Abstract
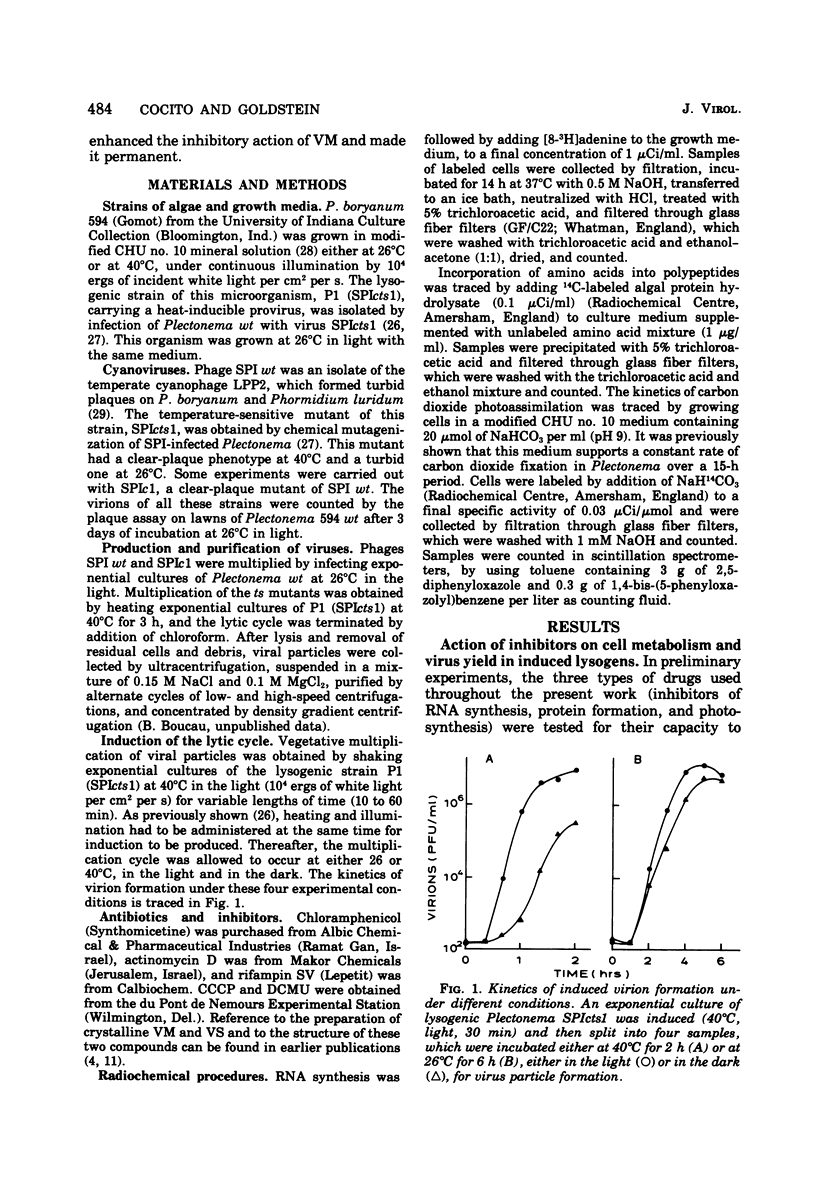
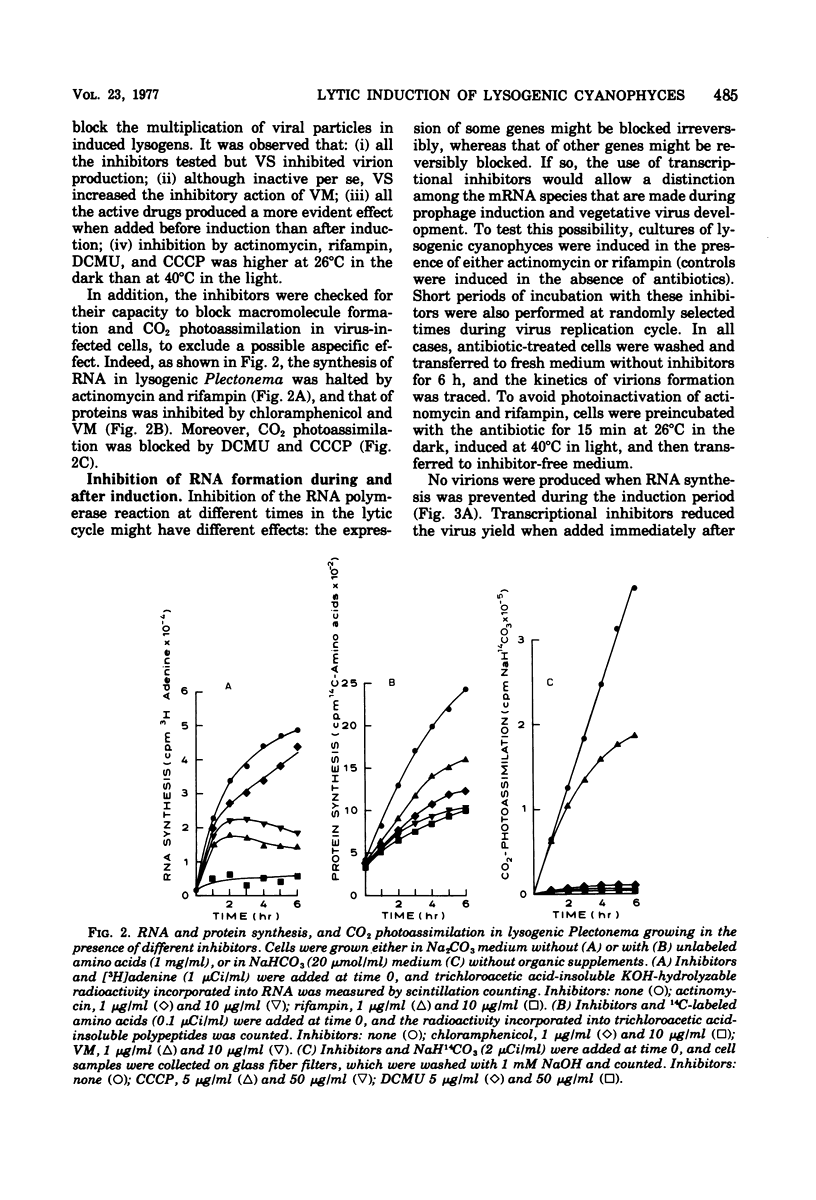
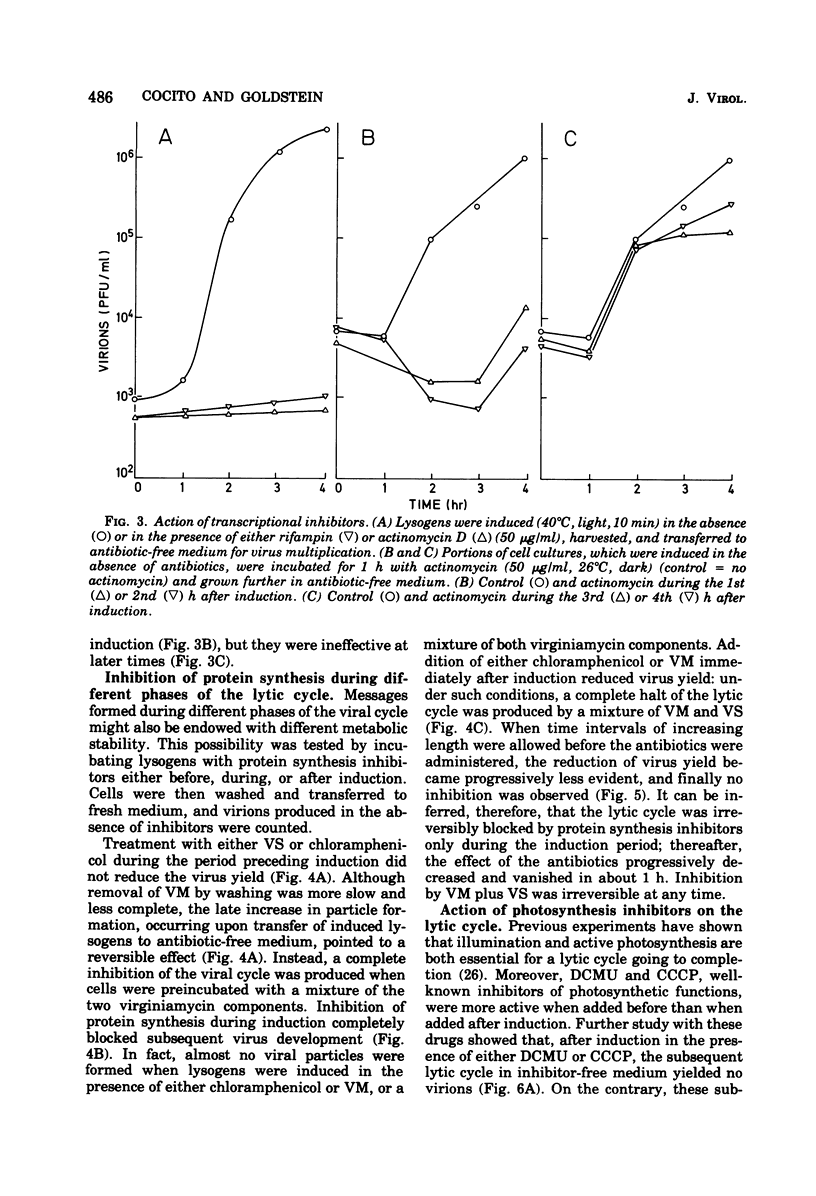
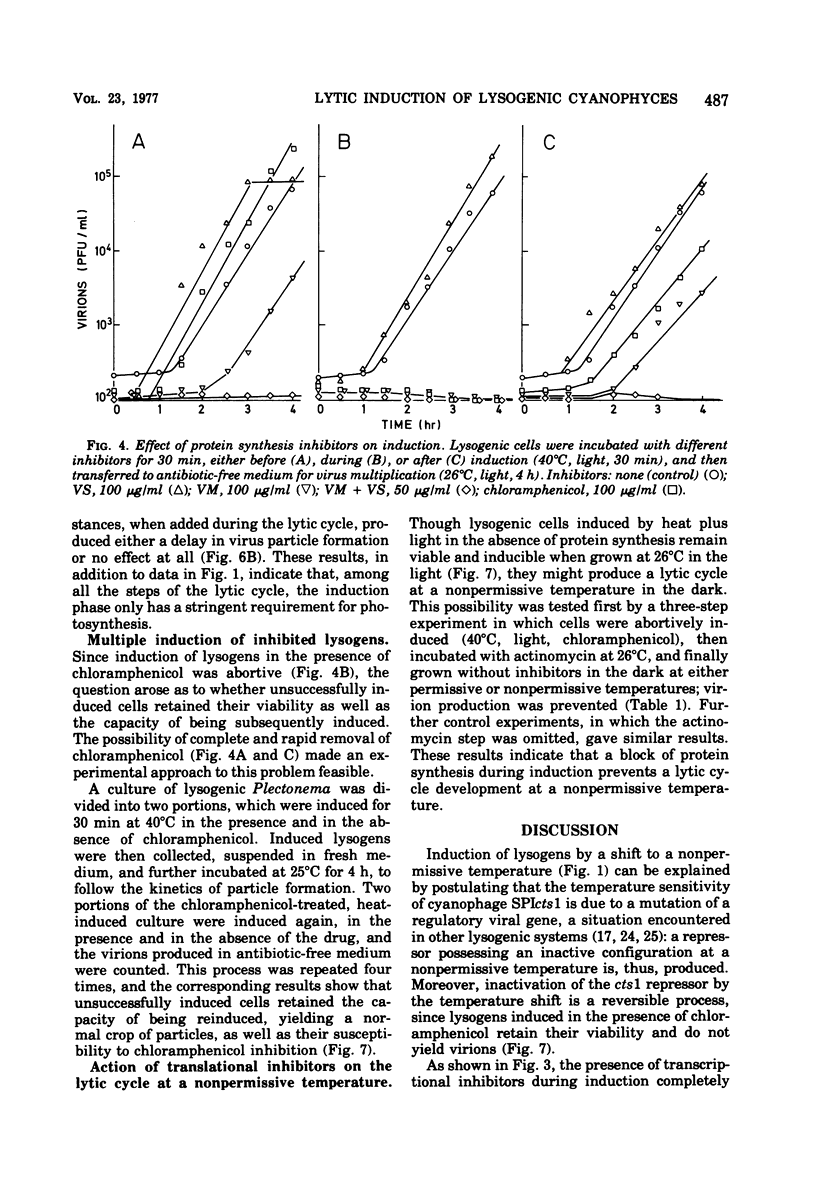
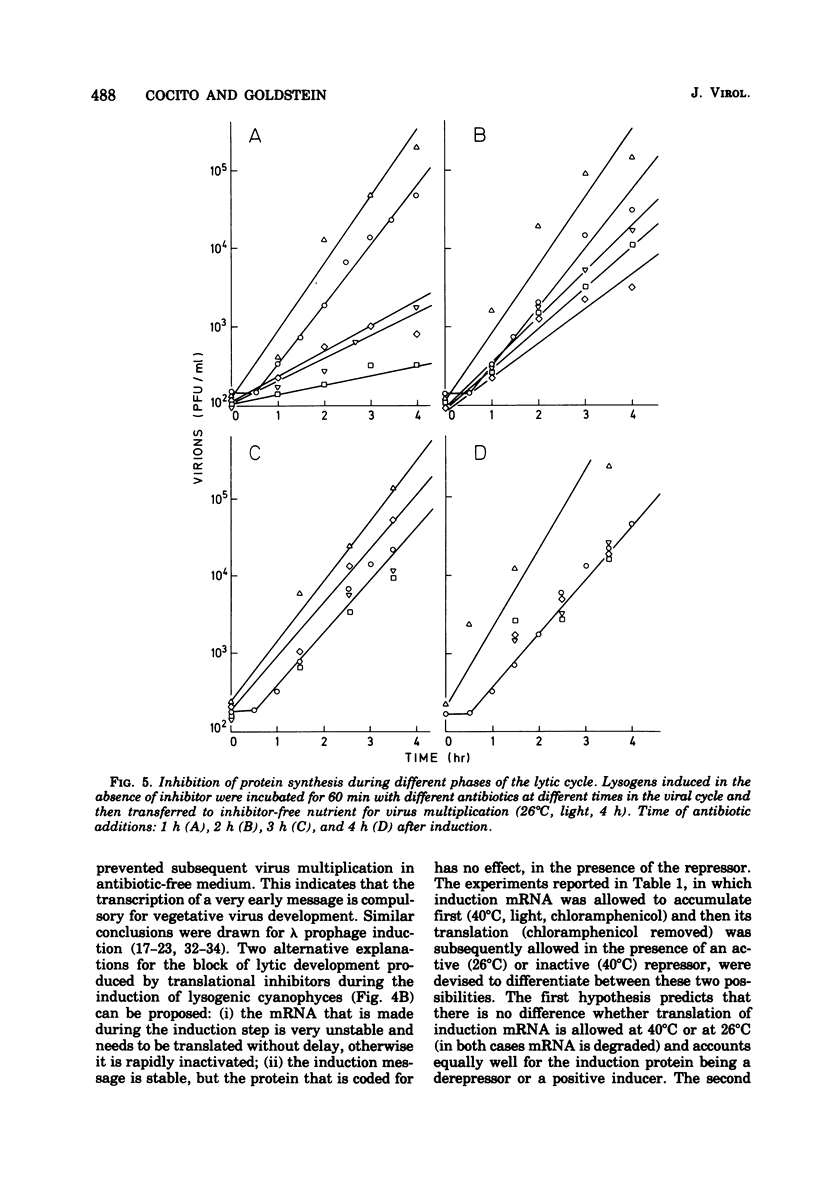
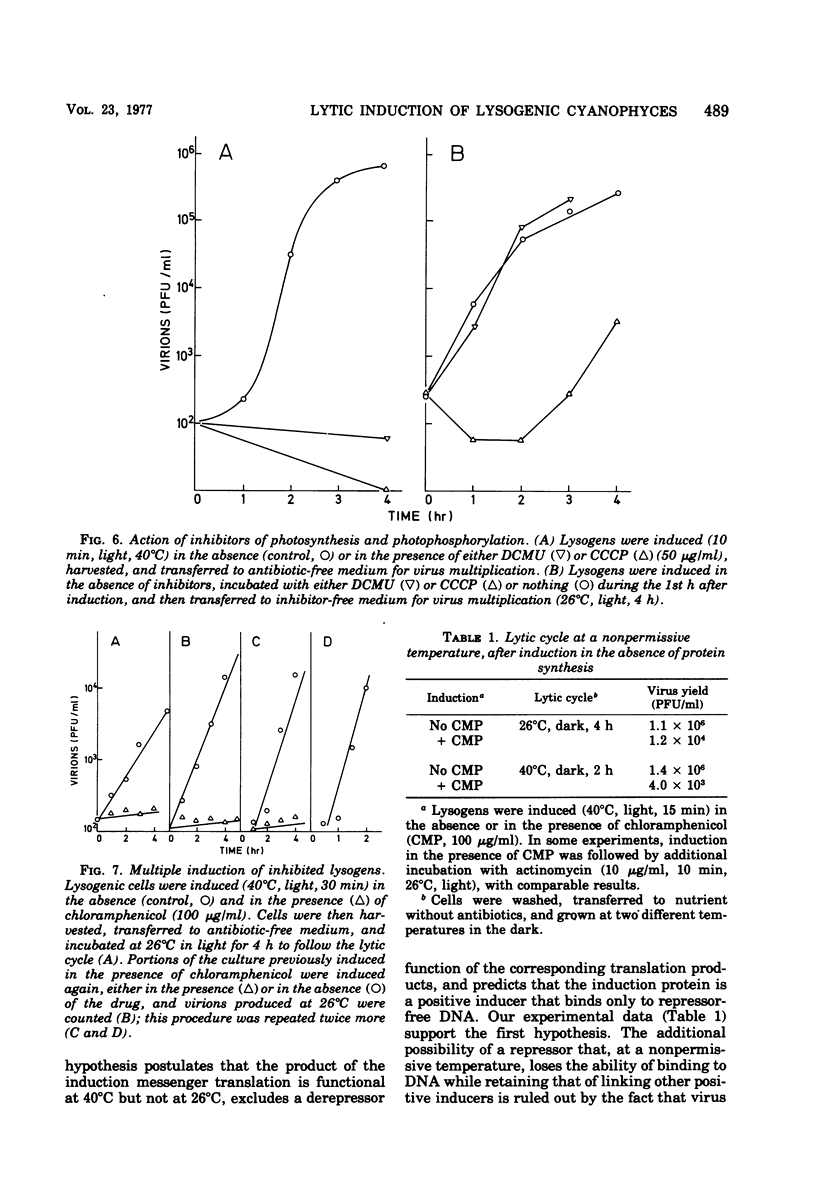
When the lysogenic strain SPIcts1 of the blue-green alga Plectonema boryanum carrying a temperature-sensitive mutation in the LPP2 prophage was heated at a nonpermissive temperature in the light, a lytic cycle occurred, with production of infectious viral particles. Inhibitors of transcription, translation, and photosynthetic functions interfered with this process and produced different effects when administered at different phases of the viral cycle. The presence of the inhibitors during the temperature shift did not allow a successful induction to take place; lysogens submitted to such a process produced a normal virus yield, however, when the drugs were removed and the temperature was shifted again. Incubation with the inhibitors during the early postinduction period reduced the virus yield; at later times, however, the inhibitory action rapidly declined. When cells were induced in the presence of chloramphenicol, incubated with actinomycin, and then grown in the dark, at either permissive or nonpermissive temperatures, virus multiplication was equally inhibited. These data indicate that: (i) provirus induction in lysogenic cyanophyces relies on the synthesis of early viral proteins; (ii) induction of mRNA is unstable and becomes rapidly inactivated when its translation is prevented; and (iii) inhibition of photosynthesis prevents the induction message from being expressed. It is suggested that the SPIcts1 prophage codes for a mutated repressor, which is reversibly inactivated at a nonpermissive temperature, and that the repressor must be inactivated at the same time that the message coded for by very early genes is translated, for a successful induction of the lytic cycle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. M., Jr Algal viruses. Adv Virus Res. 1972;17:243–277. doi: 10.1016/s0065-3527(08)60752-6. [DOI] [PubMed] [Google Scholar]
- Cannon R. E., Shane M. S., Bush V. N. Lysogeny of a blue-green alga, Plectonema boryanum. Virology. 1971 Jul;45(1):149–153. doi: 10.1016/0042-6822(71)90121-8. [DOI] [PubMed] [Google Scholar]
- Cannon R. E., Shane M. S. The effect of antibiotic stress on protein synthesis in the establishment of lysogeny of Plectonema boryanum. Virology. 1972 Jul;49(1):130–133. doi: 10.1016/s0042-6822(72)80014-x. [DOI] [PubMed] [Google Scholar]
- Cocito C. G., Bronchart R., Van Pel B. Phenotypic and genotypic changes induced in eucaryotic cells by protein inhibitors. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1688–1694. doi: 10.1016/0006-291x(72)90804-2. [DOI] [PubMed] [Google Scholar]
- Cocito C. Formation and decay of polyribosomes and ribosomes during the inhibition of protein synthesis and recovery. Biochimie. 1971;53(9):987–1000. doi: 10.1016/s0300-9084(71)80067-6. [DOI] [PubMed] [Google Scholar]
- Cocito C. Formation of ribosomal particles in virginiamycin sensitive and resistant mutants of Bacillus subtilis. Biochimie. 1973;55(2):153–161. doi: 10.1016/s0300-9084(73)80387-6. [DOI] [PubMed] [Google Scholar]
- Cocito C., Fraselle G. The properties of virginiamycin-resistant mutants of Bacillus subtilis. J Gen Microbiol. 1973 May;76(1):115–125. doi: 10.1099/00221287-76-1-115. [DOI] [PubMed] [Google Scholar]
- Cocito C., Kaji A. Virginiamycin M, a specific inhibitor of the acceptor site of ribosomes. Biochimie. 1971;53(6):763–770. doi: 10.1016/s0300-9084(71)80117-7. [DOI] [PubMed] [Google Scholar]
- Cocito C. Metabolism of macromolecules in bacteria treated with virginiamycin. J Gen Microbiol. 1969 Aug;57(2):179–194. doi: 10.1099/00221287-57-2-179. [DOI] [PubMed] [Google Scholar]
- Cocito C. Origin and metabolic properties of the RNA species formed during the replication cycle of virus 2C. J Virol. 1974 Dec;14(6):1482–1493. doi: 10.1128/jvi.14.6.1482-1493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocito C., Shilo M. Macromolecule metabolism and photosynthetic functions in blue-green algae treated with virginiamycin, an inhibitor of protein synthesis. Antimicrob Agents Chemother. 1974 Aug;6(2):136–143. doi: 10.1128/aac.6.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocito C. The action of virginiamycin on nucleic acid and protein synthesis in Bacillus subtilis infected with bacteriophage 2C. J Gen Microbiol. 1969 Aug;57(2):195–206. doi: 10.1099/00221287-57-2-195. [DOI] [PubMed] [Google Scholar]
- Cocito C. The ribosomal cycle in bacteria treated with an inhibitor of protein synthesis. Biochimie. 1973;55(3):309–316. doi: 10.1016/s0300-9084(73)80130-0. [DOI] [PubMed] [Google Scholar]
- Cocito C., Voorma H. O., Bosch L. Interference of virginiamycin M with the initiation and the elongation of peptide chains in cell-free systems. Biochim Biophys Acta. 1974 Mar 27;340(3):285–298. doi: 10.1016/0005-2787(74)90274-3. [DOI] [PubMed] [Google Scholar]
- Echols H. Developmental pathways for the temperate phage: lysis vs lysogeny,. Annu Rev Genet. 1972;6(0):157–190. doi: 10.1146/annurev.ge.06.120172.001105. [DOI] [PubMed] [Google Scholar]
- Echols H. Lysogeny: viral repression and site-specific recombination. Annu Rev Biochem. 1971;40:827–854. doi: 10.1146/annurev.bi.40.070171.004143. [DOI] [PubMed] [Google Scholar]
- Eisen H., Brachet P., Pereira da Silva L., Jacob F. Regulation of repressor expression in lambda. Proc Natl Acad Sci U S A. 1970 Jul;66(3):855–862. doi: 10.1073/pnas.66.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A., Isaacs L. N., Echols H., Sly W. S. DNA replication and messenger RNA production after induction of wild-type lambda bacteriophage and lambda mutants. J Mol Biol. 1966 Aug;19(1):174–186. doi: 10.1016/s0022-2836(66)80059-1. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Marcaud L., Sheldrick P., Luzzati D., Gros F. Studies of the messenger RNA of bacteriophage lambda, I. Various species synthesized early after induction of the prophage. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1013–1020. doi: 10.1073/pnas.61.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naono S., Gros F. On the mechanism of transcription of the lambda genome during induction of lysogenic bacteria. J Mol Biol. 1967 May 14;25(3):517–536. doi: 10.1016/0022-2836(67)90203-3. [DOI] [PubMed] [Google Scholar]
- Padan E., Shilo M. Cyanophages-viruses attacking blue-green algae. Bacteriol Rev. 1973 Sep;37(3):343–370. doi: 10.1128/br.37.3.343-370.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Shilo M., Oppenheim A. B. Lysogeny of the blue-green alga Plectonema boryanum by LPP2-SPI cyanophage. Virology. 1972 Feb;47(2):525–526. doi: 10.1016/0042-6822(72)90294-2. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Hopkins N. The operators controlled by the lambda phage repressor. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1282–1287. doi: 10.1073/pnas.60.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M., Echols H. The role of the N gene of phage lambda in the synthesis of two phage-specific proteins. Proc Natl Acad Sci U S A. 1968 Jun;60(2):707–712. doi: 10.1073/pnas.60.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimon A., Oppenheim A. B. Heat induction of the blue-green alga Plectonema boryanum lysogenic for the cyanophage SPlcts1. Virology. 1975 Apr;64(2):454–463. doi: 10.1016/0042-6822(75)90123-3. [DOI] [PubMed] [Google Scholar]
- Rimon A., Oppenheim A. B. Isolation and genetic mapping of temperature-sensitive mutants of cyanophage LPP2-SPI. Virology. 1974 Dec;62(2):567–569. doi: 10.1016/0042-6822(74)90417-6. [DOI] [PubMed] [Google Scholar]
- Safferman R. S., Morris M. E. Observations on the Occurrence, Distribution, and Seasonal Incidence of Blue-green Algal Viruses. Appl Microbiol. 1967 Sep;15(5):1219–1222. doi: 10.1128/am.15.5.1219-1222.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safferman R. S., Morris M. E., Sherman L. A., Haselkorn R. Serological and electron microscopic characterization of a new group of blue-green algal viruses (LPP-2). Virology. 1969 Dec;39(4):775–780. doi: 10.1016/0042-6822(69)90015-4. [DOI] [PubMed] [Google Scholar]
- Skalka A., Butler B., Echols H. Genetic control of transcription during development of phage gamma. Proc Natl Acad Sci U S A. 1967 Aug;58(2):576–583. doi: 10.1073/pnas.58.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K., Hradecna Z., Szybalski W. Asymmetric distribution of the transcribing regions on the complementary strands of coliphage lambda DNA. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1618–1625. doi: 10.1073/pnas.57.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa T. Effect of ultraviolet irradiation on bacteriophage lambda immunity. J Mol Biol. 1967 Jan 28;23(2):247–263. doi: 10.1016/s0022-2836(67)80031-7. [DOI] [PubMed] [Google Scholar]
- Van Pel B., Bronchart R., Kebers F., Cocito C. Structure and function of cytoplasmic organelles in transiently and permanently bleached Euglena. Exp Cell Res. 1973 Mar 30;78(1):103–110. doi: 10.1016/0014-4827(73)90043-8. [DOI] [PubMed] [Google Scholar]
- Van Pel B., Cocito C. Formation of chloroplast ribosomes and ribosomal RNA in Euglena incubated with protein inhibitors. Exp Cell Res. 1973 Mar 30;78(1):111–117. doi: 10.1016/0014-4827(73)90044-x. [DOI] [PubMed] [Google Scholar]
- Varon M., Cocito C., Seijffers J. Effect of virginiamycin on the growth cycle of Bdellovibrio. Antimicrob Agents Chemother. 1976 Jan;9(1):179–188. doi: 10.1128/aac.9.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]


