Abstract
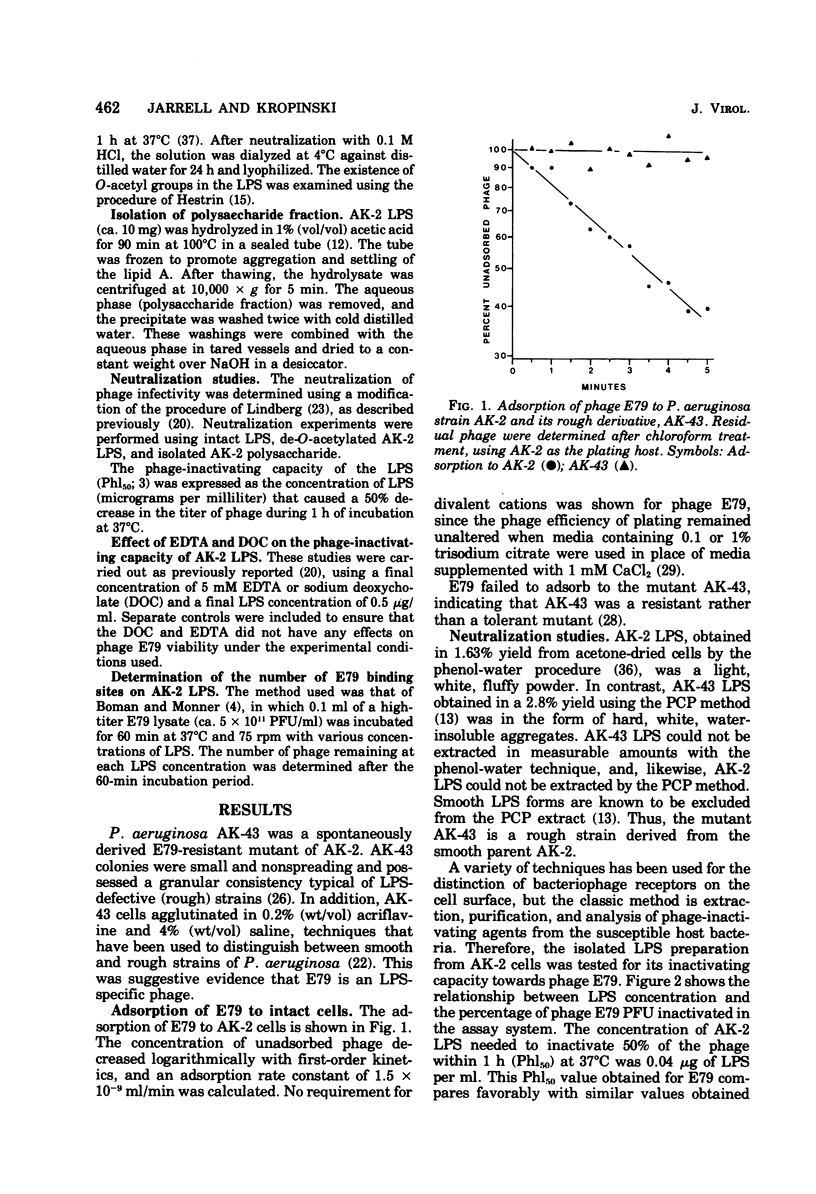
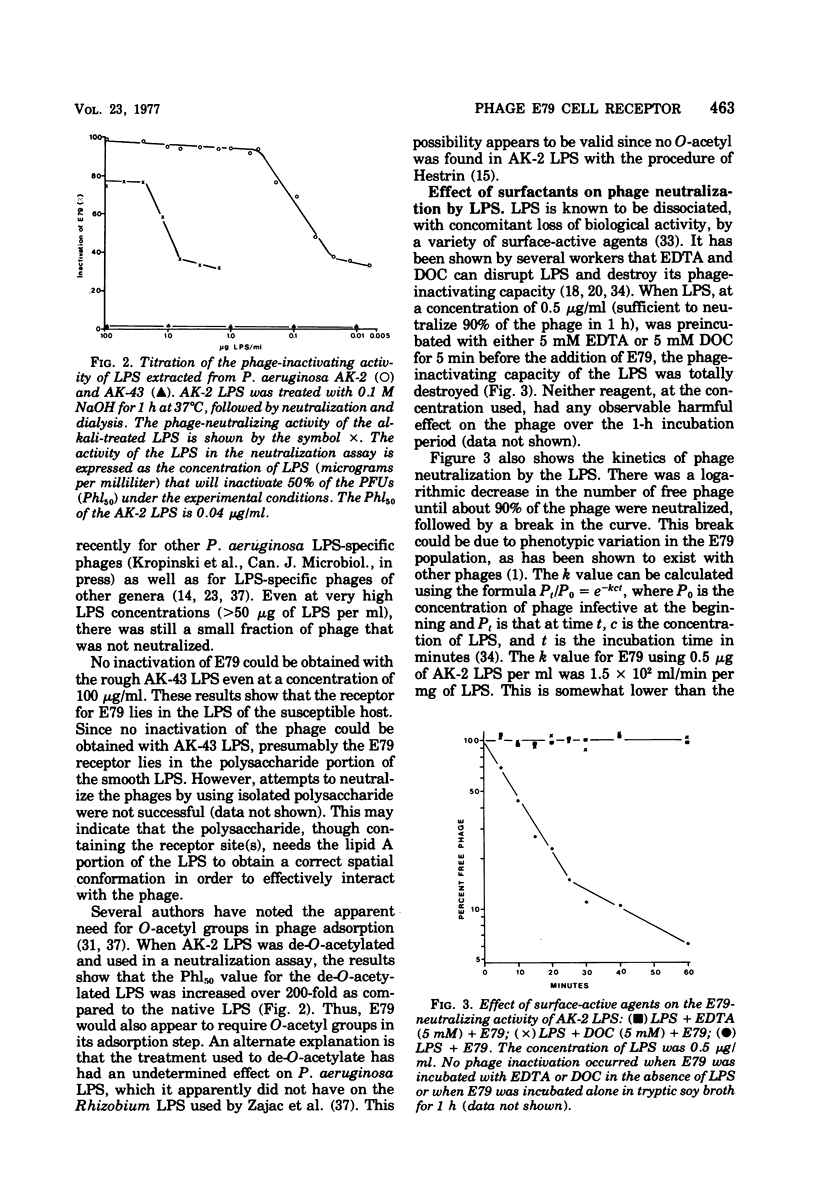
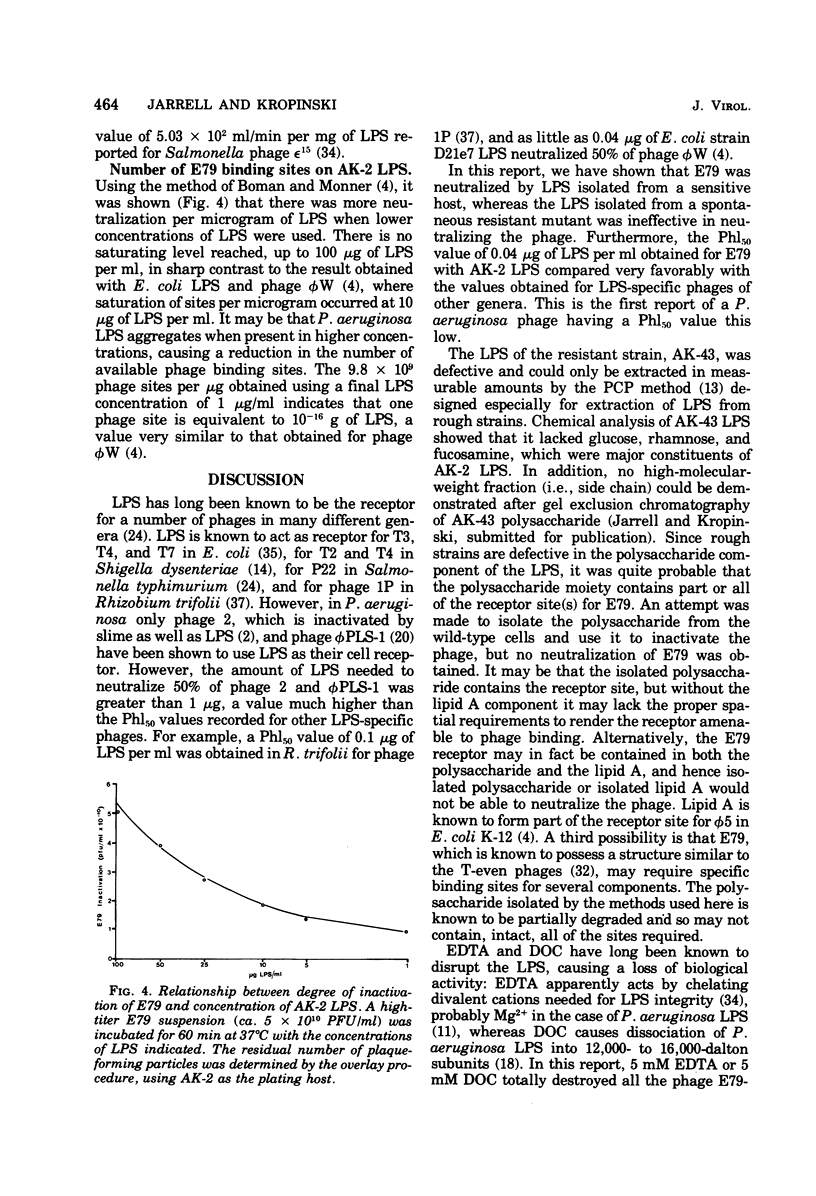
Bacteriophage E79 was shown to interact with the lipopolysaccharide (LPS) of Pseudomonas aeruginosa strain PAO. LPS isolated from an E79-sensitive, smooth strain inactivated the phage, exhibiting a Phl50 value (concentration of LPS that caused a 50% decrease in the titer of phage during 1 h of incubation at 37 degrees C) of 0.04 microgram/ml, whereas the LPS isolated from a rough mutant derived from the wild type showed no neutralizing activity towards E79. EDTA and sodium deoxycholate were demonstrated to abolish the neutralizing capacity of the smooth LPS. One E79 receptor site was shown to be equivalent to 10(-16) g of LPS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUMER J., DIRKX J. [Isolation of bacteriophage receptor substances in Shigella]. Ann Inst Pasteur (Paris) 1960 Jun;98:910–914. [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Reese J. F., Imaeda T. Interaction of Pseudomonas bacteriophage 2 with the slime polysaccharide and lipopolysaccharide of Pseudomonas aeruginosa strain B1. J Virol. 1971 Sep;8(3):311–317. doi: 10.1128/jvi.8.3.311-317.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Monner D. A. Blocking of bacteriophages phi W and phi 5 with lipopolysaccharides from Escherichia coli K-12 mutants. J Bacteriol. 1975 Feb;121(2):465–470. doi: 10.1128/jb.121.2.465-470.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. A pilus-dependent Pseudomonas aeruginosa bacteriophage with a long noncontractile tail. Virology. 1973 Feb;51(2):489–492. doi: 10.1016/0042-6822(73)90448-0. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Basic characterization of a Pseudomonas aeruginosa pilus-dependent bacteriophage with a long noncontractile tail. J Virol. 1973 Nov;12(5):1139–1148. doi: 10.1128/jvi.12.5.1139-1148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E., Pitt T. L. Pilus-dependence of four Pseudomonas aeruginosa bacteriophages with non-contractile tails. J Gen Virol. 1974 Jul;24(1):1–15. doi: 10.1099/0022-1317-24-1-1. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. The adsorption of the Pseudomonas aeruginosa filamentous bacteriophage Pf to its host. Can J Microbiol. 1973 May;19(5):623–631. doi: 10.1139/m73-103. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. The fluorescent staining of bacteriophage nucleic acids. J Gen Microbiol. 1966 Sep;44(3):383–391. doi: 10.1099/00221287-44-3-383. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Melling J. Loss of sensitivity to EDTA by Pseudomonas aeruginosa grown under conditions of Mg-limitation. J Gen Microbiol. 1968 Dec;54(3):439–444. doi: 10.1099/00221287-54-3-439. [DOI] [PubMed] [Google Scholar]
- Fensom A. H., Meadow P. M. Evidence for two regions in the polysaccharide moiety of the lipopolysaccharide of Pseudomonas aeruginosa 8602. FEBS Lett. 1970 Jul 29;9(2):81–84. doi: 10.1016/0014-5793(70)80318-0. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Goldhar J., Eulan E., Kacewman B. Receptors for T2, T4 and T6 phages in Sh. dysenteriae 7 and other serotypes of Sh. dysenteriae. Zentralbl Bakteriol Orig A. 1975 Nov;233(3):335–341. [PubMed] [Google Scholar]
- HOLLOWAY B. W., EGAN J. B., MONK M. Lysogeny in Pseudomonas aeruginosa. Aust J Exp Biol Med Sci. 1960 Aug;38:321–329. doi: 10.1038/icb.1960.34. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Stanisich V. Pseudomonas genetics. Annu Rev Genet. 1971;5:425–446. doi: 10.1146/annurev.ge.05.120171.002233. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Properties of R plasmids determining gentamicin resistance by acetylation in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Sep;6(3):239–252. doi: 10.1128/aac.6.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K., Kropinski A. M. The isolation and characterization of a lipopolysaccharide-specific Pseudomonas aeruginosa bacteriophage. J Gen Virol. 1976 Oct;33(1):99–106. doi: 10.1099/0022-1317-33-1-99. [DOI] [PubMed] [Google Scholar]
- Key B. A., Gray G. W., Wilkinson S. G. The purification and chemical composition of the lipopolysaccharide of Pseudomonas alcaligenes. Biochem J. 1970 Dec;120(3):559–566. doi: 10.1042/bj1200559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Chadwick J. S. The pathogenicity of rough strains of Pseudomonas aeruginosa for Galleria mellonella. Can J Microbiol. 1975 Dec;21(12):2084–2088. doi: 10.1139/m75-297. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A. Studies of a receptor for felix O-1 phage in Salmonella minnesota. J Gen Microbiol. 1967 Aug;48(2):225–233. doi: 10.1099/00221287-48-2-225. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEAD T. H., VAN DEN ENDE M. Bacteriophage inhibition by extracts from phageinsensitive bacteria of the genus Pseudomonas. J Hyg (Lond) 1953 Mar;51(1):108–124. doi: 10.1017/s0022172400015539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa Y., Maruo B. Isolation and characterization of T-even ghost-tolerant mutants of Escherichia coli. J Bacteriol. 1976 Jul;127(1):40–45. doi: 10.1128/jb.127.1.40-45.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. H., Metcalf E. S., Todd J. K. Characteristics of bacteriophages attacking psychrophilic and mesophilic pseudomonads. J Virol. 1968 Apr;2(4):357–364. doi: 10.1128/jvi.2.4.357-364.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M. F116: a DNA bacteriophage specific for the pili of Pseudomonas aeruginosa strain PAO. Virology. 1973 Oct;55(2):558–560. doi: 10.1016/0042-6822(73)90203-1. [DOI] [PubMed] [Google Scholar]
- SLAYTER H. S., HOLLOWAY B. W., HALL C. E. THE STRUCTURE OF PSEUDOMONAS AERUGINOSA PHAGES B3, E79, AND F116. J Ultrastruct Res. 1964 Oct;11:274–281. doi: 10.1016/s0022-5320(64)90032-2. [DOI] [PubMed] [Google Scholar]
- Shaw D. R., Chatterjee A. N. O-Acetyl groups as a component of the bacteriophage receptor on Staphylococcus aureus cell walls. J Bacteriol. 1971 Oct;108(1):584–585. doi: 10.1128/jb.108.1.584-585.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Uetake H. In vitro interaction between phage and receptor lipopolysaccharide: a novel glycosidase associated with Salmonella phage epsilon15. Virology. 1973 Mar;52(1):148–159. [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]
- Zajac E., Russa R., Lorkiewicz Z. Lipopolysaccharide as receptor for rhizobium phage 1P. J Gen Microbiol. 1975 Oct;90(2):365–367. doi: 10.1099/00221287-90-2-365. [DOI] [PubMed] [Google Scholar]



