Abstract
cDNAs for glutathione-independent prostaglandin D synthase were isolated from cDNA libraries of human brain. The longest cDNA insert was 837 base pairs long and contained a coding region of 570 base pairs corresponding to 190 amino acid residues with a calculated Mr of 21,016. Between two cDNA inserts isolated from the two different libraries, nucleotide substitutions were observed at 16 positions, including conservative amino acid substitutions at 2 positions and nonconservative substitutions at 5 positions, indicating genetic heterogeneity of this enzyme in humans. The computer-assisted homology search revealed that the enzyme is a member of the lipocalin superfamily, comprising secretory hydrophobic molecule transporters, showing the greatest homology (28.8-29.4% identity; 51.3-53.1% similarity) to alpha 1-microglobulin among the members of this superfamily. In a phylogenetic tree of the superfamily, this enzyme, alpha 1-microglobulin, and the gamma chain of the complement component C8 form a cluster separate from the other 14 members. The two distinctive characteristics of glutathione-independent prostaglandin D synthase, as compared to the other members of this superfamily, are its enzymatic properties and its association with membranes that were probably acquired after evolutionary divergence of the two lipocalins. Based on the observed sequence homology, the tertiary structure of the enzyme was deduced to consist of an eight-stranded anti-parallel beta-barrel forming a hydrophobic pocket. Furthermore, the Cys-65 residue in the pocket, which is conserved only in the human and rat enzymes but not in other lipocalins, was considered to be a putative active site of the enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Halim M. S., von Holst H., Meyerson B., Sachs C., Anggård E. Prostaglandin profiles in tissue and blood vessels from human brain. J Neurochem. 1980 May;34(5):1331–1333. doi: 10.1111/j.1471-4159.1980.tb09980.x. [DOI] [PubMed] [Google Scholar]
- Ali S., Clark A. J. Characterization of the gene encoding ovine beta-lactoglobulin. Similarity to the genes for retinol binding protein and other secretory proteins. J Mol Biol. 1988 Feb 5;199(3):415–426. doi: 10.1016/0022-2836(88)90614-6. [DOI] [PubMed] [Google Scholar]
- Bedard P. A., Yannoni Y., Simmons D. L., Erikson R. L. Rapid repression of quiescence-specific gene expression by epidermal growth factor, insulin, and pp60v-src. Mol Cell Biol. 1989 Mar;9(3):1371–1375. doi: 10.1128/mcb.9.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D. E., Means A. R., Wright E. J., Singh S. P., Tiver K. K. Molecular cloning of the cDNA for two major androgen-dependent secretory proteins of 18.5 kilodaltons synthesized by the rat epididymis. J Biol Chem. 1986 Apr 15;261(11):4956–4961. [PubMed] [Google Scholar]
- Cancedda F. D., Dozin B., Rossi F., Molina F., Cancedda R., Negri A., Ronchi S. The Ch21 protein, developmentally regulated in chick embryo, belongs to the superfamily of lipophilic molecule carrier proteins. J Biol Chem. 1990 Nov 5;265(31):19060–19064. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cowan S. W., Newcomer M. E., Jones T. A. Crystallographic refinement of human serum retinol binding protein at 2A resolution. Proteins. 1990;8(1):44–61. doi: 10.1002/prot.340080108. [DOI] [PubMed] [Google Scholar]
- Dente L., Ciliberto G., Cortese R. Structure of the human alpha 1-acid glycoprotein gene: sequence homology with other human acute phase protein genes. Nucleic Acids Res. 1985 Jun 11;13(11):3941–3952. doi: 10.1093/nar/13.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna D. T., McLean J. W., Wion K. L., Trent J. M., Drabkin H. A., Lawn R. M. Human apolipoprotein D gene: gene sequence, chromosome localization, and homology to the alpha 2u-globulin superfamily. DNA. 1987 Jun;6(3):199–204. doi: 10.1089/dna.1987.6.199. [DOI] [PubMed] [Google Scholar]
- Goh Y., Urade Y., Fujimoto N., Hayaishi O. Content and formation of prostaglandins and distribution of prostaglandin-related enzyme activities in the rat ocular system. Biochim Biophys Acta. 1987 Sep 25;921(2):302–311. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hayaishi O. Sleep-wake regulation by prostaglandins D2 and E2. J Biol Chem. 1988 Oct 15;263(29):14593–14596. [PubMed] [Google Scholar]
- Henzel W. J., Rodriguez H., Singer A. G., Stults J. T., Macrides F., Agosta W. C., Niall H. The primary structure of aphrodisin. J Biol Chem. 1988 Nov 15;263(32):16682–16687. [PubMed] [Google Scholar]
- Holden H. M., Rypniewski W. R., Law J. H., Rayment I. The molecular structure of insecticyanin from the tobacco hornworm Manduca sexta L. at 2.6 A resolution. EMBO J. 1987 Jun;6(6):1565–1570. doi: 10.1002/j.1460-2075.1987.tb02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Schneider M., Epp O., Mayr I., Messerschmidt A., Pflugrath J., Kayser H. Crystallization, crystal structure analysis and preliminary molecular model of the bilin binding protein from the insect Pieris brassicae. J Mol Biol. 1987 May 20;195(2):423–434. doi: 10.1016/0022-2836(87)90661-9. [DOI] [PubMed] [Google Scholar]
- Ichiyoshi Y., Endo H., Yamamoto M. Length polymorphism in the 3' noncoding region of rat hepatic alpha 2u-globulin mRNAs. Biochim Biophys Acta. 1987 Oct 9;910(1):43–51. doi: 10.1016/0167-4781(87)90093-5. [DOI] [PubMed] [Google Scholar]
- Ito S., Narumiya S., Hayaishi O. Prostaglandin D2: a biochemical perspective. Prostaglandins Leukot Essent Fatty Acids. 1989 Sep;37(4):219–234. doi: 10.1016/0952-3278(89)90033-1. [DOI] [PubMed] [Google Scholar]
- Julkunen M., Seppälä M., Jänne O. A. Complete amino acid sequence of human placental protein 14: a progesterone-regulated uterine protein homologous to beta-lactoglobulins. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8845–8849. doi: 10.1073/pnas.85.23.8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumeyer J. F., Polazzi J. O., Kotick M. P. The mRNA for a proteinase inhibitor related to the HI-30 domain of inter-alpha-trypsin inhibitor also encodes alpha-1-microglobulin (protein HC). Nucleic Acids Res. 1986 Oct 24;14(20):7839–7850. doi: 10.1093/nar/14.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent B. C., Nilsson M. H., Båvik C. O., Jones T. A., Sundelin J., Peterson P. A. Characterization of the rat retinol-binding protein gene and its comparison to the three-dimensional structure of the protein. J Biol Chem. 1985 Sep 25;260(21):11476–11480. [PubMed] [Google Scholar]
- Lee K. H., Wells R. G., Reed R. R. Isolation of an olfactory cDNA: similarity to retinol-binding protein suggests a role in olfaction. Science. 1987 Feb 27;235(4792):1053–1056. doi: 10.1126/science.3493528. [DOI] [PubMed] [Google Scholar]
- Monaco H. L., Zanotti G., Spadon P., Bolognesi M., Sawyer L., Eliopoulos E. E. Crystal structure of the trigonal form of bovine beta-lactoglobulin and of its complex with retinol at 2.5 A resolution. J Mol Biol. 1987 Oct 20;197(4):695–706. doi: 10.1016/0022-2836(87)90476-1. [DOI] [PubMed] [Google Scholar]
- Newcomer M. E., Jones T. A., Aqvist J., Sundelin J., Eriksson U., Rask L., Peterson P. A. The three-dimensional structure of retinol-binding protein. EMBO J. 1984 Jul;3(7):1451–1454. doi: 10.1002/j.1460-2075.1984.tb01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. C., Rao A. G., Howard O. M., Sodetz J. M. The eighth component of human complement: evidence that it is an oligomeric serum protein assembled from products of three different genes. Biochemistry. 1987 Aug 25;26(17):5229–5233. doi: 10.1021/bi00391a003. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Nakashima H., Kanehisa M., Ooi T. Detection of weak sequence homology of proteins for tertiary structure prediction. Protein Seq Data Anal. 1987;1(2):107–116. [PubMed] [Google Scholar]
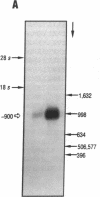
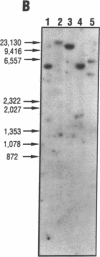
- Ogorochi T., Narumiya S., Mizuno N., Yamashita K., Miyazaki H., Hayaishi O. Regional distribution of prostaglandins D2, E2, and F2 alpha and related enzymes in postmortem human brain. J Neurochem. 1984 Jul;43(1):71–82. doi: 10.1111/j.1471-4159.1984.tb06680.x. [DOI] [PubMed] [Google Scholar]
- Ogorochi T., Ujihara M., Narumiya S. Purification and properties of prostaglandin H-E isomerase from the cytosol of human brain: identification as anionic forms of glutathione S-transferase. J Neurochem. 1987 Mar;48(3):900–909. doi: 10.1111/j.1471-4159.1987.tb05602.x. [DOI] [PubMed] [Google Scholar]
- Papiz M. Z., Sawyer L., Eliopoulos E. E., North A. C., Findlay J. B., Sivaprasadarao R., Jones T. A., Newcomer M. E., Kraulis P. J. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. 1986 Nov 27-Dec 3Nature. 324(6095):383–385. doi: 10.1038/324383a0. [DOI] [PubMed] [Google Scholar]
- Pervaiz S., Brew K. Homology and structure-function correlations between alpha 1-acid glycoprotein and serum retinol-binding protein and its relatives. FASEB J. 1987 Sep;1(3):209–214. doi: 10.1096/fasebj.1.3.3622999. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Reed R. R., Feinstein P. G., Snyder S. H. Molecular cloning of odorant-binding protein: member of a ligand carrier family. Science. 1988 Jul 15;241(4863):336–339. doi: 10.1126/science.3388043. [DOI] [PubMed] [Google Scholar]
- Riley C. T., Barbeau B. K., Keim P. S., Kézdy F. J., Heinrikson R. L., Law J. H. The covalent protein structure of insecticyanin, a blue biliprotein from the hemolymph of the tobacco hornworm, Manduca sexta L. J Biol Chem. 1984 Nov 10;259(21):13159–13165. [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmale H., Holtgreve-Grez H., Christiansen H. Possible role for salivary gland protein in taste reception indicated by homology to lipophilic-ligand carrier proteins. Nature. 1990 Jan 25;343(6256):366–369. doi: 10.1038/343366a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Esch F. A chick neural retina adhesion and survival molecule is a retinol-binding protein. J Cell Biol. 1986 Jun;102(6):2295–2301. doi: 10.1083/jcb.102.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M., Fex J., Urade Y., Hayaishi O. Brain-type prostaglandin D synthetase occurs in the rat cochlea. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7677–7680. doi: 10.1073/pnas.84.21.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujihara M., Urade Y., Eguchi N., Hayashi H., Ikai K., Hayaishi O. Prostaglandin D2 formation and characterization of its synthetases in various tissues of adult rats. Arch Biochem Biophys. 1988 Feb 1;260(2):521–531. doi: 10.1016/0003-9861(88)90477-8. [DOI] [PubMed] [Google Scholar]
- Urade Y., Fujimoto N., Hayaishi O. Purification and characterization of rat brain prostaglandin D synthetase. J Biol Chem. 1985 Oct 15;260(23):12410–12415. [PubMed] [Google Scholar]
- Urade Y., Fujimoto N., Kaneko T., Konishi A., Mizuno N., Hayaishi O. Postnatal changes in the localization of prostaglandin D synthetase from neurons to oligodendrocytes in the rat brain. J Biol Chem. 1987 Nov 5;262(31):15132–15136. [PubMed] [Google Scholar]
- Urade Y., Nagata A., Suzuki Y., Fujii Y., Hayaishi O. Primary structure of rat brain prostaglandin D synthetase deduced from cDNA sequence. J Biol Chem. 1989 Jan 15;264(2):1041–1045. [PubMed] [Google Scholar]
- Wolfe L. S. Eicosanoids: prostaglandins, thromboxanes, leukotrienes, and other derivatives of carbon-20 unsaturated fatty acids. J Neurochem. 1982 Jan;38(1):1–14. doi: 10.1111/j.1471-4159.1982.tb10847.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]