Abstract
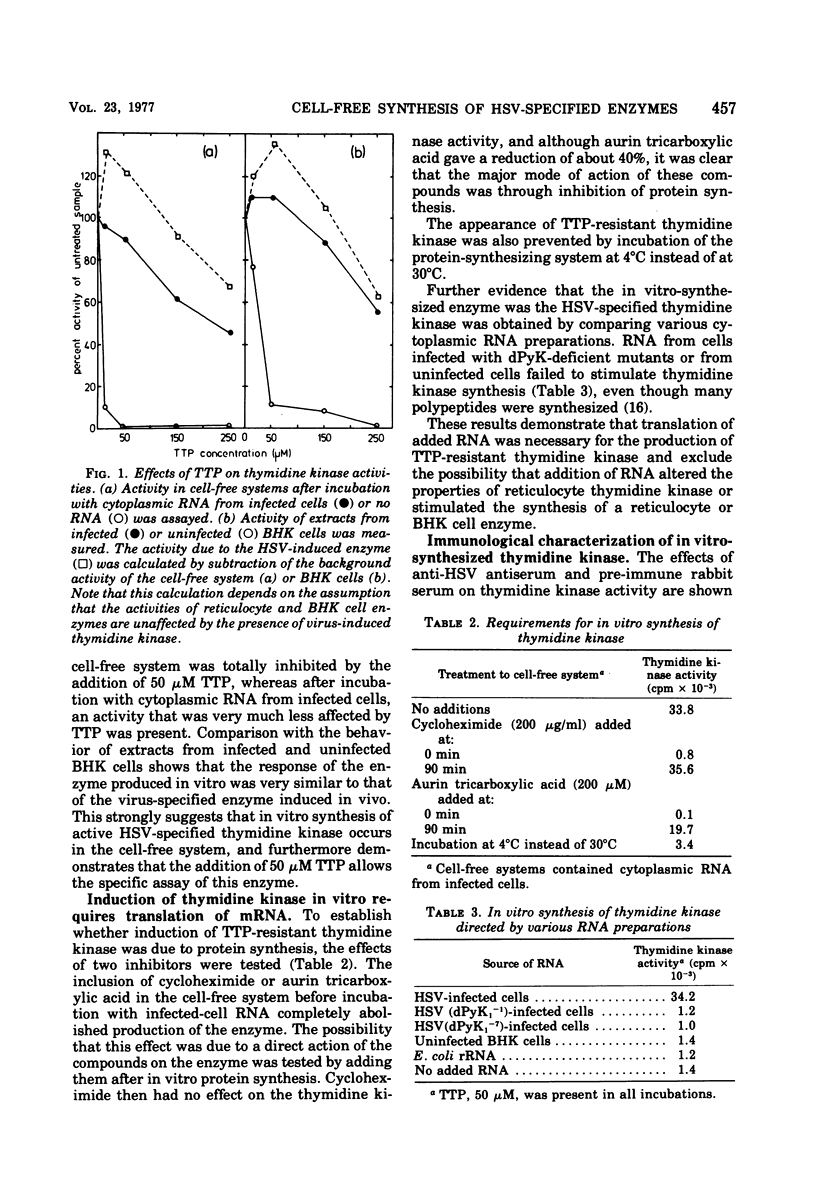
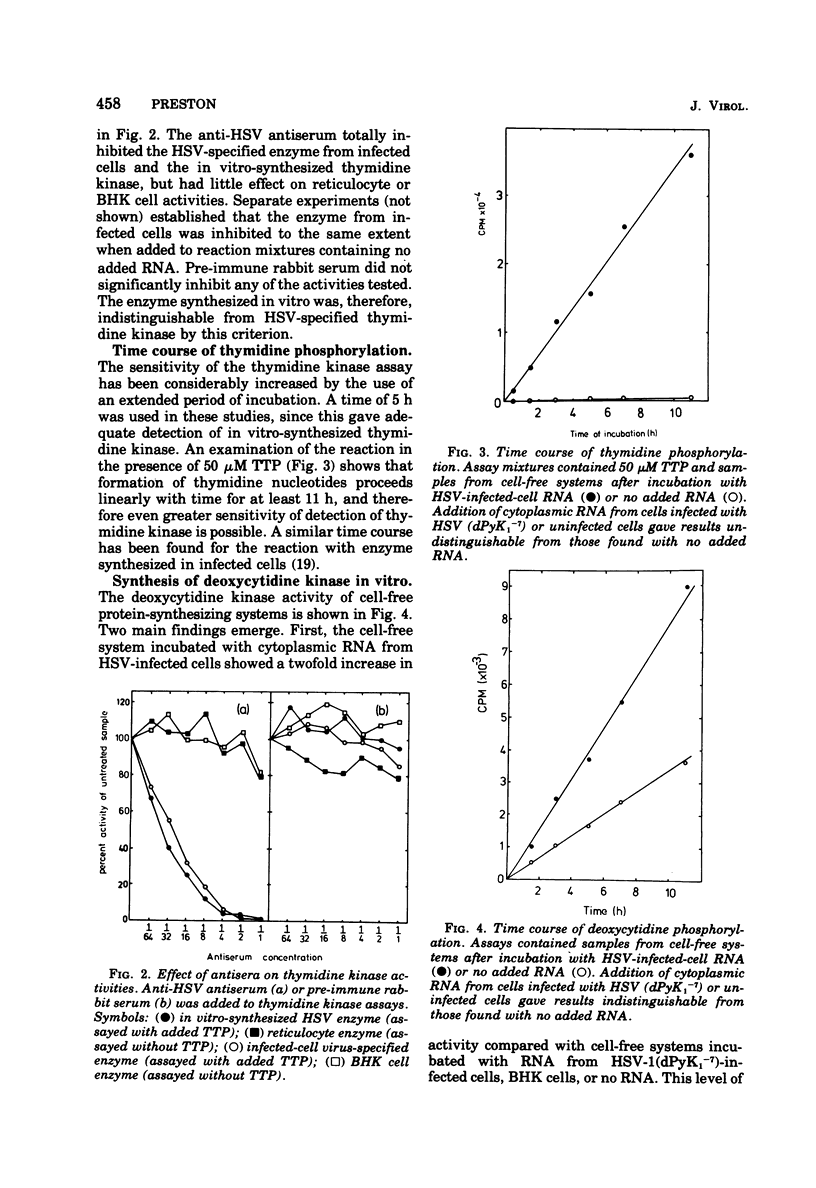
The incubation of a cell-free protein-synthesizing system prepared from rabbit reticulocytes with cytoplasmic RNA from herpes simplex virus (HSV)-infected cells resulted in increased thymidine kinase activity. This enzyme activity was specifically inhibited by anti-HSV antiserum and was relatively unaffected by TTP, an inhibitor of cellular thymidine kinases. Induction of the new activity was prevented by addition of inhibitors of eucaryotic protein synthesis, and no new activity was detected when RNA from cells infected with pyrimidine deoxyribonucleoside kinase-deficient mutants, instead of wild-type HSV, was added. An increased deoxycytidine kinase activity with similar properties to the HSV-specified enzyme activity was also present in cell-free systems incubated with RNA from HSV-infected cells. Phosphorylation of thymidine and deoxycytidine at 30 degrees C continued for longer than 11 h. The findings are consistent with the accurate synthesis in vitro of enzymically active HSV-specified pyrimidine deoxyribonucleoside kinase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Atkins J. F., Dunn J. J. Bacteriophage T3 and T7 early RNAs are translated by eukaryotic 80S ribosomes: active phage T3 coded S-adenosylmethionine cleaving enzyme is synthesized. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2752–2756. doi: 10.1073/pnas.73.8.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck O. E., Gassen H. G. Translation of bacteriophage T4 mRNA into active lysozyme by a wheat germ cell-free system. Biochem Biophys Res Commun. 1977 Jan 10;74(1):16–24. doi: 10.1016/0006-291x(77)91369-9. [DOI] [PubMed] [Google Scholar]
- Cremer K. J., Summers W. C., Gesteland R. F. Cell-free synthesis of herpes simplex virus proteins. J Virol. 1977 Jun;22(3):750–757. doi: 10.1128/jvi.22.3.750-757.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Steiner D. F., Chick W. L. Partial purification and characterization of the mRNA for rat preproinsulin. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3539–3543. doi: 10.1073/pnas.73.10.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J., Perera P. A., Morrison J. M., Gentry G. A., Subak-Sharpe J. H. Herpes virus-specified proteins. In: strategy of the viral genome. Ciba Found Symp. 1971:355–376. [PubMed] [Google Scholar]
- Herrlich P., Schweiger M. T3 and T7 bacteriophage deoxyribonucleic acid-directed enzyme synthesis in vitro. J Virol. 1970 Dec;6(6):750–753. doi: 10.1128/jvi.6.6.750-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus-specific polypeptides studied by polyacrylamide gel electrophoresis of immune precipitates. J Gen Virol. 1974 Feb;22(2):171–185. doi: 10.1099/0022-1317-22-2-171. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. PROPERTIES OF DEOXYTHYMIDINE KINASE PARTIALLY PURIFIED FROM NONINFECTED MOUSE FIBROBLAST CELLS. Virology. 1965 May;26:16–27. doi: 10.1016/0042-6822(65)90021-8. [DOI] [PubMed] [Google Scholar]
- Kellems R. E., Alt F. W., Schimke R. T. Regulation of folate reductase synthesis in sensitive and methotrexate-resistant sarcoma 180 cells. In vitro translation and characterization of folate reductase mRNA. J Biol Chem. 1976 Nov 25;251(22):6987–6993. [PubMed] [Google Scholar]
- Klemperer H. G., Haynes G. R., Shedden W. I., Watson D. H. A virus-specific thymidine kinase in BHK-21 cells infected with herpes simplex virus. Virology. 1967 Jan;31(1):120–128. doi: 10.1016/0042-6822(67)90015-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Ovalbumin messenger ribonucleic acid translation. Comparable rates of polypeptide initiation and elongation on ovalbumin and globin messenger ribonucleic acid in a rabbit reticulocyte lysate. J Biol Chem. 1973 Mar 25;248(6):2095–2106. [PubMed] [Google Scholar]
- Preston C. M., Szilagyi J. F. Cell-free translation of RNA synthesized in vitro by a transcribing nucleoprotein complex prepared from purified vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1002–1009. doi: 10.1128/jvi.21.3.1002-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. The cell-free synthesis of herpesvirus-induced polypeptides. Virology. 1977 May 1;78(1):349–353. doi: 10.1016/0042-6822(77)90109-x. [DOI] [PubMed] [Google Scholar]
- Sakiyama S., Buchanan J. M. In vitro synthesis of deoxynucleotide kinase programmed by bacteriophage "T4-RNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1376–1380. doi: 10.1073/pnas.68.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. P., Wagner M., Summers W. C. Possible peptide chain termination mutants in thymide kinase gene of a mammalian virus, herpes simplex virus. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4081–4084. doi: 10.1073/pnas.72.10.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Tse T. P. Isolation of rat liver albumin messenger RNA. J Biol Chem. 1976 Dec 10;251(23):7461–7467. [PubMed] [Google Scholar]
- Thang M. N., Thang D. C., De Maeyer E., Montagnier L. Biosynthesis of mouse interferon by translation of its messenger RNA in a cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3975–3977. doi: 10.1073/pnas.72.10.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. C., Walsh M. L., Pennica D., Cohen P. S., Ennis H. L. Transcription-translation and translation-messenger RNA decay coupling: separate mechanisms for different messengers. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1126–1130. doi: 10.1073/pnas.73.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Chambers D. A. A DNA-directed cell-free system for beta-galactosidase synthesis; characterization of the de novo synthesized enzyme and some aspects of the regulation of synthesis. Cold Spring Harb Symp Quant Biol. 1969;34:753–761. doi: 10.1101/sqb.1969.034.01.085. [DOI] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]


