Abstract
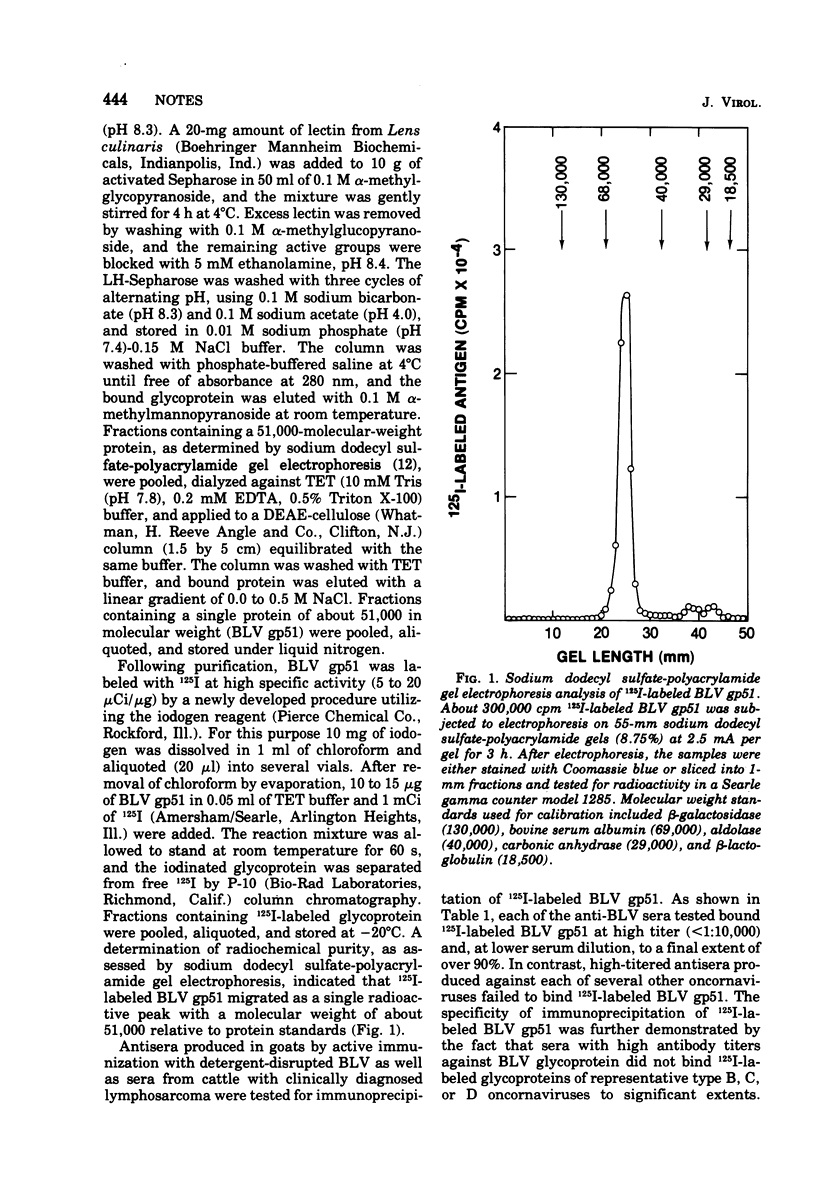
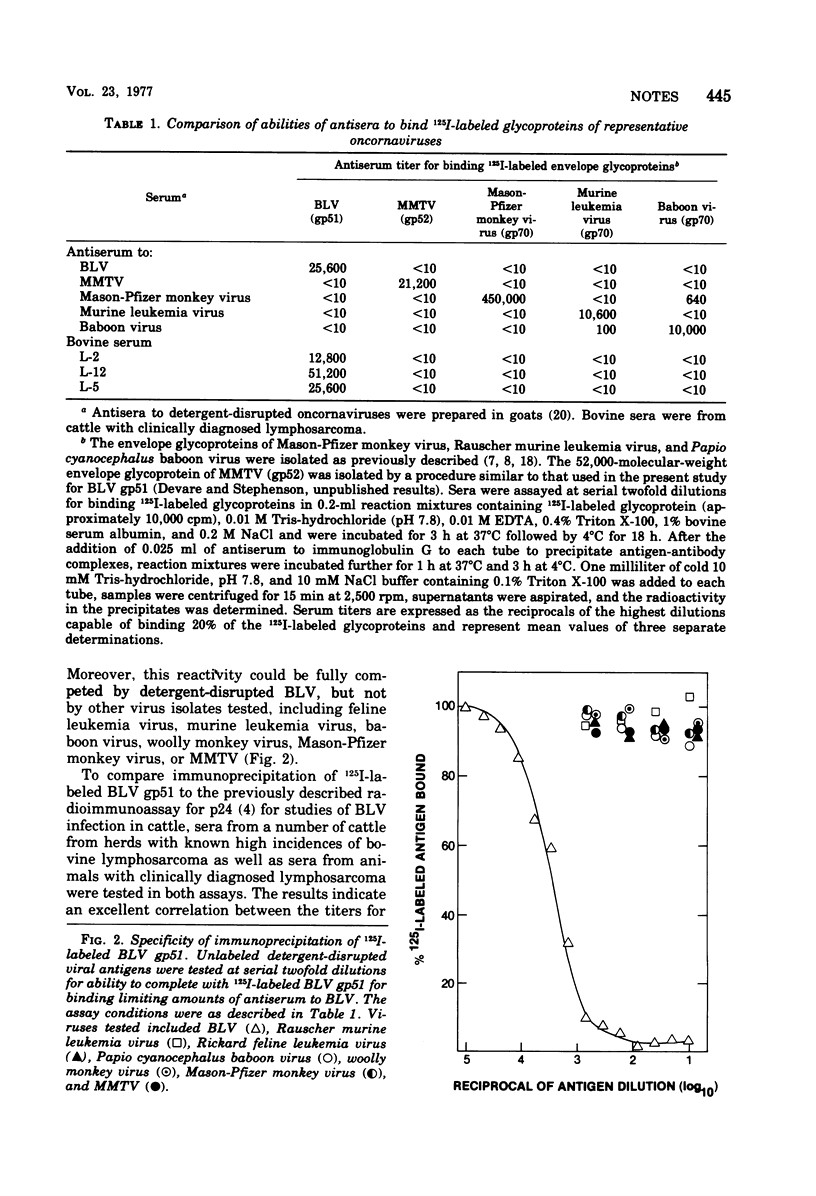
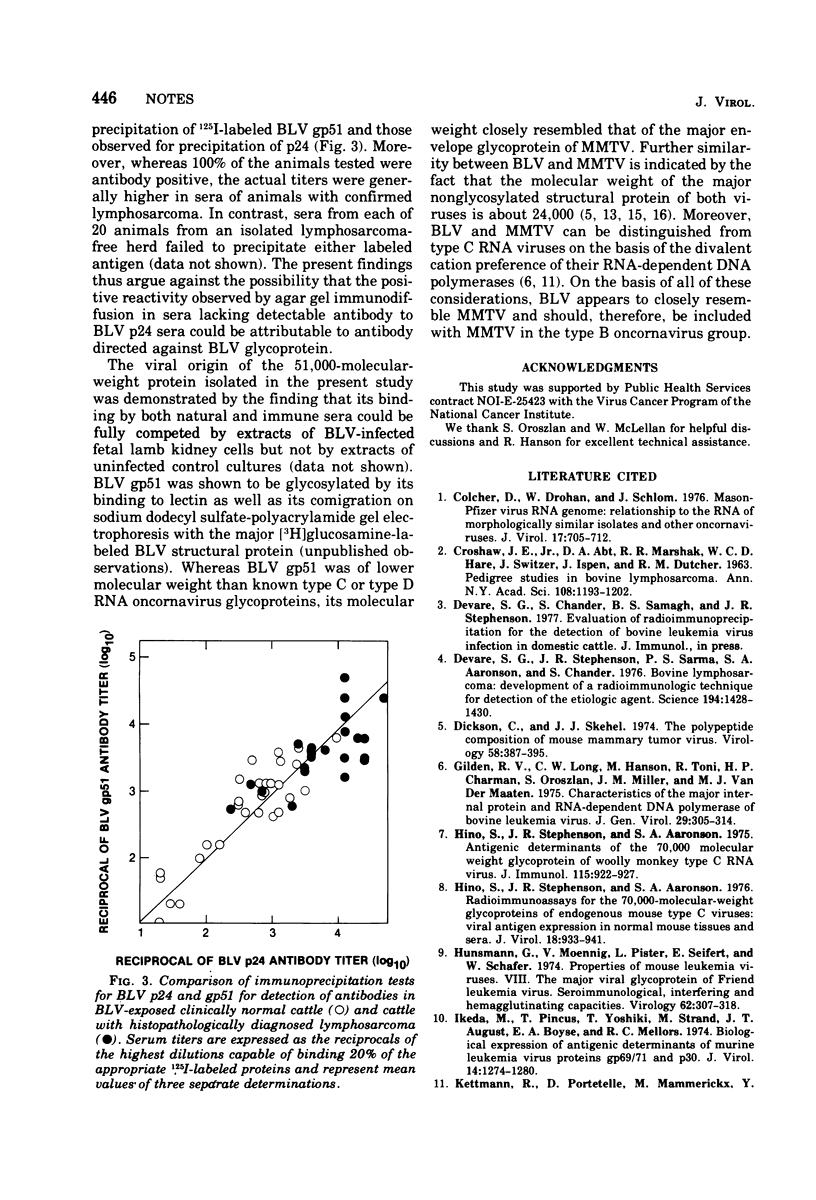
The major envelope glycoprotein of bovine leukemia virus was isolated by lectin-bound Sepharose and DEAE-cellulose column chromatography. This protein was shown to have a molecular weight of about 41,000 and to lack detectable immunological cross-reactivity with glycoproteins of other oncornaviruses. Sera obtained from 100% of cattle examined with clinically diagnosed lymphosarcoma contained high-titered antibody to 125I-labeled bovine leukemia virus glycoprotein, whereas sera from animals in a disease-free herd were antibody negative.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CROSHAW J. E., Jr, ABT D. A., MARSHAK R. R., HARE W. C., SWITZER J., IPSEN J., DUTCHER R. M. PEDIGREE STUDIES IN BOVINE LYMPHOSARCOMA. Ann N Y Acad Sci. 1963 Nov 4;108:1193–1202. doi: 10.1111/j.1749-6632.1963.tb13444.x. [DOI] [PubMed] [Google Scholar]
- Colcher D., Drohan W., Schlom Mason-Pfizer virus RNA genome: relationship to the RNA of morphologically similar isolates and other oncornaviruses. J Virol. 1976 Mar;17(3):705–712. doi: 10.1128/jvi.17.3.705-712.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Stephenson J. R., Sarma P. S., Aaronson S. A., Charder S. Bovine lymphosarcoma: development of a radioimmunologic technique for detection of the etiologic agent. Science. 1976 Dec 24;194(4272):1428–1430. doi: 10.1126/science.188129. [DOI] [PubMed] [Google Scholar]
- Dickson C., Skehel J. J. The polypeptide composition of mouse mammary tumor virus. Virology. 1974 Apr;58(2):387–395. doi: 10.1016/0042-6822(74)90074-9. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Long C. W., Hanson M., Toni R., Charman H. P., Oroszlan S., Miller J. M., Van der Maaten M. J. Characteristics of the major internal protein and RNA-dependent DNA polymerase of bovine leukaemia virus. J Gen Virol. 1975 Dec;29(3):305–314. doi: 10.1099/0022-1317-29-3-305. [DOI] [PubMed] [Google Scholar]
- Hino S., Stephenson J. R., Aaronson S. A. Antigenic determinants of the 70,000 molecular weight glycoprotein of woolly monkey type C RNA virus. J Immunol. 1975 Oct;115(4):922–927. [PubMed] [Google Scholar]
- Hino S., Stephenson J. R., Aaronson S. A. Radiommunoassays for the 70,000-molecular-weight glycoproteins of endogenous mouse type C viruses: viral antigen expression in normal mouse tissues and sera. J Virol. 1976 Jun;18(3):933–941. doi: 10.1128/jvi.18.3.933-941.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsmann G., Moennig V., Pister L., Seifert E., Schäfer W. Properties of mouse leukemia viruses. VIII. The major viral glycoprotein of Friend leukemia virus. Seroimmunological, interfering and hemagglutinating capacities. Virology. 1974 Dec;62(2):307–318. doi: 10.1016/0042-6822(74)90394-8. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Pincus T., Yoshiki T., Strand M., August J. T., Boyse E. A., Mellors R. C. Biological expression of antigenic determinants of murine leukemia virus proteins gp69-71 and p30. J Virol. 1974 Nov;14(5):1274–1280. doi: 10.1128/jvi.14.5.1274-1280.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Portetelle D., Mammerickx M., Cleuter Y., Dekegel D., Galoux M., Ghysdael J., Burny A., Chantrenne H. Bovine leukemia virus: an exogenous RNA oncogenic virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1014–1018. doi: 10.1073/pnas.73.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Howk R. S., Scolnick E. M., Oroszlan S., Gilden R. V. Immunochemical characterization of two major polypeptides from murine mammary tumor virus. J Virol. 1974 Jun;13(6):1200–1210. doi: 10.1128/jvi.13.6.1200-1210.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper C. E., Abt D. A., Ferrer J. F., Marshak R. R. Seroepidemiological evidence for horizontal transmission of bovine C-type virus. Cancer Res. 1975 Oct;35(10):2714–2716. [PubMed] [Google Scholar]
- Ritzi E., Baldi A., Spiegelman S. The purification of a gs antigen of the murine mammary tumor virus and its quantitation by radioimmunoassay. Virology. 1976 Nov;75(1):188–197. doi: 10.1016/0042-6822(76)90017-9. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Dion A. S. Polypeptides of the mouse mammary tumor virus. I. Characterization of two group-specific antigens. Virology. 1975 Apr;64(2):471–491. doi: 10.1016/0042-6822(75)90125-7. [DOI] [PubMed] [Google Scholar]
- Steeves R. A., Strand M., August J. T. Structural proteins of mammalian oncogenic RNA viruses: murine leukemia virus neutralization by antisera prepared against purified envelope glycoprotein. J Virol. 1974 Jul;14(1):187–189. doi: 10.1128/jvi.14.1.187-189.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Hino S., Garrett E. W., Aaronson S. A. Immunological cross reactivity of Mason-Pfizer monkey virus with type C RNA viruses endogenous to primates. Nature. 1976 Jun 17;261(5561):609–611. doi: 10.1038/261609a0. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of mammalian oncogenic RNA viruses: multiple antigenic determinants of the major internal protein and envelope glycoprotein. J Virol. 1974 Jan;13(1):171–180. doi: 10.1128/jvi.13.1.171-180.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick S. R., Stephenson J. R., Aaronson S. A. Immunological characterization of a low molecular weight polypeptide of murine leukemia virus. Virology. 1973 Jul;54(1):199–206. doi: 10.1016/0042-6822(73)90129-3. [DOI] [PubMed] [Google Scholar]



