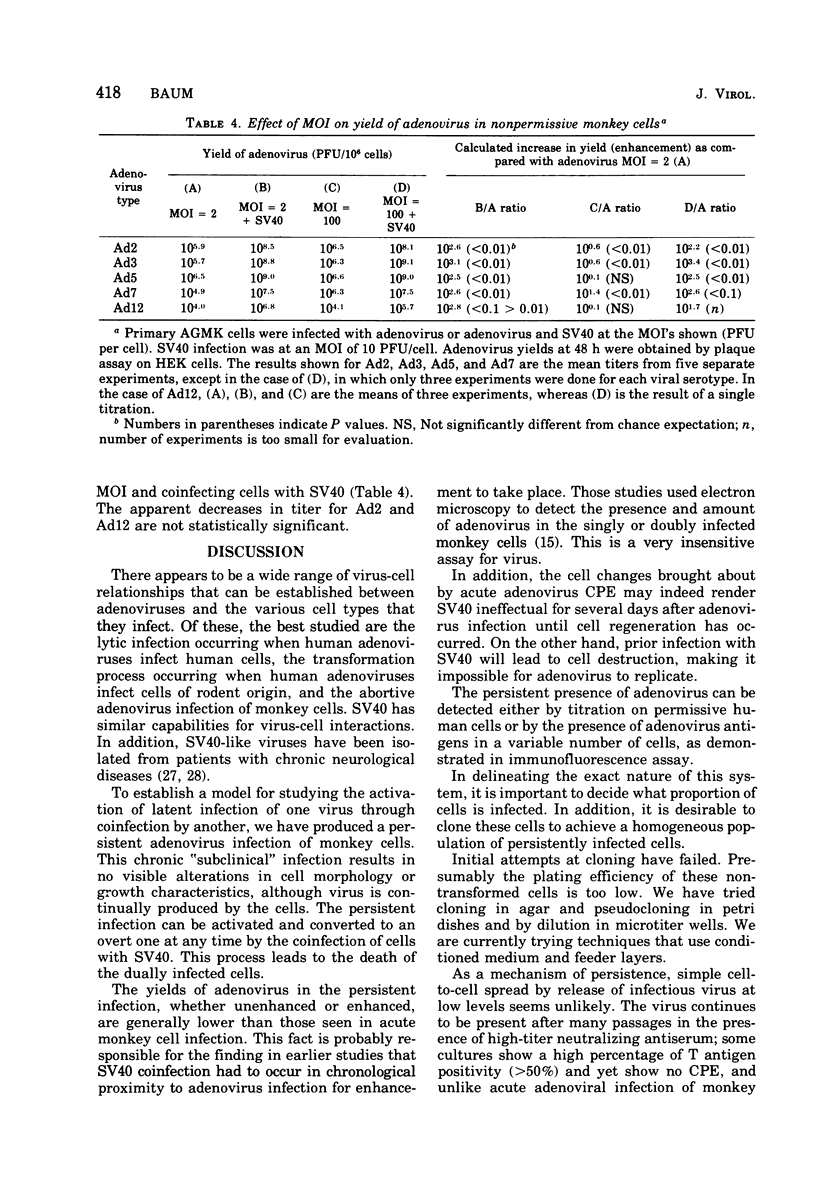
Persisten infections of monkey cells have been established by using two serotypes of human adenovirus. The persistently infected cells show no morpho logical changes, but continue ot produce low titers of infectious adenovirus. The inapparent infection can, at any time, be converted to a cytolytic productive one by superinfection with simian virus 40. Persistence in this system does not appear to result from multiple rounds of lytic infection, nor is it mediated by production of defective interfering particles. The persistently infected cells do not possess the characteristics of oncogenic transformation. Results of these studies also whow that the nonpermissiveness of monkey cells to adenovirus replication can be partially overcome by infection at high multiplicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Wiese W. H., Reich P. R. Studies on the mechanism of enhancement of adenovirus 7 infection in African green monkey cells by simian virus 40: formation of adenovirus-specific RNA. Virology. 1968 Feb;34(2):373–376. doi: 10.1016/0042-6822(68)90253-5. [DOI] [PubMed] [Google Scholar]
- Daniell E. Genome structure of incomplete particles of adenovirus. J Virol. 1976 Aug;19(2):685–708. doi: 10.1128/jvi.19.2.685-708.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. I., Baum S. G. Posttranscriptional block to adenovirus replication in nonpermissive monkey cells. Virology. 1974 Jul;60(1):45–53. doi: 10.1016/0042-6822(74)90364-x. [DOI] [PubMed] [Google Scholar]
- Friedman M. P., Lyons M. J., Ginsberg H. S. Biochemical consequences of type 2 adenovirus and Simian virus 40 double infections of African green monkey kidney cells. J Virol. 1970 May;5(5):586–597. doi: 10.1128/jvi.5.5.586-597.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Nakajima K., Oda K., Shimojo H. Complementation of translational defect for growth of human adenovirus type 2 in Simian cells by a Simian virus 40-induced factor. J Mol Biol. 1973 Dec 5;81(2):207–223. doi: 10.1016/0022-2836(73)90190-3. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- Jerkofsky M., Rapp F. Stimulation of adenovirus replication in simian cells in the absence of a helper virus by pretreatment of the cells with iododeoxyuridine. J Virol. 1975 Feb;15(2):253–258. doi: 10.1128/jvi.15.2.253-258.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F., Anderson C. W. Block to multiplication of adenovirus serotype 2 in monkey cells. J Virol. 1975 Dec;16(6):1650–1668. doi: 10.1128/jvi.16.6.1650-1668.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak S. Defective virions in human adenovirus type 12. J Virol. 1971 Apr;7(4):426–433. doi: 10.1128/jvi.7.4.426-433.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M. O., Reed G., Kern J., Gilden R. V., Huebner R. J. Transformation of rodent cells by adenovirus 19 and other group D adenoviruses. J Natl Cancer Inst. 1969 Oct;43(4):917–923. [PubMed] [Google Scholar]
- O'CONOR G. T., RABSON A. S., BEREZESKY I. K., PAUL F. J. MIXED INFECTION WITH SIMIAN VIRUS 40 AND ADENOVIRUS 12. J Natl Cancer Inst. 1963 Oct;31:903–917. [PubMed] [Google Scholar]
- O'CONOR G. T., RABSON A. S., MALMGREN R. A., BEREZESKY I. K., PAUL F. J. MORPHOLOGIC OBSERVATIONS OF GREEN MONKEY KIDNEY CELLS AFTER SINGLE AND DOUBLE INFECTION WITH ADENOVIRUS 12 AND SIMIAN VIRUS 40. J Natl Cancer Inst. 1965 May;34:679–693. [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., BAUM S. G. EVIDENCE FOR A POSSIBLE GENETIC HYBRID BETWEEN ADENOVIRUS TYPE 7 AND SV40 VIRUSES. Proc Natl Acad Sci U S A. 1964 Dec;52:1340–1347. doi: 10.1073/pnas.52.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., HUEBNER R. J., GILMORE L. K., PARROTT R. H., WARD T. G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953 Dec;84(3):570–573. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- Rapp F., Feldman L. A., Mandel M. Synthesis of virus deoxyribonucleic acid during abortive infection of simian cells by human adenoviruses. J Bacteriol. 1966 Oct;92(4):931–936. doi: 10.1128/jb.92.4.931-936.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich P. R., Baum S. G., Rose J. A., Rowe W. P., Weissman S. M. Nucleic acid homology studies of adenovirus type 7-SV40 interactions. Proc Natl Acad Sci U S A. 1966 Feb;55(2):336–341. doi: 10.1073/pnas.55.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Baum S. G., Pugh E., Hoggan M. D. Studies of adenovirus SV40 hybrid viruses. I. Assay system and further evidence for hybridization. J Exp Med. 1965 Nov 1;122(5):943–954. doi: 10.1084/jem.122.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROHL W. A., SCHLESINGER R. W. QUANTITATIVE STUDIES OF NATURAL AND EXPERIMENTAL ADENOVIRUS INFECTIONS OF HUMAN CELLS. II. PRIMARY CULTURES AND THE POSSIBLE ROLE OF ASYNCHRONOUS VIRAL MULTIPLICATION IN THE MAINTENANCE OF INFECTION. Virology. 1965 Jun;26:208–220. doi: 10.1016/0042-6822(65)90048-6. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal S. P., Rowe W. P. Enhancement of adenovirus infection in WI-38 and AGMK cells by pretreatment of cells with 5-iododeoxyuridine. Virology. 1975 Apr;64(2):513–519. doi: 10.1016/0042-6822(75)90128-2. [DOI] [PubMed] [Google Scholar]
- TRENTIN J. J., YABE Y., TAYLOR G. The quest for human cancer viruses. Science. 1962 Sep 14;137(3533):835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L., ZuRhein G. M., Albert A. E., Marsh R. F. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973 Aug 17;181(4100):674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- Weiner L. P., Herndon R. M., Narayan O., Johnson R. T., Shah K., Rubinstein L. J., Preziosi T. J., Conley F. K. Isolation of virus related to SV40 from patients with progressive multifocal leukoencephalopathy. N Engl J Med. 1972 Feb 24;286(8):385–390. doi: 10.1056/NEJM197202242860801. [DOI] [PubMed] [Google Scholar]



